Abstract
The application method of essential oils and plant extracts in micro/nanocapsules on textiles can affect the properties of the fabric due to evaporation. This paper presents a comparative study on different applications of encapsulated herbal products on cotton fabric. UV curing of nanocapsules and simultaneous encapsulation/stabilization were used to prepare a controllable release essential oils on cotton fabric. FESEM images indicated well stabilized nanocapsules on the cotton fabric in both methods. FT-IR and UV–Vis analysis confirmed the presence of the encapsulated peppermint oil on the cotton fabric. There was longer controlled release and increased stiffness found in the samples prepared with UV curing, while there was lower weight and thickness, improved water retention, and ameliorated water absorbency in the sample produced with simultaneous encapsulation/stabilization. Further, better durability and prolonged release of antimicrobial, fragrance, and insect repellent agents can be obtained by UV curing method of the textiles, while the simultaneous encapsulation/stabilization method is more suitable for disposable applications such as wound dressings or medical textiles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Application of herbal products such as plant extracts and essential oils as micro/nanocapsules on textiles has better durability of desired properties through controlling the release of core materials (Aladpoosh and Montazer 2016; Bezerra et al. 2016; Chang et al. 2013; Ghayempour et al. 2016; Javid et al. 2014; Liu et al. 2015).
A major concern in application of encapsulated materials on textiles is the used technique to graft encapsulated plant extracts and essential oils on the fabric surface. Usually, application of encapsulated materials on textiles is carried out during thermal processing at high temperature. This is not suitable for essential oils due to breaking of the capsule wall and loss of content. Ultraviolet (UV) and microwave irradiation are low-temperature curing methods that have been introduced as alternatives to thermal processing. Several studies also reported application of microwave curing in the stabilization of encapsulated plant extract on the textiles (Bischof Vukušić et al. 2011; Budimir et al. 2012; El-Molla et al. 2013; Ghayempour and Mortazavi 2015; Li et al. 2008; Zhao et al. 2011). In UV curing, the fabric surface is exposed to UV light for a few seconds to polymerize the resin component into a uniform film and to stabilize the capsules on the fabric surface. High speed, low temperature, energy savings, low pollution, and high productivity are the advantages of this method. This also prevents breaking of the capsules wall and evaporating the core content, increasing the durability of treated fabric (Calamari and Harper 2000; Li et al. 2004, 2005, 2008). UV curing was used to fix lemon fragrance microcapsules on cotton fabric by Li et al. in 2005. They padded the cotton fabric with a mixture containing an oligomer, a monomer, an initiator, and microcapsules, and passed the fabric through a UV light area. They indicated that the optimized curing process was obtained with polyurethane as the oligomer, tripropylene glycol diacrylate as the monomer, and phenyl bis(2,4,6-trimethyl benzoyl) phosphine oxide as the initiator in the presence of fragrance microcapsules (Li et al. 2005).
On the other hand, treatment of textiles with encapsulated materials in a multi-step process was carried out using a chemical binder including encapsulation, centrifuging, drying, and final stabilization. This is costly and time-consuming with unexpected side-effects. However, this has recently been solved by introducing a single-step process through simultaneous encapsulation and stabilization on the fabric (Ghayempour and Montazer 2016b).
In this paper, cotton fabric containing encapsulated peppermint oil was prepared by two different methods: (1) application of encapsulated essential oil on cotton fabric using UV curing and (2) one-step encapsulation/stabilization on the cotton fabric. The treated fabrics were characterized by field emission scanning electron microscopy (FESEM) and Fourier transform infrared (FT-IR) spectroscopy. The peppermint oil on the fabric and its release behavior from nanocapsules were investigated using UV–Vis. The washing and rubbing stability of the treated fabrics were evaluated comparing FT-IR and UV–Vis spectra before and after washing and rubbing. Finally, the physical properties of the treated fabrics such as stiffness, weight, thickness, water retention, and wettability were studied and the results reported.
Experimental
Materials
Peppermint oil was purchased from Barij Essence Co., Iran. Tragacanth gum (TG) was received from Fars province and used as a powder with average size of 200–500 μm. Triton X-100 and aluminum chloride were purchased from Merck Co., Germany. All solutions were prepared with distilled water. The used textile substrate was a 140 g m−2 bleached cotton fabric.
Encapsulation of peppermint oil in TG
Encapsulation was carried out using peppermint oil as the core material, TG as the wall material, aluminum chloride as a cross-linking agent, and Triton X-100 as an emulsifier. The mixture of TG, peppermint oil, and Triton X-100 was sonicated with a 7 mm sonotrode connected to a 400 W and 24 kHz ultrasonic homogenizer (UP400S, Hielscher, Germany). Aluminum chloride was then added to the prepared microemulsion and sonicated to form nanocapsules containing peppermint oil. The prepared nanocapsules were centrifuged with a RST 24&16 centrifuge device. They were finally washed with deionized water to remove the remaining compounds and dried at room temperature.
Application of encapsulated peppermint oil on cotton fabric using UV curing
The prepared nanocapsules were mixed with an aliphatic polyurethane acrylate resin with ratio of 2:1. The obtained mixture was then placed on the cotton fabric using a film applicator with thickness of 30 μm. The fabric was transferred to a 300 W UV curing device and exposed to UV light for 1 min. The component of polyurethane acrylate resin was polymerized into a uniform film and the nanocapsules stabilized on the fabric fibers.
Simultaneous encapsulation and stabilization of peppermint oil on the cotton fabric
Three milliliters of peppermint oil and 0.2 mL Triton X-100 0.2% were added to 100 mL Tragacanth gum 1%, and the obtained mixture was sonicated to a white microemulsion. The cotton fabric was then immersed into microemulsion for 5 min. Aluminum chloride 2% was added to the mixture for simultaneous encapsulation and stabilization of peppermint oil on the cotton fabric. The treated cotton fabric was finally washed with distilled water to remove excess raw materials from the fabric surface.
Characterization of the treated fabrics
The surface morphology of the treated cotton fabrics was studied by a FESEM device (VEGA2-TESCAN scanning electron microscopy, Czech). A FT-IR spectrophotometer (Thermo Nicolet, model: Nexus 670, USA) was used to obtain FT-IR spectra of the samples. Peppermint oil on the fabric surface was confirmed by studying UV–Vis spectra of pure peppermint oil and treated cotton fabric using a UV–Vis spectrophotometer (UV–Vis Array Spectrophotometer, Iran). The release behavior of peppermint oil from the treated cotton fabrics was investigated by stirring in 5 mL acetone for different intervals for releasing peppermint oil from nanocapsules. The comparison of UV–Vis spectra of the solution indicates the release behavior of the treated fabrics. The washing and rubbing stability of the treated fabrics were investigated by FT-IR and UV–Vis spectra before and after testing. Washing and rubbing tests were carried out according to ISO 105-C10 (2006) standard test method during a 45 min processing at 30 °C and ISO 105-X12 (2001) standard test method using a crockmeter/rubbing stability tester (SDL Atlas, M238AA, USA) respectively. The physical properties of the treated cotton fabrics including stiffness, weight and thickness were investigated according to ASTM D-1388-64 (1975), SIST EN 12127:1999 (1999) and SIST EN ISO 5084:1999 (1996) standard methods, respectively. Also, the water retention and water absorbency of samples were measured based on ASTM D2402-07 (2012) and AATCC 79 (2014) standard test methods.
Results and discussion
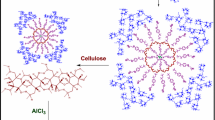
Two various methods were used to prepare cotton fabric containing encapsulated peppermint oil. Figure 1 shows a schematic of UV curing and simultaneous encapsulation/stabilization methods for preparing cotton fabric containing nanocapsules. In the UV curing method, the prepared microemulsions were converted to TG nanocapsules in the presence of aluminum ions. The size distribution of nanocapsules on the fabric surface was calculated from FESEM images. The FESEM image and structure of nanocapsules containing peppermint oil are shown in Fig. 2a, b. The nanocapsules are formed in a spherical shape, and peppermint oil was placed into TG nanocapsules through cross-linking of aluminum ions with carboxylic groups of TG. Figure 2c indicates fairly uniform size distribution of nanocapsules ranging from 55–75 nm, with maximum size distribution of 66 nm (66%). The prepared nanocapsules were stabilized on the fabric with exposure to UV light. TG with emulsifying, gelling, and stabilizing ability acts as the wall material in encapsulation processing. This can be used as a natural binder in stabilization processing (Ghayempour and Montazer 2017). Here, a direct application method was followed called simultaneous encapsulation/stabilization on the fabric surface. The nanocapsules containing peppermint oil formed on the fabric fibers and stabilized using TG.
Surface morphology of the treated cotton fabrics
FESEM images of the treated cotton fabrics produced by UV curing of nanocapsules and simultaneous encapsulation/stabilization are shown in Fig. 3. As seen in Fig. 3a, the nanocapsules containing peppermint oil successfully stabilized on the cotton fabric using acrylic resin without destroying or breaking of the nanocapsules wall. The acrylic resin covered the surface of the nanocapsules on the fabric surface as a film layer. Figure 3b shows that simultaneous encapsulation/stabilization method leads to in situ preparation of nanocapsules containing peppermint oil on the cotton fabric. This is a green and safe method to form nanocapsules on the fabric surface through ultrasound irradiation without any separated stabilization process. TG as the wall material in encapsulation processing also acts as a binder to graft the prepared nanocapsules on the fabric surface. In fact, stabilization of the nanocapsules on the cotton fabric occurs through interactions between aluminum ions and hydroxyl groups of cellulose and also the intermolecular hydrogen bindings between TG and cellulose.
Structure of the treated cotton fabrics
FT-IR was used to study peppermint oil into Tragacanth nanocapsules on the fabric surface prepared by two different methods, UV curing of nanocapsules and simultaneous encapsulation/stabilization (Fig. 4). FT-IR spectrum of TG includes the peaks related to the stretching vibrations of hydroxyl, carboxylate and carbonyl groups at 3420, 1629, and 1743 cm−1, respectively. The aliphatic C–H groups, C–O–C bonds and hydroxyl groups in raw cotton fabric cause peaks at 2900, 1058, and 3347 cm−1. FT-IR spectrum of peppermint oil is complex due to various important compounds such as menthol, menthone, menthofuran, menthyl acetate, and piperitone. The peaks at 1712 and 1676 cm−1 can be attributed to carbonyl group of menthone and piperitone, respectively (Ghayempour and Mortazavi 2014). The peaks in 1713 and 1676 cm−1 along with a peak at 1742 cm−1 related to TG appeared in the spectra of the treated cotton fabric using UV curing method. The similar peaks also appeared in spectra of the treated fabric using simultaneous encapsulation/stabilization method. These confirm peppermint oil and TG on the surface of the fabric. The nanocapsules on fabrics treated with UV curing and simultaneous encapsulation/stabilization methods create peaks at 1111 and 1110 cm−1 related to Al–O–C bond respectively (Wypych 2004). A peak appeared at 1729 cm−1 related to acrylate resin used as the binder in UV curing process.
Presence of encapsulated peppermint oil on the fabrics
The presence of peppermint oil on the fabrics was confirmed by UV–Vis spectroscopy of pure peppermint oil and treated cotton fabrics. As seen in Fig. 5, comparing UV–Vis spectra of pure peppermint oil with cotton fabric treated with both methods shows peppermint oil on the fabric. A peak at 242 nm in the spectrum of treated fabric confirms the successful encapsulation and stabilization using both different methods.
Release behavior of peppermint oil from the treated cotton fabrics
Application of encapsulated materials on the textiles is useful due to controlled release. This characteristic was investigated using UV–Vis spectra of the remaining solution after different stirring times. Figure 6a shows a prolonged release for the fabric treated with the UV curing method, confirming that not all of the peppermint oil is released from the nanocapsules on the cotton fabric. The value of solution absorbance at 242 nm increased from 0.27 to 1.43 after 72 h. A very slow release of peppermint oil from the stabilized nanocapsules on the fabric surface indicates a good durability of the performed treatment. The UV–Vis spectrum of the treated cotton fabric with simultaneous encapsulation/stabilization method and the absorbance intensity for different stirring times at 242 nm are presented in Fig. 6b. The absorbance intensity increased from 0.34 for 2 h to 1.38 for 24 h at 242 nm indicating gradual peppermint oil release from the nanocapsules on the cotton fabric. The peppermint oil was entirely released from the cotton fabric after 24 h stirring, and the absorbance intensity was maximized. This indicates good stability and controlled release of the plant extracts from the encapsulated and treated cotton fabric. Figure 6c shows the plot of relative concentration of released peppermint oil against time for the cotton fabric treated with both methods. As observed, the relative concentration of peppermint oil released from the cotton fabric treated with simultaneous encapsulation/stabilization method reaches 87% after 24 h, while this is 94% for the cotton fabric treated with the UV curing method after 72 h. Figure 7 indicates a mechanism of the controlled release of peppermint oil from nanocapsules on the cotton fabric schematically. The polymeric wall of nanocapsules and the external environment are important factors in the release rate of materials from nanocapsules on the textiles. Tragacanth gum as the wall material of nanocapsules can gradually be destroyed through diffusion or mechanical stimuli. Diffusion into Tragacanth nanocapsules performs in presence of an external hydrophilic solvent and mechanical stimuli carries out by the pressure force or friction operation. These lead to partial breakage of Al–O–C bonds and release of peppermint oil from nanocapsules. The cotton fabric treated with UV-cured nanocapsules showed better durable release behavior against the simultaneous encapsulation/stabilization method. The acrylate resin covers the fabric surface, and the film layer of stabilized nanocapsules on the fibers produced better stability and durability of the treated fabrics. The slow release of peppermint oil from cotton fabric treated with UV curing is suitable for antimicrobial and redolent applications required prolonged release. The release of peppermint oil from nanocapsules can be performed through diffusion or mechanical stimuli. On the other hand, the faster controlled release of peppermint oil from the fabric treated through simultaneous encapsulation/stabilization is appropriate for wound dressing applications, due to the fast release of wound healing agents to accelerate the wound healing processing. The release of peppermint oil from Tragacanth nanocapsules on wound dressing was previously carried out through diffusion (Ghayempour and Montazer 2016a).
Washing stability
Washing stability is an important issue for the treated fabrics that was investigated by FT-IR and UV–Vis analysis. The FT-IR spectrum of the cotton fabrics treated with two various methods before and after washing are shown in Fig. 8. The peaks related to carbonyl groups of TG at 1742 cm−1 and peaks related to peppermint oil at 1677 and 1712 cm−1 in FT-IR spectrum of washed cotton fabric using UV curing (Fig. 8a) indicates presence of TG and peppermint oil on the washed fabric. The peak related to Al–O–C bond at 1112 cm−1 also confirms the nanocapsules on the fabric surface. Similar results were obtained by the cotton fabric treated using simultaneous encapsulation/stabilization method. Figure 8b shows the similar peaks for the washed fabric; though, their intensity slightly decreased compared to the UV curing method. This indicates the higher stability of the fabric treated using UV curing method due to covering of nanocapsules with acrylate resin as a film layer. Table 1 represents the absorbance intensities in UV–Vis spectra of the cotton fabric treated with UV curing and simultaneous encapsulation/stabilization methods before and after washing. The lower absorbance intensity of the washed samples confirmed the FT-IR results. A number of nanocapsules can be removed from the fabric surface during washing reduced the absorbance intensity; nevertheless, the absorbance of 0.55 and 0.48 for the washed fabrics treated with UV curing and simultaneous encapsulation/stabilization methods proves the presence of peppermint oil. The results indicated remaining of 36.2 and 30.6% peppermint oil on the cotton fabric treated with UV curing and simultaneous encapsulation/stabilization after washing.
Rubbing stability
FT-IR and UV–Vis were used to study the rubbing stability of the treated fabrics. Figure 9a shows FT-IR spectra of the fabric treated with UV curing method before and after rubbing. The peaks at 1742, 1677/1712 and 1111 cm−1 related to TG, peppermint oil and Al–O–C in FT-IR spectrum of the rubbed treated cotton fabric indicates remaining of a few nanocapsules on the fabric after rubbing. Similar peaks appear for the fabric treated with simultaneous encapsulation/stabilization (Fig. 9b), which shows that it has relatively good rubbing stability. Table 1 also represents a lower absorbance intensity in the UV–Vis spectra for both samples during rubbing; however, these peaks indicate that some nanocapsules containing peppermint oil still remain on the fabric surface after rubbing. The amount of peppermint oil on the rubbed fabrics against un-rubbed fabrics were 24.3 and 18.5% for cotton fabric treated with UV curing and simultaneous encapsulation/stabilization methods, respectively.
Physical properties of treated cotton fabrics
Table 2 presents the physical properties of the cotton fabrics treated with UV curing and simultaneous encapsulation/stabilization methods. The results indicate that the thickness of the fabric treated with UV curing is increased more than the simultaneous encapsulation/stabilization method. This is due to placing a resin film layer on the nanocapsules in UV curing. The stiffness of the cotton fabric treated with simultaneous encapsulation/stabilization is close to that of raw cotton fabric; however, the stiffness of the UV curing method was increased to 198.12 N m−1. This is due to the resin film layer possibly affecting the stiffness of the treated fabric. The weight of the fabrics is also increased for UV curing and simultaneous encapsulation/stabilization methods, 4.1 and 3.5%, respectively. This is related to stabilization of nanocapsules along with acrylate resin in UV curing and formation of nanocapsules on the fabric in simultaneous encapsulation/stabilization. Water retention and water absorbency of the cotton fabric treated with UV curing is lower than simultaneous encapsulation/stabilization caused from the hydrophobic structure of acrylate resin. This is related to the cellulosic structure of cotton fabric and swelling of TG due to the water-swellable fraction called bassorin (Ghayempour et al. 2015; Montazer and Kahali 2016). The water retention of the cotton fabrics treated with UV curing and simultaneous encapsulation/stabilization methods enhanced 134 and 333%, respectively. The time of water absorption into raw cotton fabric was 8.22 s, which increased to 10.18 s for the UV curing method. The formed resin film layer on the fabric surface prevents water absorbing into the fabric. On the other hand, the water quickly absorbed into the cotton fabric treated with simultaneous encapsulation/stabilization due to the swelling nature of TG.
Conclusions
UV curing method is a suitable alternative for common thermal stabilization process; however, introducing a fast and low-temperature method for reducing processing time and also comparing with UV curing method is the main approach of this paper. Nanocapsules containing peppermint oil was prepared in a micromulsion method using ultrasonic irradiation and then stabilized on the cotton fabric with UV curing method. Also a simultaneous encapsulation/stabilization method was used for in situ encapsulation of peppermint oil on the cotton fabric. FESEM images indicated coverage of the nanocapsules with a resin film layer on fabric treated by UV curing. Further, nanocapsules successfully formed and stabilized on the fabric using TG with simultaneous encapsulation/stabilization method. The peaks related to TG, peppermint oil and Al–O–C bonds in FT-IR spectra and also peppermint oil in UV–Vis spectra of the treated fabrics confirmed the nanocapsules containing peppermint oil on the cotton fabrics treated with both methods. The cotton fabric treated with UV curing indicated prolonged release time and relatively better washing and rubbing stability. This suggests that this method is optimal for preparation of antimicrobial, fragrance, anti-odor, and insect repellents requiring prolonged release and stability against washing and rubbing. Nevertheless, a covering of acrylate resin as a film layer on the fabric surface leads to reducing properties of the fabric such as weight, thickness, water retention, and water absorbency. On the other hand, simultaneous encapsulation/stabilization improves physical properties of the fabric. This provides a high quality fabric with relative appropriate stability and controlled release. This can be used in different textiles; however, it is more useful for wound dressing or other disposable textiles that do not need for washing or rubbing stability and long released time.
References
AATCC Test Method 79 (2014) Absorbency of textiles, American association of textile chemists and colorists. Research Triangle Park, NC, USA
ASTM D-1388-64 (1975) Standard test method for stiffness of fabrics. New York, ASTM
ASTM D2402-07 (2012) Standard test method for water retention of textile fibers (Centrifuge procedure). ASTM International, West Conshohocken, PA
Aladpoosh R, Montazer M (2016) Nano-photo active cellulosic fabric through in situ phytosynthesis of star-like Ag/ZnO nanocomposites: investigation and optimization of attributes associated with photocatalytic activity. Carbohydr Polym 141:116–125. doi:10.1016/j.carbpol.2016.01.005
Bezerra FM, Carmona OG, Carmona CG, Lis MJ, de Moraes FF (2016) Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 23:1459–1470. doi:10.1007/s10570-016-0882-5
Bischof Vukušić S, Flinčec Grgac S, Budimir A, Kalenić S (2011) Cotton textiles modified with citric acid as efficient antibacterial agent for prevention of nosocomial infections. Croat Med J 52:68–75. doi:10.3325/cmj.2011.52.68
Budimir A, Bischof Vukusic S, Grgac Flincec S (2012) Study of antimicrobial properties of cotton medical textiles treated with citric acid and dried/cured by microwaves. Cellulose 19:289–296. doi:10.1007/s10570-011-9614-z
Calamari TA, Harper RJ (2000) Textiles, finishing. Kirk-Othmer Encyclopedia of Chemical Technology, Wiley. doi:10.1002/0471238961.0609140903011201.a01
Chang F-P, Kuang L-Y, Huang C-A, Jane W-N, Hung Y, Y-iC Hsing, Mou C-Y (2013) A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J Mater Chem B 1:5279–5287. doi:10.1039/C3TB20529K
El-Molla MM, Haggag K, El-Shall FN, Shaker NO, Alian NA (2013) Use of novel synthesized aqueous binders for pigment printing of polyester fabrics. Indian J Fibre Text Res 38:57–65
Ghayempour S, Montazer M (2016a) Micro/nanoencapsulation of essential oils and fragrances: focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J Microencapsul. doi:10.1080/02652048.2016.1216187
Ghayempour S, Montazer M (2016b) A robust friendly nano-encapsulated plant extract in hydrogel Tragacanth gum on cotton fabric through one single step in-situ synthesis and fabrication. Cellulose 23:2561–2572. doi:10.1007/s10570-016-0958-2
Ghayempour S, Montazer M (2017) Ultrasound irradiation based in-situ synthesis of star-like Tragacanth gum/zinc oxide nanoparticles on cotton fabric. Ultrason Sonochem 34:458–465. doi:10.1016/j.ultsonch.2016.06.019
Ghayempour S, Mortazavi SM (2014) Antibacterial activity of peppermint fragrance micro–nanocapsules prepared with a new electrospraying method. J Essent Oil Res 26:492–498. doi:10.1080/10412905.2014.949882
Ghayempour S, Mortazavi SM (2015) Microwave curing for applying polymeric nanocapsules containing essential oils on cotton fabric to produce antimicrobial and fragrant textiles. Cellulose 22:4065–4075. doi:10.1007/s10570-015-0765-1
Ghayempour S, Montazer M, Mahmoudi Rad M (2015) Tragacanth gum as a natural polymeric wall for producing antimicrobial nanocapsules loaded with plant extract. Int J Biol Macromol 81:514–520. doi:10.1016/j.ijbiomac.2015.08.041
Ghayempour S, Montazer M, Mahmoudi Rad M (2016) Encapsulation of Aloe Vera extract into natural Tragacanth gum as a novel green wound healing product. Int J Biol Macromol 93, Part A:344–349. doi:10.1016/j.ijbiomac.2016.08.076
International Standard, ISO 105-X12 (2001) Textiles-tests for colour fastness: colour fastness to rubbing. ISO, Switzerland
International Standard, ISO 105-C10 (2006) Textiles-tests for colour fastness: colour fastness to washing with soap or soap and soda. ISO, Switzerland
Javid A, Raza ZA, Hussain T, Rehman A (2014) Chitosan microencapsulation of various essential oils to enhance the functional properties of cotton fabric. J Microencapsul 31:461–468. doi:10.3109/02652048.2013.879927
Li S, Boyter H, Stewart N (2004) Ultraviolet (UV) curing processes for textile coloration. AATCC Rev 4:44–49
Li S, Boyter H Jr, Qian L (2005) UV curing for encapsulated aroma finish on cotton. J Text Inst 96:407–411
Li S, Lewis JE, Stewart NM, Qian L, Boyter H (2008) Effect of finishing methods on washing durability of microencapsulated aroma finishing. J Text Inst 99:177–183. doi:10.1080/00405000701489701
Liu C, Liang B, Shi G, Li Z, Zheng X, Huang Y, Lin L (2015) Preparation and characteristics of nanocapsules containing essential oil for textile application. Flavour Fragr J 30:295–301. doi:10.1002/ffj.3245
Montazer M, Kahali P (2016) A novel polyvinyl alcohol–tragacanth/nano silver hydrogel on polyester fabric through in-situ synthesis method. J Ind Text 45:1635–1651
SIST-EN ISO 5084:1999 (1996) Determination of thickness of textiles and textile products. Brussels, CEN
SIST-EN 12127 (1999) Determination of mass per unit area using small samples. Brussels, CEN
Wypych F (2004) Chemical modification of clay surfaces. In: Fernando W, Kestur Gundappa S (eds) Interface science and technology, vol 1. Elsevier, New York, pp 1–56. doi:10.1016/S1573-4285(04)80036-X
Zhao X, Min J, Jx He (2011) Effect of microwave curing on antimicrobial activity of chitosan biguanidine hydrochloride treated wool fabrics. J Text Inst 102:801–807. doi:10.1080/00405000.2010.522047
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghayempour, S., Montazer, M. Herbal products on cellulosic fabric with controlled release: comparison of in situ encapsulation and UV curing of the prepared nanocapsules. Cellulose 24, 4033–4043 (2017). https://doi.org/10.1007/s10570-017-1385-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1385-8