Abstract
Application of encapsulated essential oils in the chemical finishing of textiles is rapidly developing because of their versatility and flexibility. In this work, alginate nanocapsules containing peppermint oil were prepared by the microemulsion method and applied on cotton fabric by a microwave curing process. Effective parameters on antimicrobial activity of finished cotton fabric were optimized to obtain a good antimicrobial and fragrant textile. FT-IR spectroscopy and TGA were used to quantify the presence of nanocapsules on the cotton fabric. Also, the surface distribution, attendance and preferred position of the nanocapsules on the textile fibers were investigated by SEM images. The releasing behavior of peppermint oil from the finished cotton fabric in different washing cycles measured by GC-MS analysis indicated that the percent of peppermint oil reached 16 % after 25 washing cycles. Antimicrobial activity of the finished fabric showed 100 % bacterial reduction for both E. coli and S. aureus bacteria in optimum conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fibers and polymers not only have a resistance ability against different bacteria and disease transmission, but they also normally act as a common media for the growth, transfer and diffusion of microorganisms (Jantas and Górna 2006). Microorganisms rapidly transfer to fabric through direct contact with the skin. Also, the rough and permeable surface of the fabric provides a favorable environment for their growth. Any textile finishing method that leads to inhibiting or killing of microorganisms can be properly described as “antimicrobial finishing” (Heywood 2003).
Fragrances and essential oils have long been used in pharmacy and medicine. Nowadays, they are considered natural drugs because of their pharmaceutical effects (Wang and Chen 2005). Most essential oils are antiseptic and kill bacteria or inhibit their growth. Also, they are compatible with the human body and promote cellular rejuvenation. Among the essential oils, peppermint oil has been widely used in various fields such as foods, cosmetics and personal products because of its properties including headache and migraine pain relief; antiseptic and antispasmodic effects; treatment of colds, flu and digestive problems; and relief of itching, stress and mental illnesses (Cristina 2004).
Aromatherapy is the science and art of using natural herbal products and essential oils to balance the mind, body and spirit (Cristina 2004). A new development in aromatherapy science is the preparation of fragrant textiles using different plant extracts. These textiles play an important role in meeting physiological demands, providing comfort and happiness as well as killing pathogenic bacteria. An important problem is the volatility of essential oils and their chemical instability in relation to air, light, moisture and high temperatures (Beristain et al. 2001; Cristina 2004). The most effective method for preparing fragrant textiles is a technique based on preparing tiny packaged materials with many attractive features called micro-nanoencapsulation, which can prolong the scent lifetime through controlled release (Ghayempour and Mortazavi 2013, 2014; Teixeira et al. 2011).
The wall material is an important factor in the characterization of micro-nanocapsules and aromatherapy textiles finished with fragrant mico-nanocapsules. Among the natural polysaccharides, alginate is widely used in different fields such as the medicine, food and textile industries because of its biodegradability, compatibility and capability of drug delivery. It is a natural polysaccharide consisting of linear copolymers of 1-4 glycosidically linked β-d-mannuronic acid and α-l-guluronic acid residues (Ghayempour and Mortazavi 2014, 2015).
A major concern is the need for a technique to graft the fragrant nanocapsules on the fabric surface. Some of the studies done on the microencapsulation of essential oils and their applications in the textile industry are summarized in Table 1. For example, Monllor et al. (2007) prepared melamine formalin microcapsules containing flavor and applied them on the cotton fabric by impregnation and exhaustion methods. They showed that the efficiency of the impregnation process is higher than that of bath exhaustion.
Stabilization of nanocapsules on textiles has usually been performed during a thermal process at high temperatures (130–170 °C) over a certain period of time (1–10 min). The quick evaporation and swelling lead to breaking of the capsule walls and the escape of their contents. Loss of the fragrance from the capsules reduces the amount of aroma on the fabric and decreases its durability. A cotton fabric finished with aroma capsules by a thermal curing process can only withstand 25 washing cycles (Li et al. 2005). An alternative to the thermal process is the use of a microwave curing method for the stabilization process. Impinging a short length of microwaves on a material leads to vibration, polarization and rapid motion of the molecules, ultimately heating the material (Li et al. 2008). Bischof Vukušić et al. (2011) used microwave curing in the antimicrobial finishing of cotton fabric with citric acid and sodium hypophosphite monohydrate. They confirmed that the antibacterial activity of samples finished by microwave curing was higher than that of conventional curing. Zhao et al. (2011) finished wool fabrics with chitosan biguanidine hydrochloride using microwave curing and compared the results to those with conventional curing. The results indicated that the degree of crosslinking and antimicrobial activity of the samples was higher with microwave curing. Budimir et al. (2012) used the microwave as a drying and curing device to produce antimicrobial cotton textiles. They showed that the antimicrobial activity of the samples dried by the microwave was better than that of samples obtained by conventional drying. Also, ultraviolet and microwave curing was used by El-Molla et al. (2013) to apply new binders for pigment printing of polyester fabrics.
In this work, alginate nanocapsules containing peppermint oils were prepared by the microemulsion technique using an ultrasonic stirrer and applied on the cotton fabric using a microwave curing method. The effects of various parameters such as the amount of fragrance nanocapsules, type of binder, amount of selected binder and curing time on the antimicrobial activity of the finished cotton fabric were evaluated and the best results used as the optimized values. The presence of nanocapsules on the fabric surface was corroborated by Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM) and thermogravimetric analysis (TGA). Also, release of essential oils from the finished cotton fabric in different washing cycles was investigated by gas chromatography-mass spectrometry (GC–MS) analysis. Finally, the antimicrobial activity of the cotton fabric finished with nanocapsules containing peppermint oil was investigated.
Experimental
Materials
Peppermint oil was received from Barij Essence Pharmaceutical Co., Iran. Sodium alginate [Manutex FAV, ISP Alginates (UK) Ltd.], calcium chloride (Merck, Germany) and Tween 20 (Merck KGaA, Darmstadt, Germany) were purchased. Three types of binders [acrylate from Clariant Co., polyvinyl acetate (PVA) from Kremer and polyurethane (PU) from Edolan] were used to graft the fragrant nanocapsules on the cotton fabrics. Diammonium hydrogen phosphate (DAHP) obtained from Merck Co. was utilized as the catalyst. A cured and bleached 100 % cotton fabric (weight: 140 g m−2, warp: 22 yarn cm−1, weft: 25 yarn cm−1, warp and weft yarn count: 20 Nm warp yarn spin: 640 Tpm and weft yarn spin: 520 Tpm) was used as the fabric sample.
Preparation of nanocapsules
Fragrant nanocapsules were prepared by sodium alginate as the wall material, peppermint oil as the core material and Tween 20 as the emulsifier. For this purpose, a mixture of sodium alginate 1.5 %, 3 ml peppermint oil and Tween 20 0.1 % was stirred using a 200-W and 24-kHz ultrasonic wave-generating device (UP200H Hielscher, Germany) equipped with a sonotrode (3 mm in diameter) for 5 min. Finally, calcium chloride 5 % was added to the microemulsion, and the sonification process was continued to obtain nanocapsules. The efficiency of the encapsulation process was calculated by measuring W Nanocapsules (weight of prepared nanocapsules) and W Primary materials [weight of primary materials (peppermint oil, alginate and calcium chloride)] by Eq. 1:
Finishing process
A printing paste was prepared by mixing fragrant nanocapsules, three different binders and DAHP ((NH4)2HPO4) as the catalyst. Then, the cotton fabric was finished with printing paste and cured using a home-use 800-W microwave oven (EM-S2588B, Sanyo, Japan). The microwave curing process was carried out at different times to obtain an acceptable stabilization of the nanocapsules on the cotton fabric with minimum loss of fragrance.
SEM
A scanning electron microscope (SERON Technology ALS-2100, South Korea) was utilized to study of the surface morphology of the nanocapsules and finished cotton fabric. Each sample was fixed on a standard sample holder and sputter coated with gold. They were then examined by an SEM at suitable magnification.
FT-IR
The FT-IR spectra of the samples were measured using a BOMEN MB series 100 spectrophotometer (Hartman & Braun, Canada). Spectra were collected at a resolution of 4 cm−1 and given as the ratio of 21 single beam scans to the same number of background scans in pure KBr.
Thermal analysis
The thermal behavior of the finished cotton fabric was investigated using a thermal analysis system (DTA/TGA, BAHR-Thermoanalysis GmbH and STA 503) by heating the finished cotton fabric at 600 °C with an increasing rate of 10 °C min−1 under N2 steady flow.
Washing test
To study of the washing durability, the finished cotton fabrics were laundered on a short time program of 45 min at 30 °C according to the International Standard, ISO 105-C10 (2006) standard test method. The washing durability of the finished cotton fabrics was investigated by SEM photographs and antimicrobial assays.
Rubbing test
The rubbing strength of the finished cotton fabrics was investigated using a Crockmeter device (Electronic Crockmeter M238B, UK) according to the International Standard, ISO 105-X12 (2001) standard test method. The fabrics were rubbed for ten cycles per 10 s by a finger covered with a fabric sample at a pressure of 10 N.
Release study
To investigate the release of peppermint oil, the percent of residual peppermint oil in the finished cotton fabrics was measured by GC-MS analysis after 0, 5, 10, 15, 20 and 25 washing cycles. For this purpose, finished cotton fabrics were cut into 5 cm × 5 cm pieces and mixed with 5 ml of acetone solution to separate the fragrant nanocapsules from the fabric surface. GC-FID analysis was carried out using an HP-6980 Agilent gas chromatograph equipped with a flame ionization detector (FID) and a fused silica capillary column of HP-5MS (I.D. = 0.25 mm, 30 m long, and 0.25 µm film thickness and 5 % phenyl methyl siloxane), and GC-MS analysis was performed using an HP 6890 GC system coupled with a 5973 network mass selective detector.
Antimicrobial assay
The antimicrobial activity of the finished cotton fabrics was investigated according to the AATCC test method 100-2004 (2005). This assay was carried out using two bacterial species, Escherichia coli ATCC 1330 (American Type Culture Collection) as the gram-negative bacteria and Staphylococcus aureus ATCC 1337 as the gram-positive bacteria. A colony of bacteria preserved with a bio-loop was inoculated on a nutrient agar plate and cultivated at 37 °C for 24–48 h. Then, a colony of bacteria cultivated on the agar plate was inoculated into 20 ml of broth and cultivated at 37 °C for 18–24 h under constant stirring. The number of living bacteria in the test tube was evaluated by measuring the optical density at 580 nm. The bacteria number in the test tube was adjusted to 1–2 × 106 CFU ml−1 by dilution with the nutrient broth. Then 1 ml broth was added to 20 ml of the nutrient culture medium and cultivated at 37 °C for 2 h with shaking. The number of living bacteria was adjusted to 1 ± 0.3 × 105 CFU ml−1 by dilution with 1/20 of nutrient broth. This suspension was subsequently stored at 0 °C to be used later as an inoculator.
Finished and unfinished (control) cotton fabrics were cut into 48 ± 0.1-cm-diameter pieces. After sterilization in an autoclave at 121 °C for 15 min, 0.2 ml of the inoculator adjusted to 1 ± 0.3 × 105 CFU ml−1 was applied to fabrics and the samples cultivated at 37 °C for 18 h. The number of living bacteria on the fabric was evaluated after inoculation, and the percentage of bacteria reduction (R) was calculated using Eq. (2).
where A is the number of bacteria on the unfinished cotton fabric (control) and B the colony number of bacteria on the finished cotton fabric. For each sample, the test was repeated three times, and two separate treatments were used for each replicate.
Properties of finished cotton fabric
The physical properties of unfinished and finished cotton fabric by nanocapsules containing peppermint oil were evaluated and compared. For this purpose, the mass, stiffness and thickness of fabrics were determined according to the SIST EN 12127:1999, ASTM D-1388-64 (1975) and SIST-EN ISO 5084:1999 (1996) standard methods.
Results and discussion
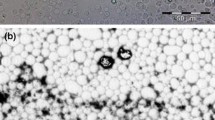
Alginate nanocapsules containing peppermint oil were prepared in different conditions, and the effective parameters according to the size and morphology of nanocapsules such as the values of materials and time of the sonification process were optimized. The results indicated that the best product obtained using sodium alginate 1.5 %, calcium chloride 5 % and Tween 20 0.1 % in the 5-min sonification process. Figure 1 displays the SEM image of fragrant nanocapsules prepared in optimum conditions. As can be seen, the nanocapsules containing peppermint oils were successfully prepared with a spherical shape and average size of 80 nm. Also, the results of encapsulation efficiency indicated that the peppermint oil was successfully encapsulated in alginate with an efficiency of 98.5 %.
The prepared nanocapsules were used as the fragrant and antimicrobial material because of their properties such as naturalness, good scent, antimicrobial activity and controlled release. To prevent the breakage or destruction of fragrant nanocapsules during the finishing process, a low-temperature curing based on microwave irradiation was used to apply nanocapsules on the cotton fabric. For this purpose, first, the effect of different parameters such as the amount of fragrance nanocapsules, type and amount of the used binder, and time of microwave curing on the antimicrobial activity of the finished cotton fabric was investigated. Then, the characterization of the finished cotton fabric in optimum conditions was evaluated by SEM, FT-IR, TGA, GC-MS and antimicrobial assays.
Effect of different parameters on the antimicrobial activity of the finished cotton fabric
Amount of fragrance nanocapsules
Two grams acrylate binder and different values of fragrance nanocapsules were used to study the effect of the nanocapsule amount on the antimicrobial activity of samples. This work was done in a 30-s curing time. The results showed that the number of bacteria decreased with an increasing amount of nanocapsules so that the maximum antibacterial activity (89 %) obtained using 10 g nanocapsules (Fig. 2a). It should be noted that the antibacterial activity did not change with a further increase of the nanocapsule amount. Therefore, 10 g nanocapsules was considered the optimum value of fragrant nanocapsules.
Types of binder
Binders play an important role in grafting the nanocapsules on the fabric surface. Therefore, selection of the suitable binder has a considerable effect on the durability, washing stability and quality of finished cotton fabric. To select the best binder, 2 g of three binder types (acrylate, PVA and PU) with 10 g nanocapsules containing peppermint oil were applied for 30 s. As can be seen in Fig. 2b, the highest antibacterial activity (88 %) obtained by the acrylate binder.
Amount of binder
In order to study the effect of the binder amount, different values of 0, 1, 2, 5 and 10 g acrylate binder were used in the finishing process. The nanocapsule value and time of curing were 10 g and 30 s, respectively. The results indicated that the greatest antibacterial reduction observed using 5 g acrylate binder (Fig. 2c). Therefore, this value was selected as the optimum value of the acrylate binder.
Time of microwave curing
In this study, we tried to apply the fragrant nanocapsules on the cotton fabric at a possible least time of microwave curing to prevent any damage to the nanocapsules structure and their core material (peppermint oil). To select the minimum required time, microwave curing was done for 20, 25, 30, 35, 40 and 45 s in the presence of 10 g nanocapsules and 5 g acrylate binder. As can be observed in Fig. 2d, 35 s is the best time for microwave curing. In these situations, the antimicrobial activity reached 100 %.
SEM
Figure 3 shows the SEM images of cotton fabrics finished with alginate nanocapsules containing peppermint oil before and after 25 washing cycles and rubbing test. Large amounts of fragrance nanocapsules grafted on the cotton fabric (Fig. 3a) indicates that the acrylate binder is bound the nanocapsules on the fiber surface well. Figure 3b indicates that many nanocapsules are remained on the fabric after 25 washing cycles. Therefore, the finishing process yielded good washing stability. The SEM image of the finished cotton fabric after the rubbing test is given in Fig. 3c. As observed, a small number of nanocapsules are remained on the fabric surface compared to before the rubbing test, and only some of the nanocapsules were protected by neighboring fibers or remained in cavities within the fiber.
FT-IR
In order to study the structure of the fabric finished with nanocapsules containing peppermint oil, infrared spectra of the unfinished fabric and finished fabric before and after 25 washing cycles were taken in the 500–4000 cm-1 region. The FT-IR spectrum of the unfinished cotton fabric is shown in Fig. 4a. As can be seen, stretching vibrations of O–H bonds of cellulose is appeared in the range of 3000–3600 cm−1, and the peaks related to aliphatic C–H and C–O–C in the cellulosic structure of cotton fabric are appeared at 2923 and 1026 cm−1, respectively (Chen et al. 2011). Figure 4b indicates the FT-IR absorption spectrum of the finished cotton fabric. The peak appeared at 1737 cm−1 is related to the stretching vibration of the carbonyl group in the structure of alginate and acrylate. Also, the peaks observed at 1647 and 1431 cm−1 are attributed to asymmetric and symmetric stretching vibrations of the carboxylate ion in calcium alginate, respectively. These results show that the nanocapsules were well grafted on the cotton fabric surface through the acrylate binder. The FT-IR spectrum of the finished cotton fabric after 25 washing cycles is shown in Fig. 4c. As observed, the peaks related to alginate nanocapsules (1737, 1647 and 1431 cm−1) are still visible in the FT-IR spectrum. Therefore, some nanocapsules are remained on the fabric after the washing test. This confirms the results of the SEM images.
Thermal analysis
TGA curves of unfinished and finished cotton fabric with fragrance nanocapsules containing peppermint oils are shown in Fig. 5. As seen, the unfinished cotton fabric showed thermal behavior with the onset temperature of degradation at about 310 °C. Also, the maximum rate of weight reduction was observed at about 350–400 °C, which can be attributed to the decomposition of cellulose. The TG curve of finished cotton fabric indicated a lesser decomposition temperature with the maximum weight reduction rate at 300–350 °C. Destroying the alginate nanocapsules containing peppermint oils and releasing the peppermint oil from nanocapsules at a lower temperature lead to changing the thermal behavior of finished cotton fabric. On the other hand, the binder plays an important role in grafting the nanocapsules on the cotton fabric; therefore, increasing the temperature leads to breaking the fabric-binder and nanocapsule-binder interactions.
Release of essential oils from the finished cotton fabric
The release behavior of fragrant nanocapsules from finished cotton fabric in different washing cycles was evaluated by GC–MS analysis, presented in Fig. 6a. As can be observed, the amount of peppermint oil after ten washing cycles decreased only 32 % in comparison with the unwashed sample. After 25 washing cycles, the percent of peppermint oil reached 16 %. The results indicate that the alginate nanocapsules have high stability. Also they are as well as grafted to fabric fibers and the finished cotton fabric have high washing durability.
Various mechanisms have been suggested for the release of the core material from the capsules, such as applying the pressure force, dissolving the materials by chemicals, changing the pH or temperature, and diffusion from the capsule walls. In this research, alginate as a biodegradable polysaccharide was used as the wall material. This biopolymer produces stable nanocapsules that helps to control the release of essential oils. Therefore, the prepared alginate nanocapsules broke or destroy according to the environmental conditions and peppermint oil leaks from them. Also, the prepared nanocapsules can be swollen in aqueous environments, and peppermint oil diffused from the nanocapsules wall.
Antimicrobial activity of fabrics finished with nanocapsules
In this study, the antimicrobial activity of cotton fabrics finished with nanocapsules containing peppermint oil was investigated using common microorganisms of textiles such as S. aureus and E. coli. For this purpose, an antibacterial test was carried out after 0, 1, 2, 5, 10, 15, 20 and 25 washing cycles and the rubbing test on the finished cotton fabrics as was an investigation of their washing and rubbing durability. As can be seen in Fig. 6b, finished cotton fabrics indicated good antibacterial activity at 25 washing cycles so that the percent of bacterial reduction at five and ten first washing cycles was 100 % by S. aureus and E. coli, respectively. Also, samples showed 95 and 96 % bacterial reduction after 25 washing cycles by S. aureus and E. coli, respectively. The complete results are presented in Table 2. Figure 7 displays the antimicrobial activity of unfinished fabric, finished fabric before the washing test, finished fabric after 25 washing cycles and finished fabric after the rubbing test. The results indicate that fabrics finished with nanocapsules containing peppermint oil exhibit 100 % antimicrobial activity. Peppermint oil contains a variety of volatile molecules such as terpenes, terpenoids, and phenol-derived aromatic and aliphatic components. These components and menthols characterize the flavor quality of peppermint oil and act as prooxidants affecting inner cell membranes and organelles such as mitochondria, thus killing bacteria (Bakkali et al. 2008).
The finished cotton fabric displayed lower antimicrobial activity after washing because of the release of peppermint oil from nanocapsules during the washing process. Also, the rubbing test led to breaking and destruction of many nanocapsules grafted on the fabrics and reduction of the antimicrobial activity. The partial antimicrobial activity of the blank sample was caused by the blanching process done on it. On the other hand, the gram-positive bacterium, S. aureus, exhibited better activity in all the tests compared with the gram-negative bacterium, E. coli. This difference can be explained by their structure and cell wall.
Properties of fabric
The properties of unfinished and finished cotton fabrics such as mass, stiffness and thickness were investigated. The results indicated that the mass of finished cotton fabric increased about 5.2 % against unfinished cotton fabric. This increasing of mass can be attributed to the presence of fragrant nanocapsules on the fabric surface. Also, the stiffness of finished cotton fabric slightly increased in comparison with unfinished cotton fabric, which is related to the addition of nanocapsules and binder to the fabric. The results of thickness measurements indicated that there was no significant difference in thickness between finished and unfinished cotton fabric. This result is due to placing the nanosize capsules between fibers of cotton fabric during the finishing process.
Conclusions
Encapsulation is a new technique used for textile finishing with antimicrobial materials. In this study, the prepared nanocapsules containing peppermint oil by the microemulsion method were stabilized on the cotton fabric by a microwave curing finishing process. The effective parameters concerning the antimicrobial activity such as the amount of nanocapsules, type and amount of binder, and time of the curing process were optimized. The results indicated that the washing durability of the finished cotton fabrics under optimized conditions was 25 washing cycles. The evaluation of peppermint oil release in different washing cycles indicated that the percent of peppermint oil reached 16 % after 25 washing cycles. Infrared spectra of fabric samples, SEM images and TGA results revealed the presence and alignment of nanocapsules on the cotton fabric. The antimicrobial activity maintained even after the washing and rubbing tests. Also, the finished cotton fabrics obtained a durable aroma of peppermint oil in addition to antimicrobial activity.
References
AATCC test method 100–2004 (2005) Antibacterial finishes on textile materials: assessment of AATCC technical manual. American Association of textile chemists and colorists, Research Triangle Park
ASTM D-1388-64 (1975) Method A: standard test method for stiffness of fabrics. ASTM, New York
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475. doi:10.1016/j.fct.2007.09.106
Beristain CI, García HS, Vernon-Carter EJ (2001) Spray-dried encapsulation of cardamom (Elettaria cardamomum) essential oil with mesquite (Prosopis juliflora) gum. LWT Food Sci Technol 34:398–401. doi:10.1006/fstl.2001.0779
Bischof Vukušić S, Flinčec Grgac S, Budimir A, Kalenić S (2011) Cotton textiles modified with citric acid as efficient antibacterial agent for prevention of nosocomial infections. Croat Med J 52:68–75. doi:10.3325/cmj.2011.52.68
Bonet Aracil MÁ, Monllor P, Capablanca L, Gisbert J, Díaz P, Montava I (2015) A comparison between padding and bath exhaustion to apply microcapsules onto cotton. Cellulose 22:2117–2127. doi:10.1007/s10570-015-0600-8
Budimir A, Bischof Vukusic S, Grgac Flincec S (2012) Study of antimicrobial properties of cotton medical textiles treated with citric acid and dried/cured by microwaves. Cellulose 19:289–296. doi:10.1007/s10570-011-9614-z
Chen X, Yu J, Zhang Z, Lu C (2011) Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr Polym 85:245–250. doi:10.1016/j.carbpol.2011.02.022
Cristina ED (2004) Understanding true aromatherapy: understanding essential oils. Home Health Care Manag Pract 16:474–479. doi:10.1177/1084822304265851
El-Molla MM, Haggag K, El-Shall FN, Shaker NO, Alian NA (2013) Use of novel synthesized aqueous binders for pigment printing of polyester fabrics. Indian J Fibre Text Res 38:57–65
Ghayempour S, Mortazavi SM (2013) Fabrication of micro–nanocapsules by a new electrospraying method using coaxial jets and examination of effective parameters on their production. J Electrostat 71:717–727. doi:10.1016/j.elstat.2013.04.001
Ghayempour S, Mortazavi SM (2014) Antibacterial activity of peppermint fragrance micro–nanocapsules prepared with a new electrospraying method. J Essent Oil Res 26:492–498. doi:10.1080/10412905.2014.949882
Ghayempour S, Mortazavi SM (2015) Preparation and investigation of sodium alginate nanocapsules by different microemulsification devices. J Appl Polym Sci. doi:10.1002/app.41904
Heywood D (2003) Textile finishing. Society of Dyers and Colourists, Bradford
Hong K, Park S (1999) Melamine resin microcapsules containing fragrant oil: synthesis and characterization. Mater Chem Phys 58:128–131. doi:10.1016/S0254-0584(98)00263-6
Hong K, Park S (2000) Preparation of poly(l-lactide) microcapsules for fragrant fiber and their characteristics. Polymer 41:4567–4572. doi:10.1016/S0032-3861(99)00677-1
International Standard, ISO 105-C10 (2006) Textiles-Tests for colour fastness: Colour fastness to washing with soap or soap and soda. ISO, Geneva
International Standard, ISO 105-X12 (2001) Textiles-Tests for colour fastness: Colour fastness to rubbing. ISO, Geneva
Jantas R, Górna K (2006) Antibacterial finishing of cotton fabrics. Fibres Text East Eur 14:88–91
Javid A, Raza ZA, Hussain T, Rehman A (2014) Chitosan microencapsulation of various essential oils to enhance the functional properties of cotton fabric. J Microencapsul 31:461–468
Lee A, Han C, Yi E (2014) Preparation and characterization of melamine-formaldehyde microcapsules containing Citrus unshiu essential oil. Fibers Polym 15:35–40. doi:10.1007/s12221-014-0035-0
Li S, Boyter H, Qian L (2005) UV curing for encapsulated aroma finish on cotton. J Text Inst 96:407–411. doi:10.1533/joti.2005.0116
Li S, Lewis JE, Stewart NM, Qian L, Boyter H (2008) Effect of finishing methods on washing durability of microencapsulated aroma finishing. J Text Inst 99:177–183. doi:10.1080/00405000701489701
Monllor P, Bonet MA, Cases F (2007) Characterization of the behaviour of flavour microcapsules in cotton fabrics. Eur Polym J 43:2481–2490. doi:10.1016/j.eurpolymj.2007.04.004
Rajendran R, Radhai R, Kotresh TM, Csiszar E (2013) Development of antimicrobial cotton fabrics using herb loaded nanoparticles. Carbohydr Polym 91:613–617. doi:10.1016/j.carbpol.2012.08.064
Rodrigues SN, Martins IM, Fernandes IP, Gomes PB, Mata VG, Barreiro MF, Rodrigues AE (2009) Scentfashion®: microencapsulated perfumes for textile application. Chem Eng J 149:463–472. doi:10.1016/j.cej.2009.02.021
SIST-EN 12127 (1999) Determination of mass per unit area using small samples. CEN, Brussels
SIST-EN ISO 5084:1999 (1996) Determination of thickness of textiles and textile products. CEN, Brussels
Teixeira CSNR, Martins IMD, Mata VLG, Filipe Barreiro MF, Rodrigues AE (2011) Characterization and evaluation of commercial fragrance microcapsules for textile application. J Text Inst 103:269–282. doi:10.1080/00405000.2011.566312
Thilagavathi G, Krishna Bala S, Kannaian T (2007) Microencapsulation of herbal extracts for microbial resistance in healthcare textiles. Indian J Fibre Text Res 32:351–354
Wang CX, Chen SL (2005) Aromachology and its application in the textile field. Fibres Text East Eur 13:41–44
Zhao X, Min J, Jx He (2011) Effect of microwave curing on antimicrobial activity of chitosan biguanidine hydrochloride treated wool fabrics. J Text Inst 102:801–807. doi:10.1080/00405000.2010.522047
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghayempour, S., Mortazavi, S.M. Microwave curing for applying polymeric nanocapsules containing essential oils on cotton fabric to produce antimicrobial and fragrant textiles. Cellulose 22, 4065–4075 (2015). https://doi.org/10.1007/s10570-015-0765-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0765-1