Abstract
Cellulose nanofibers (CNF) have been suggested in the literature as a potential barrier coating layer for paper and paperboard. However, due to its rheological properties and solids content, the material is difficult to apply to paper at significant coat weight levels. The use of CNF as a coating to improve the structure and barrier properties of paperboard was investigated. Two forms of CNF were used: (1) refiner produced material and (2) material produced with an ultra-fine grinder. Carboxymethyl cellulose (CMC) was used for some samples as an additive. The rheology of these suspensions was characterized. These materials were applied onto the surface of paperboard using a draw-down rod coater in two layers. Scanning electron microscope (SEM) was used to see the coverage of the paper by the CNF layer. Air permeability, water penetration and barrier properties of the samples were characterized. The steady shear viscosity of CNF suspension decreased after the addition of CMC. Although CNF at 2% solid content without CMC could not be spread out onto the surface of paper uniformly, 3% CNF along with CMC was successfully coated on the surface of paper: CMC acts as a dispersant that produces a uniform suspension with minimal flocs. The ground CNF with CMC produced the best samples with good coverage as revealed by SEM images. The results show that the structure and barrier properties of coated paperboards improved considerably by the application of CNF coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paper is widely used as a packaging material for its low cost, biodegradability and its ability to be recycled. Nevertheless, because of its nature and depending on the application, it usually needs to be coated with other materials to obtain good barrier properties. Food packaging papers, for instance, should be sufficient barriers against oxygen, water, water vapor, and grease (Paunonen 2013). Currently, coating materials are largely produced from fossil-derived synthetic plastics which are not necessarily environmentally friendly and sometimes lead to difficulties in recycling processes (Anthony et al. 2015; Lavoine et al. 2014). With increasing environmental concerns and the need for sustainable development in the future, the use of renewable materials is expected to grow.
In order to improve the barrier performance of paper in packaging industries to meet the requirements including consumer convenience and environmental issues, innovative modified packaging materials are being developed. Several bio-materials including polysaccharides (cellulose, starch, hemicellulose and chitosan), proteins (collagen, zein and soybean) and lipids have been researched (Anthony et al. 2015). Recently, the development of cellulose nanofiber (CNF) has offered a new alternative to the polymer coating to form a barrier layer. CNF is produced through mechanical treatment including homogenizing (Turbak et al. 1983, grinding (Taniguchi and Okamura 1998), microfluidisation (Zimmermann et al. 2010), and disk refining (Johnson et al. 2016). Compared to the other kinds of nanocellulose such as nanocrystalline cellulose (CNC), CNF has a broader size distribution and wider fibril diameter. After drying, it is able to form a dense percolating network by strong hydrogen bonds and this suggests that these films could potentially have barrier properties which can be considered as an interesting alternative to fossil fuel based materials. In addition, the enhancement of mechanical properties and their use as aqueous suspensions provides opportunities for CNF application as coating material for cellulosic substrates. A recent review of the potential to use CNF as a barrier coating is given by Lavoine et al. (2012). Syverud and Stenius (2009) reported that applying CNF layer lowered sheet porosity and resulted in a denser structure which decreased air permeability. Kumar et al. (2014) report quite good oxygen permeability results for CNF films. Lavoine et al. (2014) show some improvements in mechanical properties of board samples coated with CNF. Hassan et al. (2016) studied the effect of CNF coating on the bagasse paper sheet properties and reported that an increase in tensile strength and a decrease in porosity of paper sheet was observed. Xu et al. (2016) reported that using CNF in coating color improved the surface strength, however it decreased the water resistance of coated papers. Despite this promise, CNF suspensions have to be applied at a low solid content (lower than 1%) because of extremely high viscosities at higher solids contents (Rautkoski et al. 2015). This means that in practice a large amount of water must be evaporated in the drying section of coating machine that gives rise to high drying costs. When the solids content of CNF is increased, the entanglement of fibers result in high flocculation and the coating cannot be spread out on the base paper evenly.
To address this problem, carboxymethyl cellulose (CMC) was studied here because of its ability to disperse fibers and reduce their flocculation (Dimic-Misic et al. 2014; Liimatainen et al. 2009; Yan et al. 2006); CMC can improve the uniformity of fiber suspension and modify the suspension rheology (Nazari and Bousfield 2016; Pahimanolis et al. 2013). CMC is an anionic derivative of cellulose which is prepared by introducing carboxymethyl groups along the cellulose chain. It has been used in different industries such as textile and food industries. Owing to the very good film making and water retention characteristic, CMC has been used in papermaking industry as a surface sizing agent, coating binder and wet end additives (Beghello et al. 1997; Blomstedt 2007). It is inexpensive and readily available (Chen et al. 2016). The sorption of CMC on the surface of cellulosic fibers is believed to be done through hydrogen bonding which is influenced by the degree of substitution (DS) and molecular weight of CMC. Moreover, two forms of CNF, refiner CNF (rCNF) and grinder CNF (gCNF) were used to see whether the type of CNF can affect the coating properties particularly when it is used in a blend with CMC. Hamada and Bousfield (2010) coated two kinds of CNF, refiner and homogenizer produced CNF onto the surface of synthetic fiber sheet and mentioned that the contact angle on the homogenizer one was lower than refiner CNF and the homogenizer CNF showed a higher ink absorption rates. Richmond et al. (2014) reported that using refiner CNF in coating formulation increased steady shear viscosity. A slight decrease in air permeability was also observed.
Therefore, the goals of this study are: 1 to investigate the effect of CMC on the dispersion properties of CNF and 2 to study the effect of different CNF types with and without CMC at higher solids content on structure and barrier properties of paperboard.
Materials and methods
Refiner-produced CNF (rCNF) was prepared at the University of Maine Process Development Center using a 20 in. GL&V disk refiner with no pretreatment from bleached softwood kraft pulp as described by others (Richmond et al. 2013). The consistency of rCNF was 3% which was diluted using deionized water to obtain different solids content. The grinder-produced CNF (gCNF) was produced by taking the refiner CNF and circulating it in a bench scale ultra-fine friction grinder (Masuko Supermasscolloider, MKCA 6-2, Japan) for 120 min. It was prepared in 1.5% consistency where the solids content was adjusted by the addition of water or by using a laboratory centrifuge (Sorvall RC 6 plus, Thermo Electron Corporation, USA). Carboxymethyl cellulose (CMC) with molecular weight of 450,000 g/mol and a degree of substitution of 0.7 was donated by CPKelco (Finnfix 4000 G). The solution of CMC was prepared by dispersing an appropriate amount of CMC powder in deionized water at a concentration of 1.5% by stirring for 3 h at 600 rpm at room temperature. The CMC solution was added to the CNF suspensions after it was prepared. The CMC was evenly distributed in the CNF indicating the CMC is highly compatible with the CNF.
Morphology of CNF suspension
Morphology of CNF material was examined using transmission electron microscope (TEM) (Philips CM10). CNF sample was first diluted by deionized water and treated for 1 min in an ultrasound bath (BAUSCH&LOM8, 100-C). The diluted CNF suspension was dropped on a carbon coated copper grid and the excess water was removed by a paper tip. The sample was then stained with a 1% (v/v) uranyl acetate aqueous solution (UA) for at least 1 min and again the excess UA was removed by a paper tip. Then the grid was allowed to dry at room temperature and examined at an accelerate voltage of 100 kv. A camera (ORIUS, GATAN) was used to capture the pictures at different magnifications. Digital image analysis (Image J) was used to determine the dimension of nanocellulose fibrils. For both types of CNF, 250 measurements were selected randomly and evaluated from TEM images at the same magnification.
Gravimetric water retention (GWR) of CNF suspension
The gravimetric method (Abo-Academi type method) was used to measure dewatering properties of CNF suspension in accordance with TAPPI standard T701 pm-01. ÅA-GWR (Model 150, Kaltec Scientific, USA) with polycarbonate membrane (5 µm) and 0.5 bar external pressure was applied. The GWR of 1.5 and 3% CNF suspension including rCNF and gCNF with and without CMC in two times (90 and 180 s) were determined. The average of three experiments was reported.
Viscosity characterization
A stress-controlled Bohlin Gemini rheometer (Malvern Instruments Ltd, UK) was used to study the steady shear viscosity of different CNF suspensions. The diameter of plate in the parallel plate geometry was 40 mm and 2 mm gap was chosen in this test. The shear rate measurements were conducted in the range of 0.01–100 s−1. The test was performed at room temperature.
Paper coating process
A wood free bleached base paper with grammage of 200 g/m2 was selected for coating experiments. CNF suspensions with different solids contents from 1 to 3 wt% to achieve different coating weights with or without CMC (4 wt% based on dry weight of CNF) were prepared and applied on the surface of paper using a draw-down rod coater (BYC-Gardner, 2101, USA) in two layers. Coating speed was 50 mm/s and wire-wound rod #52 was used. The coated papers were dried in oven at 105 °C for 5 min. After determining the coat weight, the samples were conditioned at 50% RH and temperature of 23 °C for barrier and structure properties tests using standard procedures.
Paper properties
The surface structure of uncoated and coated papers was observed by scanning electron microscopy (SEM) (HITACHI TM 3000). 15 kV accelerating voltage was applied. The roughness of the paperboard surface was determined using Sheffield roughness (Model: PS-10-20-40, Sheffield corporation, USA) according to TAPPI standard T538 om-97. The average of 10 measurements was reported. A Gurley densometer (Lorentzen & Wettre, Sweden) was used to measure the air resistance of coated paper according to TAPPI standard T460 om-02. Hercules sizing tester (Hercules incorporated, USA) was used to measure the aqueous resistance of paperboard in accordance with TAPPI standard T530 om-96. This method employs a dark color dye solution as the penetrant to permit optical detection of the liquid as it moves through the sheet. The test was done on one side and the coated side was in contact to the ink (reflectance endpoint 80%). Water vapor transmission rate (WVTR) was characterized according to ASTME 96-95 using the water method. Conditioned coated paperboards were cut into 6 cm diameter circles and placed above 25 mL of distilled water in a closed container. The weight of the dish before and after test was determined and the WVTR was measured using the following equation:
where WVTR is water vapor transmission rate, G is the weight change, t is time in which G occurred and A is the test area. The average of three measurements was reported.
Grease resistance of uncoated and coated paperboards was evaluated using Kit test (T559 pm-96). Test solutions with different viscosities including castor oil, toluene and heptane (numbered from 1 to 12) were used. The highest numbered solution that remains on the surface of the paper without causing failure is reported. The number 12 (the most aggressive) represents the highest grease resistance. This test was performed in triplicate.
Results and discussion
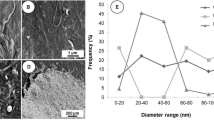
TEM images and size distribution of rCNF and gCNF fibrils are shown in Figs. 1 and 2, respectively. They reveal that a finer size distribution of CNF was achieved after grinding compared to the refiner produced material. While the rCNF had a significant number of fibrils less than 100 nm, it also had large fiber fragments on the order of 1 – 10 µm that seem to connect the fine scale fibers. For the grinder produced material, the number of these large scale fiber fragments is reduced and there is a wide range of fiber sizes. However, it should be mentioned that TEM images could not cover the entire range of diameters of CNF. A number of the fibers appear to have a morphology more similar to a ribbon: the fiber appears to be the flat remains of the cell wall. These images are quite similar to what others have reported for mechanically produced CNF materials (Richmond et al. 2014). The measurement of diameter using image J showed that the average diameter for rCNF was 0.166 µm while for gCNF it was around 0.036 µm.
The water retention of coating is one of the most important properties that affects the properties of coating materials and as a result coated papers. Figure 3 shows the gravimetric water retention (GWR) of both rCNF and gCNF with and without CMC in two times (90 and 180 s). The results show that increasing the solids content of CNF suspension from 1.5 to 3%, decreases the water that goes through the membrane. This result is expected in that the filtercake thickness increases faster for high solids suspensions compared to low solids for the same water amount through the membrane. It is also evident that there was an increase in the GWR of CNF suspension when CMC was added to the suspension. It is because CMC is a water soluble polymer leading to an increase in the viscosity of the liquid phase which results in a decrease of the water released in the GWR test. Laine et al. (2002) measured the water retention value (WRV) of fiber suspension using centrifugal force and reported that the addition of CMC increased the WRV significantly. Liimatainen et al. (2009) studied the absorption of CMC onto the surface of fibers and reported that after the addition of CMC, the WRV determined using vacuum filtration and gravity driven filtration system increased between 1 and 4%. Compared to the rCNF, gCNF decreased more the water transfer from the sample: this result can be attributed to the higher amount of fines in the latter one (Dimic-Misic et al. 2014). Laivins and Scallan (1996) reported that compared to the fibers, fines swell much more resulting in higher WRV.
The steady-shear viscosity of CNF suspension as a function of shear rate is shown in Fig. 4. All CNF suspensions showed a shear thinning behavior when subjected to increasing shear rates. This phenomenon has been reported by many others (Lasseuguette et al. 2008; Nazari and Bousfield 2016; Rezayati Charani et al. 2013; Turbak et al. 1983). In addition, the viscosity increased as the solids content increased. gCNF showed higher viscosity over that of rCNF. This is due to the higher fibrillation of gCNF (see Fig. 1) leading to a higher surface areas and an increased water uptake (Aulin et al. 2010). It is also evident that CNF together with CMC showed a lower viscosity compared with CNF aqueous suspension alone except for 1% rCNF for which a significant difference was not observed when CNF was used alone or with CMC. This was not expected because as mentioned above CMC is a water soluble material which increases the liquid phase viscosity. However, on the other hand, the reason for this observation can be attributed to the ability of CMC to decrease the friction at the fiber–fiber contact resulting in lower flocculation of nanofibrils (Ahola et al. 2008; Beghello 1998; Liimatainen et al. 2009; Nazari 2015). Yan et al. (2006) studied the impact of CMC on fiber flocculation and mentioned that the mutual repulsion between the surface charges leads to an electrostatic repulsion resulting in a reduced friction coefficient between the fibers, which in turn, decreases the flocculation. Dimic-Misic et al. (2014) studied the effect of partial and total replacement of CMC with MFC/CNF in coating colors and found that CMC helps to disperse the CNF in coating colors and lowers its tendency to flocculate. Schenker et al. (2016) reported that dispersability of the micro and nanofibrillated cellulose (MNFC) is increased after the addition of CMC polymer.
With increasing the solids content of CNF suspension, the coat weight increased as shown in Fig. 5. This is expected because the rod coater is a volume controlled coating method. CNF suspension mixed with CMC showed a higher coat weight and the highest coat weight (7.8 g/m2) was achieved with 3% gCNF and CMC. Higher coat weight can affect paper properties especially barrier properties provided that a full coverage is achieved. CNF at 3% solids content could not be coated on the surface of paper evenly and due to lots of chunks between base paper and wire-wound rod, a good coverage was not achieved. However, using CMC, 3% CNF was successfully coated on the surface of paper uniformly. At solids content higher than 3 wt% even when CMC was added, defects in the coating layer was easily observed and because of flocs, CNF could not be spread out evenly. Lavoine et al. 2014 coated cardboard sheets with 2% MFC using bar coating process and reported that due to the viscosity of MFC suspension, a whole coverage of paper surface was not obtained by applying one layer of MFC. They also stated that coat weight of 1 and 14 g/m2 were achieved after applying one and ten layers, respectively. Aulin et al. (2010) used 0.85 wt% MFC suspension for paper-coating process and reported that the highest coat weights obtained in a single coating step were 1.3 and 1.0 g/m2 for unbleached and greaseproof paper, respectively.
The SEM micrographs of the surface of coated papers are presented in Fig. 6. For the rCNF case at 2% solids content with CMC in Fig. 6b, regions of the base paper are seen that proves that a complete coverage was not obtained, however for the gCNF at 2% solids content with CMC in Fig. 6c, good coverage seems to have been obtained. For the 3% rCNF and gCNF mixed with CMC, good coverage is noted in Fig. 6e. CMC addition does generate a suspension that is more uniform than the suspensions without CMC; these suspensions flow in a uniform manner in the rod coater. The cross sectional images, Fig. 6f, g, show a well-defined CMC-containing CNF layer formed on the surface of base paperboard.
The structure and barrier properties of paperboards coated with rCNF and gCNF with and without CMC are shown in Tables 1 and 2, respectively. In addition, the influence of coat weight on these properties is plotted in Figs. 7, 8, 9 and 10.
Sheffield roughness value of paperboards are reported to decrease after being coated with CNF (Afra et al. 2016). Both rCNF and gCNF showed the same trend here, however paperboards coated with gCNF showed lower roughness values than those coated with rCNF (Tables 1 and 2). It is in response to the finer size distribution and lower amount of large fragments in gCNF suspension which affect the roughness of surface. After the addition CMC to the CNF suspension, the roughness decreased more and compared to the samples without CMC, smoother surface was observed. These results are consistent with the results presented by Beghello et al. (1997) who showed that adding a small amount of CMC (0.2–0.5%) to the top and bottom layers of paperboard decreases the roughness of surface.
The air resistance of uncoated and coated paper at different coat weights is shown in Fig. 7. Increase in the time reflects a closed up sample with likely few open or uncoated areas. Coated paperboards show large increase in the time compared to the uncoated sample particularly when gCNF was applied. Furthermore, the air resistance increased considerably with the increase of coat weight and thickness of coated samples (see Tables 1, 2). For 1.6 and 2.6 g/m2 coat weight, the air resistance was 80 and 1400 Gurley sec whereas for 6.9 and 7.8 g/m2 coat weight, the air resistance increased to about 740 and 4700 Gurley sec for rCNF and gCNF, respectively. This can be attributed to an increase in the tortuous path provided by nano particles (Aulin et al. 2010; Dufresne 2012). It is also believed that the crystalline structure of CNF plays an important role to improve the barrier properties. Fendler et al. (2007) and Syverud and Stenius (2009) studied the barrier properties of nanocellulose films and composites and mentioned that good barrier properties of cellulose and nanocellulose can be related to their crystalline structure. Since the gCNF has fine flexible ribbon like structure, it is easy to see how this material will form quite a dense layer that will block the flow of air. This means that the pores on the surface of paper have been much more blocked than rCNF-coated paper leading to higher air barrier properties. It is well known that the connected pores on the surface of paper have an important effect on air permeability (Syverud and Stenius 2009). Hamada and Mitsuhashi (2016) studied the effect of CNF prepared from bamboo and hardwood bleached kraft pulp as a coating on woven and nonwoven fabric and reported that the resistance to the air permeability increased upon coating with CNF. They also mentioned that hardwood-derived CNF showed higher air resistance due to more uniform and finer network formed by the shorter hardwood-derived CNFs. The highest air resistance was achieved with 2% gCNF with CMC. CMC changes the coating process in two ways: (1) it decreases the flocculation between nanofibers and (2) it reduces the dewatering of the suspension in front of the rod. Both are important to give a uniform CNF coating. CNF even in a very low solids content tends to be flocculated and SEM images show that a full coverage cannot be obtained. However, mixed with CMC, a tendency to make a film on the surface of paper with a much better coverage was observed.
Figure 8 shows the results of aqueous resistance of paperboards as a function of coat weight. It is obvious that coating with CNF improved this characteristic considerably. However, it should be noted that applying CNF alone did not improve this property over that of uncoated paper. When gCNF along with CMC was used the aqueous resistance increased considerably. This increase in aqueous resistance was not observed when rCNF was used even when CMC was added. The highest resistance was achieved with 3% gCNF with CMC compared to uncoated paperboard (420 vs 90 s, respectively). These results are determined by the ability of the coating to spread in a uniform manner, not the inherent properties of the coatings. As mentioned earlier when CNF is applied on the surface of base paperboard, it does not cover the paper surface evenly because of its agglomeration tendency. The higher flocculation of CNF was seen when the consistency increased and consequently the barrier properties deteriorated most likely because of the pinholes formed in the coating. However, it was not the case when gCNF along with CMC was applied and a uniform coating with a good coverage (see Fig. 6c, e) improved the aqueous resistance of paperboard considerably. As suggested by others, the formation of a dense structure formed by fibrils results in a high barrier properties (Aulin et al. 2010; Syverud and Stenius 2009; Yousefi et al. 2013). Hassan et al. (2016) applied CNF and a mixture containing of CNF and 10% chitosan as a coating on the surface of paper sheet and reported that the water absorption of coated paper sheet measured using Cobb test method decreased by 33%. They found no significant difference between the water absorption of papers coated with CNF and CNF with chitosan.
The water vapor transmission rate (WVTR) is defined as the volume of water vapor penetrating a surface unit sample of defined thickness during 24 h under specified temperature, controlled relative humidity and under a vapor pressure difference. Relative humidity, temperature and coat weight are some of the most important parameters which affect WVTR. In this study, relative humidity and temperature were kept constant (50% RH and 23 °C) and coat weight was changed with the change of the CNF solids content. Figure 9 shows the WVTR of uncoated and coated papers at different coat weights. Despite the fact that an improvement in barrier properties (lower WVTR) is expected when coat weight is increased (Song et al. 2014), a noticeable reduction compared to uncoated paper with increasing the coat weight was not observed. It is probably related to the hydrophilic characteristic of CNF. Although crystalline cellulose has a good barrier properties, with increase of the humidity, the amorphous cellulose absorbs water resulting in weakening of hydrogen bonding in cellulose chains and subsequent swelling. This results in higher mobility of cellulose chains and fibril network and as a result more sites for water molecule permeation (Aulin et al. 2010). Hassan et al. (2016) reported 16% increase in water vapor permeability (WVP) of bagasse paper sheets when they were coated with CNF. The reason for this increase was reported to be because of the hydrophilic nature of CNF. Song et al. (2014) applied a composite made from modified CNF with grafted hydrophobic monomers on it and PLA on the paper surface and discussed its impact on WVTR. They reported that at the highest coat weight (40 g/m2) and less amount of CNF (1%), the lowest WVTR was obtained (34 g/m2 day). Aulin et al. 2012 prepared a NFC-nanoclay hybrid films and reported that the clay particles enhanced the barrier performance. They reported that WVTR was considerably reduced even at 50 and 80% RH. Therefore it seems that CNF is not able to improve WVTR alone and using additives like clay to increase the tortuous path or doing modification to decrease the hydrophilic characteristic of CNF is necessary.
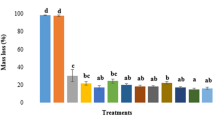
Figure 10 presents the grease resistance of coated papers as a function of coat weight. This property was evaluated according to the Kit test. The results showed that base paper coated with 2% gCNF together with CMC achieved the highest grease resistance compared to that of uncoated paper. A high coat weight together with the homogeneity of coating and a good coverage of paper surface might be the most important reasons. The nano size and web structure of CNF on the surface of paper decrease the pores and consequently decrease the migration of grease through the paper. As with the air resistance data, this sample also showed the highest air resistance. The results are in agreement with those of Aulin et al. (2010) who showed that when the air permeability decreases, i.e., increased coat weight, the oil resistance increases. Compared to gCNF, rCNF did not improve grease resistance of paperboard even when CMC was used. This might be due to the imperfections on the surface of paper after coating with rCNF. Lavoine et al. (2014) studied the barrier properties of cardboard coated with microfibrillated cellulose and reported that the grease resistant (Kit number) of 5 and 10 layers-coated boards were 1.5 and 2.5, respectively. They also stated that Polyethylene (PE) coated cardboard had a Kit number of 12. Song et al. (2015) applied coatings of sodium alginate and alkali-treated potato starch on the surface of paper based packaging and reported both of these renewable materials were effective to improve oil resistance. They also mentioned that the pores coverage of paper surface after coating particularly in higher coat weight is the main reason to decrease the oil penetration.
Conclusion
The influence of applying two forms of CNF (refiner and grinder CNF) at higher solids content with and without CMC on the structure and barrier properties of paperboards was investigated. Application of CNF with higher solids content as a coating material was achieved when it was applied with CMC. In fact, CMC reduced the fiber–fiber contact and consequently decreased the flocculation of micro and nanofibers resulted in a more homogeneous coating on the surface of paper. The highest coat weight as well as full surface coverage obtained with gCNF along with CMC resulted in a distinct improvement in barrier properties. This result is due to a dense and uniform structure of coating on the surface of paperboard that blocks pores. The exception, however, is WVTR property. The hydrophilic characteristic of CNF is probably the main reason for this observation.
References
Afra E, Mohammadnejad S, Saraeyan A (2016) Cellulose nanofibrils as coating matrial and its effect on paper properties. Prog Org Coat 101:455–460
Ahola S, Myllytie P, Osterberg M, Teerinen T, Laine J (2008) Effect of polymer adsorption on cellulose nanofibril water binding capacity and aggregation. BioResources 3(4):1315–1328
Anthony R, Xiang Z, Runge T (2015) Paper coating performance of hemicellulose-rich natural polymer from distiller’s grains. Prog Org Coat 89:240–245
ASTM Standard Test Method for Water Vapor Transmission of Materials, E96-95, 1995, ASTM International, http://www.astm.org
Aulin C, Gallstedt M, Lindstrom T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574
Aulin C, Salazar-Alvarez G, Lindstrom T (2012) High strength, flexible and transparent nanofibrillated cellulose-nanoclay biohybrid films with tunable oxygen and water vapor permeability. Nanoscale 4:6622–6628
Beghello L (1998) Some factors that influence fiber flocculation. Nord Pulp Pap Res J 13:272–279
Beghello L, Long LY, Eklund D (1997) Laboratory study on carboxymethyl cellulose as a wet-end additive in paperboard making. Pap Puu 79:55–57
Blomstedt M (2007) Modification of cellulosic fibers by carboxymethyl cellulose-effects on fibers and sheet properties. Dissertation, Helsinki University of Technology
Chen B, Zheng Q, Zhu J, Li J, Cai Z, Chen L, Gong S (2016) Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv 6:96518–96526
Dimic-Misic K, Salo T, Paltakari J, Gane P (2014) Comparing the rheological properties of novel nanofibrillar cellulose-formulated pigment coating colours with those using traditional thickener. Nord Pulp Pap Res J 29(2):253–270
Dufresne A (2012) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter GmbH 10
Fendler A, Villanueva MP, Giminez E, Lagaron JM (2007) Characterization of the barrier properties of composites of HDPE and purified cellulose fibers. Cellulose 14:427–438
Hamada H, Bousfield DW (2010) Nanofibrillated cellulose as a coating agent to improve print quality of synthetic fiber sheets. Tappi J 9(11):25–29
Hamada H, Mitsuhashi M (2016) Effect of cellulose nanofibers as a coating agent for woven and nonwoven fabrics. Nord Pulp Pap Res J 31(2):255–260
Hassan EA, Hassan ML, Abou-zeid RE, El-Wakil NA (2016) Novel nanofibrillated cellulose/chitosan nanoparticles nanocomposites films and their use for paper coating. Ind Crops and Prod 93:219–226
Johnson DA, Paradis MA, Bilodeau M, Crossley B, Foulger M, Gelinas P (2016) Effect of cellulosic nanofibrils on papermaking properties of fines papers. Tappi J 15(6):395–402
Kumar V, Bollstrom R, Yang A, Chen Q, Chen G, Salminen P, Bousfield DW, Toivakka M (2014) Comparison of nano and microfibrillated cellulose films. Cellulose 21:3443–3456
Laine J, Lindstrom T, Nordmark GG, Risinger G (2002) Studies on the topochemical modification of cellulosic fibers. Nord Pulp Pap Res J 17:50–56
Laivins GV, Scallan AM (1996) The influence of drying and beating on the swelling of fines. J Pulp Paper Sci 22(5):178–184
Lasseuguette E, Roux D, Nishiyama Y (2008) Rheological properties of microfibrillar suspension of TEMPO-oxidized pulp. Cellulose 15:425–433
Lavoine N, Desloges I, Dufresne A, Bras J (2012) Microfibrillated cellulose – Its barrier properties and applications in cellulosic materials: A review. Carbohydr Polym 90:735–764
Lavoine N, Bras J, Desloges I (2014) Mechanical and barrier properties of cardboard and 3d packaging coated with microfibrillated cellulose. J Appl Polym Sci 131:40106
Liimatainen H, Haavisto S, Haapala A, Niinimaki J (2009) Influence of adsorbed and dissolved carboxymethyl cellulose on fiber suspension dispersing, dewater ability, and fines retention. BioResources 4(1):321–340
Nazari B (2015) New applications for cellulose nanofibers: Rheological challenges. Dissertation, University of Maine
Nazari B, Bousfield DW (2016) Cellulose nanofibers influence on properties and processing of paperboard coating. Nord Pulp Pap Res J 31(3):511–520
Pahimanolis N, Salminen A, Penttila PA et al (2013) Nanofibrillated cellulose/carboxymethyl cellulose composite with improved wet strength. Cellulose 20:1459–1468
Paunonen S (2013) Strength and barrier enhancements of cellophane and cellulose derivative films: A review. BioResources 8(2):3098–3121
Rautkoski H, Pajari H, Koskela H, Sneck A, Moilanen P (2015) Use of cellulose nanofibrils (CNF) in coating colors. Nord Pulp Pap Res J 30(3):511–518
Rezayati Charani P, Dehghani-Firouzabadi M, Afra E, Blademo A, Naderi A, Lindstrom T (2013) Production of microfibrillated cellulose from unbleached kraft pulp of Kenaf and Scotch Pine and its effect on the properties of hardwood kraft: microfibrillated cellulose paper. Cellulose 20:2559–2567
Richmond F, Haugwout C, Bousfield DW (2014) The use of cellulose nanofibers in paper coating formulation. TAPPI Paper Con
Schenker M, Schoelkopf J, Mangin P, Gane P (2016) Rheological investigation of complex micro and nanofibrillated cellulose (MNFC) suspension: Discussion of flow curves and gel stability. Tappi J 15(6):405–416
Song Z, Xiao H, Zhao Y (2014) Hydrophobic-modified nano-cellulose fiber/PLA biodegradable composites for lowering water vapor transmission rate (WVTR) of paper. Carbohydr Polym 111:442–448
Song Z, Xiao H, Li Y (2015) Effects of renewable materials coating on oil resistant properties of paper. Nord Pulp Pap Res J 30:344–349
Syverud K, Stenius P (2009) Strength and barrier properties of MFC films. Cellulose 16:75–85
Taniguchi T, Okamura K (1998) New films produced from microfibrillated natural fibers. Polym Int 47:291–294
TAPPI Standard Test Method for Grease Resistance Test for Paper and Paperboard (1996) Tappi press. Atlanta, GA
TAPPI Standard Test Method for Size Test for Paper by Ink Resistance (Hercules type method) (2007) Tappi press. Atlanta, GA
TAPPI Standard Test Method Gravimetric Methods for Measuring Dewatering of Coating Colors (Abo-Academi-type methods) (2001) Tappi press. Atlanta, GA
TAPPI Standrd Test Method for Air Resistance of Paper (Gurley method) (2006) Tappi press. Atlanta, GA
Turbak AF, Snyder FW, Sandberg KR (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci Symp 37:815–827
Xu Y, Kuang Y, Salminen P, Chen G (2016) The influence of nano-fibrillated cellulose as a coating component in paper coating. BioResources 11(2):4342–4352
Yan H, Lindstrom T, Christiernin M (2006) Some ways to decrease fiber suspension flocculation and improve sheet formation. Nord Pulp Pap Res J 21:36–43
Yousefi H, Faezipour M, Hedjazi S, Mazhari Mousavi M, Azusa Y, Heidari A (2013) Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fiber/ground cellulose nanofibers of canola straw. Ind Crops and Prod 43:732–737
Zimmermann T, Bordeanu N, Strub E (2010) Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr Polym 79(4):1086–1093
Acknowledgments
The authors would like to thank the industrial sponsors of University of Maine Paper Surface Science Program (PSSP) for financial support and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazhari Mousavi, S.M., Afra, E., Tajvidi, M. et al. Cellulose nanofiber/carboxymethyl cellulose blends as an efficient coating to improve the structure and barrier properties of paperboard. Cellulose 24, 3001–3014 (2017). https://doi.org/10.1007/s10570-017-1299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1299-5