Abstract
Bacterial cellulose (BC) as a never-dried biopolymer synthesized in abundance by Gluconacetobacter xylinus is in a pure form which requires no intensive processing to remove unwanted impurities and contaminants such as lignin, pectin and hemicellulose. In contrast to plant cellulose, BC, with several remarkable physical properties, can be grown to any desired shape and structure to meet the needs of different applications. BC has been commercialized as diet foods, filtration membranes, paper additives, and wound dressings. This review article presents an overview of BC structure, biosynthesis, applications, state-of-the-art advances in enhancing BC production, and its material properties through the investigations of genetic regulations, fermentation parameters, and bioreactor design. In addition, future prospects on its applications through chemical modification as a new biologically active derivative will be discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, a water-insoluble polysaccharide, is the most abundant macromolecule on earth (Brown 2004) and is mostly produced by vascular plants. Microorganisms also produce cellulose which possesses considerably different properties and, therefore, has applications other than those of plant cellulose. Many cellulose-producing bacteria, including the genera Gluconacetobacter, Rhizobium, Agrobacterium, Rhodobacter and Sarcina have been reported (Brown 2004; Morgan et al. 2013). Bacterial cellulose (BC) was first reported by Adrian Brown (1886) while working with Bacterium aceti in 1886. A solid mass was formed at the surface of fermentation medium, which was not expected in his routine work. The solid mass was referred to as the “vinegar plant’’ or “mother” and was commonly used in home-made vinegar production. The constituent was later identified as cellulose and the name Bacterium xylinum was assigned to the microorganism responsible for its synthesis. Since its discovery, several names were given to this bacterium including Acetobacterium xylinum (Ludwig 1989) and Bacterium xylinodes (Henneberg 1906). It was later named as Acetobacter xylinum (Bergey 1925) and became the official name according to the International Code of Nomenclature of Bacteria (Sneath 1958). Now the Gram-negative, strictly aerobic bacterium is referred to as Gluconacetobacter xylinus (Valla et al. 2009), which is considered as a subspecies of Acetobacter aceti (Cannon and Anderson 1991).
Unlike plant cellulose, BC does not require extra processing to remove unwanted impurities and contaminants such as lignin, pectin and hemicellulose, thus being able to retain a greater degree of polymerization (Nishi et al. 1990). BC also demonstrates unique properties, including high degree of crystallinity, water retention value, tensile strength, and moldability (Klemm et al. 2001; Yamanaka et al. 1989; Yoshino et al. 1996). In the time period from 1946 to 1963, Hestrin et al. published many papers on the synthesis of BC which were summarized in a book chapter (Ohad et al. 1962). Chawla et al. (2009) also summarized the effects of fermentation parameters and bioreactor configuration on BC production. However, further discussions about more recent progress of BC, including biosynthesis manipulation, new bioreactor designs, production methods, modifications and applications are still needed.
Therefore, the objective of this article is to provide an extensive overview of BC chemistry, structure, advances in enhancing BC production, and its material property improvements through the investigations of genetic regulations, fermentation parameters, and bioreactor design as well as future prospects on its applications as high value products.
Bacterial cellulose biosynthesis
Cellulose producing strain
The accurate determination of the chemical composition and structural properties of BC was conducted by Hibbert and Barsha (1931). They concluded that the BC was chemically identical to the cellulose from plants. Although BC can be produced from the species of Achromobacter, Alcaligenes, Aerobacter, Agrobacterium, Azotobacter, Gluconacetobacter, Pseudomonas, Rhizobium, Sarcina, Dickeya and Rhodobacter (Brown 2004; Deinema and Zevenhuizen 1971; Morgan et al. 2013; Jahn et al. 2011), only Gluconacetobacter species can produce cellulose at commercial levels. The amount of BC produced from Acetobacter spp. varies from 1.0 to 4.0 % (w/v) of fermentatation media. Selection of commercially useful strains of cellulose producing organisms was initially driven by its use as a food product (Kuga and Brown 1988; Okiyama et al. 1992b). Nata de Coco, for example, is a popular dessert in Philippine, which consists in mixing BC in the fruit juices with other plant extracts (Lapuz et al. 1967). This microorganism was later identified as G. xylinus and the optimal culture conditions were pH at 5–5.5 and 28 °C with ammonium salts as nitrogen source and either glucose or sucrose as carbon source (Masaoka et al. 1993). The detailed mechanism of bacterial cellulose synthesis from G. xylinus (ATCC 23769 and 53582) and its location on the cell surface was investigated by Brown and his co-workers (Brown et al. 1976; Benziman et al. 1980; White and Brown 1981; Bureau and Brown 1987).
Ross et al. (1991) also reviewed cellulose biosynthesis and its function in bacteria. They outlined the “machinery” necessary for cellulose synthesis, regulatory mechanism as well as function and genetics. The biochemistry of cellulose synthesis in bacteria, plants, and algae were compared. The detailed mechanism was summarized in our Genetics and Enzyme regulation section (Ross et al. 1985, 1991).
Gluconacetobacter xylinus
Masaoka et al. (1993) performed an extensive study on cellulose producing bacterial strains of the genus Gluconacetobacter and Agrobacterium to find the best cellulose producer. The cellulose production in static culture was found to be proportional to the surface area of the culture with a constant culture volume. Culture volume and depth (above 4.5 cm) had no effect on production rate since growth occurs at the air–liquid surface (Okiyama et al. 1992a). It has been asserted that the purpose of this extracellular material is to provide a firm, floating material in which the embedded cells of G. xylinus, an obligate aerobe, may benefit from close contact with air surface (Cook and Colvin 1980). Williams and Cannon (1989) reported that the cellulose pellicles promoted the colonization of G. xylinus on the substrate and provided protection from the access of competitors. Cellulose also protects G. xylinus from the negative effects of UV lights due to its opacity (Williams and Cannon 1989).
Colvin and Leppard (1977) suggested a cycle of lipid-phosphate-carbohydrate intermediates in cellulose biosynthesis, where glucose reacts to form glucose-6-phosphate, then glucose-1-phosphate, uridine diphosphoglucose (UDP-glc), and finally cellulose. This biosynthesis of bacterial cellulose forms a multi-regulation network containing several key enzymes to control cellulose synthesis (such as glucokinase, isomerase, phosphoglucomutase, UDPG-pyrophosphorylase and cellulose synthase), in which the branched hexose monophosphate pathway (HMP) and tricarboxylic acid cycle are involved (Yu et al. 2012). The simplified pathway is shown in Fig. 1.
BC can also be synthesized in a cell-free system. Glaser (1958) first reported that in vitro cellulose synthesis can be obtained with only UDPG, ATP, and broken cells. This system was also reported later by Ben-Hayyim and Ohad (1965), Colvin and Leppard (1977), Swissa et al. (1980) and Lin et al. (1985). However, the production efficiency was low, which could be due to the requirement of membrane potential during cellulose synthesis (Delmer et al. 1982).
In nature, most cellulose is produced as crystalline cellulose, which is defined as cellulose I. In an attempt to determine the relationship between polymerization and crystallization of β-1, 4-glucans into microfibrils of G. xylinus, Benziman et al. (1980) added Calcofluor White ST, a commercial brightener for cellulose, to the production medium. This disrupted the assembly of crystalline cellulose I fibrils while accelerating the polymerization process. Once the chemical agent was washed away, ribbon production and polymerization rates restored to normal. They concluded that the polymerization and crystallization are coupled in a consecutive process, and the rate of polymerization was limited by the rate of crystallization.
Deslandes and Marchessault (1983) continued this work and developed a mechanism for the self-assembly of cellulose microfibrils. The first step is the polymerization of several β-1, 4-glucan chains at an extrusion site on the cell surface. A single G. xylinus cell may polymerize up to 200,000 glucose molecules per second into β-1, 4-glucan chains on the lined-up extrusion sites, which later secreted into the surrounding medium (Hestrin and Schramm 1954). Parallel glucan chains then aggregate and crystallize into microfibrils, and finally these microfibrils aggregate into discontinuous bundles (Iguchi et al. 2000). Native cellulose microfibrils occur in a spectrum of dimensions, ranging from 1 to 25 nm in width (corresponding to 10–250 chains) and from 1 to 9 μm in length (2,000–18,000 glucose residues) (Haigler 1985). During bacterial cellulose synthesis, bacterial cellulose synthase (Bcs) A and B play key roles to promote cellulose fiber elongation. Morgan et al. (2013) presented the crystal structure of a complex of BcsA and BcsB containing a translocating polysaccharide, which demonstrates BcsA–BcsB complex forms a cellulose-conducting channel to extend polysaccharide by one glucose.
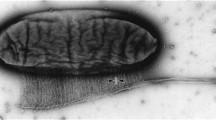
Dark field microscopy was employed to observe cellulose formation (Brown et al. 1976). Cellulose is secreted from the bacteria in the form of a ribbon, composed of approximately 46 microfibrils, at a rate of 2 μm/min. Electron micrographs of the cell envelope indicated that the presence of 50–80 porelike sites arranged in a regular row along the long axis of the cell and in evident juxtaposition with the extracellular cellulosic ribbon. A complementary row of particles within the interior of the outer membrane has been described for freeze-etched preparations. Brown (1985) presumed that these discrete structures are the sites of extrusion for cellulosic microfibrils in groups of 10–15 chains. Microfibrils, rather than individual β-1, 4-glucan chains, are supposed to be the initial form of the produced cellulose. Based on these observations, the authors concluded that the cellulose is not assembled at a distance from the cell, as it was believed earlier.
Other BC producing strains
In addition to G. xylinus, Escherichia coli and Salmonella spp. were also reported as cellulose producing microorganisms, which is related to adhesion and multicellular (biofilm) behavior (Zogaj et al. 2001; Coucheron 1991). The cellulose synthesis genes (bcsA, bcsB, bcsZ and bcsC) of these species were similar to those in G. xylinus (Solano et al. 2002). However, the amounts of BC produced by these strains were lower than that from G. xylinus. Kitamura and Yamaya (1987) also reported that BC can be produced anaerobically by pathogenic, Gram-positive Sarcina ventriculi. The produced BC from S. ventriculi remains tightly associated with the cell membrane, which may protect themselves from strong acid in mammal’s intestine.
Genetics and enzyme regulation
The cellulose synthesis enzyme complex has been observed in all cellulose-synthesizing organisms and these enzyme complexes are termed as terminal complexes (TCs) (Brown 1985; Okuda 2002). Four genes were identified from a cellulose deficient mutant (Cel−) and named AxCeSA, AxCeSB, AxCeSC (Wong et al. 1990) and AxCeSD (Delmer 1999). The complimentary fragment contained acs operon (acs A–D), consisting of four open reading frames encoding four proteins with molecular weight of 84.4, 85.3, 141.0, and 17.3 kDa, respectively. The first three proteins participate in the production of cellulose by G. xylinus while the fourth one plays a role in the crystallization of cellulose (Wong et al. 1990; Saxena et al. 1994). Moreover, three other upstream genes and two enzymes have been identified. From these genes, ORF1 is presumed to encode an enzyme with cellulase activity (CMCax, endoglucanase) (Kawano et al. 2005) and the function of second gene (ORF2) involves the construction of high-order structures (cellulose I) of BC (Nakai et al. 2002). The endoglucanase identified in bacteria and plants was predicted to act as an editor/chain terminator during cellulose synthesis (Delmer 1999). However, G. xylinus replicates only cellulose negative cells when either gene was disrupted (Standal et al. 1994). The third gene was thought to be partially responsible for the switching mechanism between cellulose I and II production (Nakai et al. 2002). Aloni et al. (1982) reported that an unknown protein and GTP is crucial for stimulating the activity of cellulose synthase. The enzyme was later identified as diguanylate cyclase (Ross et al. 1985). The diguanylate cyclase catalyses the formation of cyclic diguanylic acid (c-di-GMP), while phosphosdiesterase, as a negative control, catalyses the degradation of c-di-GMP (Ross et al. 1991). Nakai et al. (2012) reported that some cellulases such as carboxymethylcellulase play essential roles, which can reduce cellulose production and form small particulate cellulose after its function was blocked. Bhowmick et al. (2011) also reported that the expression of gcpA gene, a biofilm associated gene, may regulate cellulose synthesis in Salmonella enterica. It indicates the biofilm associated gene is a potential alternative pathway to regulate BC production. Sunagawa et al. (2013) discovered that the function of cellulose complementing factor (Ccp), a kind of membrane protein in Gluconacetobacter species, is the same as cellulose synthase complex (also called terminal complex). It means that the processing of cellulose synthesis may have other unknown components.
Coucheron (1991) found an insertion sequence in G. xylinus that altered cells to cellulose-negative mutants. Using DNA hybridization analysis, seven copies of a specific genetic insertion sequence element, IS 1031, were found in the wild-type strain. Once these elements are inserted, the cell could lose its ability to produce cellulose.
In order to enhance the production of cellulose, recombinant DNA was used to produce a faster growing organism, usually E. coli, with same cellulose-producing ability of G. xylinus. Fujiwara et al. (1992) constructed a shuttle vector, pUF106, which contained genes from both G. xylinus and E. coli. The vector also contained an ampicillin resistance gene, which makes it easy to select transformed variants. No further work on the application of this vector was found. DeWulf et al. (1996) conducted genetic engineering to produce a mutant of G. xylinus (LMG 1518), which was limited in its ability to produce gluconic acid and related species, and hence prevented dramatic decrease in pH during cultivation. The resulting pellicle size was twice as that of wild-type strain. This novel strain is beneficial to the cellulose production industry.
Production of BC
The current methods of BC production are static culture (Shezad et al. 2010; Kralisch et al. 2010), agitating culture (Yan et al. 2008; Tse et al. 2010), and the airlift reactor (Song et al. 2009). Large scale, semi-continuous, and continuous fermentation will be dominant to meet commercial demand. In all cases, the main objective is to achieve maximum production of BC with optimum form and suitable properties for the application for which it is intended.
It was previously believed that the wild strains of G. xylinus were not able to produce cellulose in a submerged and aerated system due to the accumulation of cellulose-negative (Cel−) mutants (Johnson and Neogi 1989; Oikawa et al. 1995; Mikkelsen et al. 2009; Hong et al. 2012). Unlike wild-type cells (Cel+), Cel− cells produce water-soluble polysaccharides and they are identified as acetan (Valla and Kjosbakken 1981; Griffin et al. 1996). However, through genetic modification or strain selection, several strains can now produce cellulose in an agitated and aerated bioreactor (Toyosaki et al. 1995; Hwang et al. 1999). BC produced by these new strains under agitated and airlift cultures, however, is formed as reticulated cellulose slurry with limited applications.
Static cultures for BC production
Static cultivation is a relatively simple and widely used method of cellulose pellicle production. The medium is placed into shallow trays, inoculated, and cultivated for 5–20 days until the cellulose nearly fills the tray. G. xylinus produces a gelatinous BC pellicle, which has a denser surface on the side exposed to air. The BC production is directly related to the area of air/liquid surface when the depth is less than 4.5 cm (Okiyama et al. 1992b). An external factor, named the wall effect, should be eliminated as it is the strongest limiting factor for BC production (Hornung et al. 2006). Schramm and Hestrin (1954) explained that the floating property of BC pellicle is due to the entrapped CO2 bubbles generated from metabolism of bacteria.
The traditional static culture represents an expensive way of BC production that may hinder its industrial application since the productivity is low and long cultivation time is required. Shezad et al. (2010) proposed a new static culture system based on a simple fed-batch strategy to increase the BC productivity to a suitable level for commercial applications, using waste from beer fermentation broth (WBFB) as nutrient source. It was found that WBFB is a better medium than the chemically defined medium for BC production in a fed-batch culture. Seven hundred and fifty g of BC sheet was obtained with 2.5 cm thickness after 30 days of cultivation using WBFB, representing a 2–3 times increase in BC production compared to batch cultivation. The scanning electron micrographs of the sheets showed that the fibrils of BC produced in the fed-batch culture are more crowded and thinner than those produced using chemically defined medium (CDM) (Zhou et al. 2007a). With the same purpose, Kralisch et al. (2010) developed a novel, efficient bioreactor for a semi-continuous production of planar bacteria-produced nanocelluloses (BNC) named Horizontal Lift Reactor (HoLiR) equipped with fleeces and foils of selectable length and adjustable height. This process combines the advantages of static cultivation and continuous harvesting under steady-state cultivation conditions. JeNaCell®, the commercial product from the HoLiR, is characterized by comparable material properties as BNC produced from traditional static cultures. Meanwhile, significant cost reduction using HoLiR compared with static cultivation in Erlenmeyer flasks was accomplished.
Submerged fermentation
Several attempts have been made to produce BC using the submerged fermentation, which is more convenient for scale-up production. However, there are two main concerns: (1) insufficient oxygen supply and irregular shape of produced BC, and (2) simultaneous accumulation of non-BC-producing mutants in agitation culture (Schramm and Hestrin 1954; Park et al. 2003). Moreover, the insolubility of the cellulose product poses obstacles for nutrition transfer resulting in inhomogeneity. G. xylinus BPR2001, as ATCC 700178, which was reported by Toyosaki et al. (1995) exhibited 1.8-fold higher BC productivity than the commonly used strain, G. xylinus ATCC 23769 cultivated under agitation. Other strains, such as G. xylinus BPR 3001A, G. hansenii KCTC 10505BP, and G. xylinus NUST4.1 were also reported for their applicability in the agitated fermentation (Park et al. 2003; Naritomi et al. 2002; Zhou et al. 2007b). BC pellets instead of pellicles were obtained in agitated cultures.
Airlift bioreactors have stood out as solution for reducing shear stress and avoiding shutdowns in BC production. Song et al. (2009) studied the scale-up BC production in a modified airlift-type bubble column bioreactor, which had a low shear stress and high oxygen transfer rate (kLa), using saccharified food wastes (SFW) as fermentative medium. With the addition of 0.4 % agar and aeration rate of 1.0 vvm, 5.6 g/l BC was produced in a 50 l spherical type bubble column bioreactor after 3 days of cultivation. In relation with structural properties, Hu and Catchmark (2010a) investigated the formation of spherelike BC particles under shaking conditions. They found that not all the G. xylinus strains can produce these spherelike particles. Results showed that the JCM 9730 strain could form spherelike cellulose particles under agitated culture with a rotational speed above 100 rpm. Approximately, spheres with 0.1–1 and 10 mm diameters were produced at a rotational speed of 200 and 150 rpm, when cultured in 100 ml of medium in a 250 and 150 ml Erlenmeyer flask, respectively. Field emission scanning electron microscope (FESEM) analysis revealed that cellulose particles produced at 150 rpm were hollow with a layered outer shell, while, particles produced at 125 rpm were solid, but the central region was not layered.
Bioreactor design
To meet commercial scale production, though scaling-up of static culturing is challenging, several modifications were proposed. Yoshino et al. (1996) designed a new culture vessel, which provided an oxygen-permeable silicone membrane surface in the bottom. By doing this, the rate of cellulose production was doubled since BC pellicles can be formed on the liquid–air surface and on the oxygen-permeable silicone membrane. They also found that the rate of cellulose production on the silicone membrane depended strongly on the degree of roughness of the membrane surface. The rate of BC production was about five times higher on a glossy silicone membrane than on an embossed surface (Yoshino et al. 1996).
Hornung et al. (2007) developed a novel bioreactor, which involves the generation of an aerosol spray of glucose and its even distribution to the living bacteria on the medium-air interface. The average growth was 2 mm/day or around 9 g cellulose dry mass/day. BC produced in the aerosol bioreactor showed higher mechanical strength than the traditionally produced product from a static beaker or box culture. Table 1 summarizes different types of bioreactor which are used to produce bacterial cellulose.
Airlift bioreactor
Airlift bioreactor, which requires lower power supply when compared with agitated bioreactor, is another option for BC production. Chao et al. (1997) first reported the implementation of airlift bioreactor for BC production. Air or oxygen-enriched air was supplied from the bottom and hence drove the circulation of culture medium. Later, different configuration of airlift bioreactor has been applied to enhance BC production. Chao et al. (2000) employed a 50-l internal-loop airlift reactor and obtained 3.8 g/l BC after 67 h fermentation. Cheng et al. (2002) reported a modified airlift reactor (equipped with three wire-mesh draft tubes), which yielded 7.7 g/l BC after 72 h fermentation. The highest BC concentration to date was 10.4 g/l with a 0.22 g/l/h production rate by using air-lift reactors (Chao et al. 2001b).
Rotating disk bioreactor
In agitation or airlift bioreactors, the adhesion of BC to the shaft and the upper part of the vessel causes the homogeneity problem and increases the difficulty of removing the BC product. Another concern is that the produced BC pellets exhibit low mechanical strength which limits its possible applications when compared to BC pellicle. Serafica et al. (2002) first reported the BC production in a rotating disk bioreactor, which consists of a cylindrical inlet for inoculation and several circular disks mounted on a rotating central shaft (Bungay et al. 1991). The rotating disk bioreactor was designed that the half area of its disks was submerged in the medium and the other half exposed to the atmosphere. With continuous rotation, the surface of disks is alternately located between the medium and atmosphere. The advantage of this design is that the produced BC will attach on the disks and restore its mechanical strength, in addition to aeration. Later, Krystynowicz et al. (2002) investigated the optimal fermentation condition (medium volume, rotating speed, and number of disks) for BC production. The maximum BC yield was obtained when rotating speed is at 4 rpm with a surface/medium ratio of 0.71. Attempts of adding different solid particles were conducted in order to obtain novel BC composites. Serafica et al. (2002) reported that the incorporation of solid particles into the cellulose matrix was related to the rotating speed and their concentrations. Paper fibers of ordinary cellulose can be incorporated to create composites with enhanced strength and increase the toughness of BC (Mormino and Bungay 2003). Lin and Cheng (2012) reported a semi-continuous approach by using rotating disk bioreactor containing PCS, a kind of plastic matrix, which can enhance cell adhesion. BC can be produced semi-continuously, and its productivity is approximately 2.58 mg/cm2/day for at least five consecutive runs without the need of reinoculation.
Cell immobilization and biofilm reactors
The productivity of BC can be improved by using techniques such as immobilized-cell reactors, cell-recycle reactors, and hollow fiber reactors. Biofilm reactor, one of the immobilized-cell reactors, is an excellent example of high biomass density systems, which may reduce the capital costs.
Biofilm reactors, moreover, show several advantages over suspended cell reactors, particularly in providing high biomass density, operation convenience, and production yield. Cheng et al. (2009b) successfully enhanced BC production by using a plastic composite support (PCS) biofilm reactor. The results demonstrated that the PCS biofilm reactor yielded BC production (7.05 g/l) that was 2.5-fold greater than the control (2.82 g/l). Moreover, mechanical strength analysis also indicated that the produced BC, similar to pellicle form, improved its tensile strength to a point comparable to that observed in pellicle form, which may broaden its potential applications. However, it is not clear to what extent PCS components are incorporated into the cellulose matrix. Solid nutrient supports present the capability to introduce desired additives into the produced cellulose potentially allowing new engineered materials to be created. Other studies concerning BC production were summarized in Table 2.
Long-term fermentation
A main limitation of batch fermentation is the time required to restart a new batch. Microorganisms need time to accustom themselves to new fermentation condition. Intense researches have been conducted to develop continuous fermentation processes in order to: minimizing the time for cleaning and sterilizing the bioreactor, omitting the time for seed culture preparation, and shortening the lag time for cell activation and accumulation time of enough biomass.
Naritomi et al. (1998) conducted BC fermentation in a continuous manner with ethanol addition. Results demonstrated that, with the presence of 10 g/l of ethanol, 0.95 g/l/h BC production rate and 46 % of BC yield was achieved with a dilution rate of 0.07/h. The BC production was only 0.6 g/l after 36-h fermentation for control in batch fermentation. Ethanol functioned as an energy source for ATP generation, but not as a substrate for BC synthesis. For repeated batch fermentation, Naritomi et al. (2002) studied the broth exchange ratio on BC production. The results indicated that the highest BC production rate (0.43 g/l/h) and BC yield (28 %) were achieved when the broth exchange ratio was 0.9. With a larger broth exchange ratio, the ATP content in cells was low and resulted in a lower BC production rate.
Naritomi et al. (1997) patented a process for continuously preparing BC at a production rate of 0.4 g/l/h, by maintaining the concentration of the residual sugar in the culture broth at 20 g/l. More recently, Ruka et al. (2012) proposed a 7-day cultivation period which reached the highest BC productivity. The BC yield reaches a maximum level at approximately 14 days (10 g/l). Lin and Cheng (2012) demonstrated that plastic composite support (PCS) as solid support features high potential for semi-continuous production of BC. The result shows that BC can be produced semi-continuously, and its productivity reached is approximately 2.58 mg/cm2/day, which can be maintained at least five consecutive runs.
Culture media
Previous studies have illustrated that G. xylinus has the capability to grow and produce cellulose on a variety of substrates. This leads to many investigations concerning optimal composition of medium for bacterial growth and cellulose production. The standard medium used for BC cultivation, the Hestrin-Schramm medium (Schramm and Hestrin 1954), is expensive and requires additional supplements for effective cultivation (Kongruang 2008).
Saccharified liquid medium, created by the enzymatic treatment of food wastes, appears as an inexpensive alternative to complex commercial BC production medium. Products from beets (molasses, sugar syrup, and saccharose), corn (starch, hydrolyzed starch, glucose syrup, and glucose), and potatoes (starch and starch hydrolyzates) can be used for BC production (Kongruang 2008).
Ruka et al. (2012) reported that the Yamanaka et al. (1989) and Zhou et al. (2007a) modified media produced high levels of cellulose, and more specifically the Yamanaka-mannitol combination reached the highest cellulose yield. The results also showed that glucose, mannitol and sucrose were the carbon sources that produced consistently high yields of cellulose, regardless of the composition of the media. The maximum BC production was set at 10 g produced in 14 days using a large surface area and high cellulose-producing media.
Carbon and nitrogen source
Tarr and Hibbert (1931) tested a number of carbohydrates in a culture of G. xylinus and determined that fructose provides the best yield. Glucose, sorbitol, and mannitol demonstrated an applicable production, while sorbose, mannose, cellobiose, erythritol, ethanol, and acetate completely failed to form any pellicles (Hestrin et al. 1947).
Embuscado et al. (1994) examined combinations of sugars and nitrogen materials at one time. They reported that their specific strain of G. xylinus, isolated from nata, cannot utilize glucose effectively, while fructose and sucrose worked well. The optimal substrates in terms of yield were fructose 5 %, followed by 2.5 % fructose plus 2.5 % sucrose. Addition of more than 5 % sugar did not increase the production of cellulose. Among the nitrogen sources studied, yeast extract or a combination of yeast extract and peptone were most effective.
Oikawa et al. (1995) reported that d-mannitol was an effective substrate for cellulose production of G. xylinus. The optimal medium containing polypeptone and yeast extract, in addition to mannitol, produced three times more cellulose than a glucose medium under identical culture conditions. The author presumed that mannitol is first converted to fructose before entering the cellulose synthesis pathway, which agrees with earlier study by Hestrin et al. (1947). Recent studies have investigated the replacement of carbon and nitrogen sources in Hestrin-Schramm media. Mikkelsen et al. (2009) evaluated mannitol, glucose, glycerol, fructose, sucrose and galactose as carbon sources in HS medium. They found that after 96-h of fermentation, the highest yields of BC 3.83 and 3.75 g/l were obtained with sucrose and glycerol, respectively. They also observed that different carbon sources did not markedly alter the micro-architecture of the resulting cellulose pellicles.
In order to compensate its low sugar conversion yield and to reduce the feed-stock cost of BC production, BC has been produced by fermenting the hydrolysates of agricultural wastes such as hemicelluloses (Hong et al. 2012), konjac powder (Hong and Qiu 2008), rice bark (Goelzer et al. 2009) and waste cotton fabrics (Kuo et al. 2010). Several successful efforts have been made to use certain industrial food wastes as growth medium for the BC producer organisms, which is not only a cheap way but also works as a basin for environmental cleaning (Khan et al. 2007). Thin stillage (TS) is a wastewater from rice wine distillery rich in carbon sources and organic acids. Wu and Liu (2012) discovered that TS, when employ to replace distilled water for preparing Hestrin and Schramm medium (the traditional BC production medium), can enhance the BC production 2.5-fold to a concentration of 10.38 g/l, with a sugar-BC conversion yield of 57 % (0.57 g BC/g reducing sugar) after 7 days of static cultivation. In 2012, Ha and Park (2012) further improved the BC production, 15.28 g/l of BC was obtained after 15 days of cultivation.
Many researchers studied the pretreatment of cellulosic wastes to enhance the enzymatic saccharification and, thus, obtain a richer carbon source to increase BC production (Kuo and Lee 2009; Kuo et al. 2010; Hong et al. 2012). Kuo and Lee (2009) assessed four cellulose dissolution agents, NaOH/urea solution, N-methylmorpholine-N-oxide (NMMO), ionic liquid 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) and 85 % phosphoric acid, to dissolve cotton cellulose. After 72 h, the saccharification yield ranged from 87 to 96 % for the regenerated celluloses while only around 23 % could be achieved for the untreated cellulose. The significant hydrolysis enhancement obtained with NaOH/urea-cellulose was mainly due to crystal structure switch from cellulose I to a more easily digestible cellulose II. Later, Kuo et al. (2010) employed different cellulose solvents, including ionic liquid 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), 85 % concentrated phosphoric acid, N-methylmorpholine oxide (NMMO) monohydrate, and NaOH/urea solution, to dissolve the waste fabrics and thereby obtaining a fourfold enhancement of saccharification rate and reducing sugars yield, through cellulase hydrolysis. The hydrolysate obtained was employed as carbon source to grow G. xylinus in a static culture for the production of bacterial cellulose pellicle. Higher BC yields (40–65 %) were obtained in cotton enzymatic hydrolysate cultures compared to that achieved with G. xylinus in glucose-based cultures. It was proposed that the culture with cotton hydrolysate should have a higher carbon source concentration than the glucose culture, since additional soluble cello-oligosaccharides would be present in the cotton hydrolysate. Hong et al. (2012) explored cotton-based waste textiles as alternative feedstock for production of BC by static culture of G. xylinus. To obtain a better enzymatic hydrolysis rate they suggested the cellulosic fabrics pretreatment with the ionic liquid (IL) 1-allyl-3-methylimidazolium chloride ([AMIM]Cl). A BC yield of 10.8 g/l was obtained from the cotton cloth hydrolysate with a reducing sugar concentration of 17 g/l. This result was 83 % higher than that from the glucose-based control medium, and with a 79 % higher tensile strength. They concluded that waste cotton pretreated with [AMIM]Cl has potential to serve as a high quality carbon source for BC production.
Yeast extract and peptone are the most commonly used nitrogen sources in BC production as they provide nitrogen and growth factors for Gluconacetobacter strains. However, many researchers are trying to find efficient substitutes due to their high cost. Matsuoka et al. (1996) determined that corn steep liquor (CSL) was the most effective undefined nutrient and they found that lactate and methionine had the greatest effect within CSL. The defined medium based on this analysis was able to synthesize cellulose at 90 % of the rate of the undefined medium.
Buffering capacity is also important for BC production. Insoluble BC often attaches to pH probe and leads to inaccurate reading (Shoda and Sugano 2005). Noro et al. (2004) pointed out the buffering capacity of CSL, which could maintain the pH within the optimal range during the production of BC. Jung et al. (2010a) doubled BC production (from 1.53 to 3.12 g/l) using molasses as carbon source and corn steep liquor as nitrogen source when compared with the results obtained from complex medium.
Effect of pH
The effect of pH is well documented in many studies (Hwang et al. 1999; Embuscado et al. 1994; Hutchens et al. 2007). BC production was affected due to the carbon flow directed into gluconic acid synthesis at low pH (Masaoka et al. 1993). Results from static fermentation proved that the pH usually drops to 3.5 during fermentation, which is out of optimal pH range for BC production (Klemm et al. 2001). A strict control of pH is therefore important for BC production. Embuscado et al. (1994) reported that the optimal pH for cellulose production was 4.5 and no cellulose production was observed when pH was below 3.5. A pH-shifting method was later proposed by Hwang et al. (1999). They reported that when the pH was at 4.0, G. xylinus will accumulate gluconic acid, and a preferred environment for both biomass and BC production can be achieved by shifting pH from 4.0 to 5.5 during cellulose production phase in batch cultures. The optimal pH for BC production may vary with carbon source. Hutchens et al. (2007) reported that when bacteria were cultured on glucose, the optimal initial pH was 5.5. However, the optimal pH was 6.5 when mannitol was used.
Under static batch cultivation, the pH of the culture medium decreases because of the respiratory metabolism of G. xylinus, which involves the ethanol oxidation to acetic acid and the glucose conversion into gluconic acid. This fact makes it very important to control the pH within the optimum range for cell growth and cellulose production (Kongruang 2008; Ha et al. 2011). However, in the case of fed-batch cultivation (static culture), pH remains almost constant, which can be due to the periodic addition of fresh medium (Shezad et al. 2010). Jagannath et al. (2008) studied the effect of pH on the thickness of bacterial cellulose produced from a coconut water medium in static fermentation. There was no appreciable cellulose formation at pH 3.5 even after 20 days. Maximum thickness of BC (10.2 ± 0.26 mm) was obtained at pH 4.0 with 10 % sucrose and 0.5 % ammonium sulphate concentrations, associated with a high water holding capacity (87.4 ± 4.6 %) and a very low hardness (4.2 ± 0.1 N).
Effect of oxygen
It is generally accepted that cellulose synthesis and secretion require highly aerobic conditions. Therefore, cells tend to float and reach the oxygen-rich surface (Aloni et al. 1982). Colonies of G. xylinus in static culture produce a visible film of cellulose pellicles which cover the medium surface (Ross et al. 1991). In agitated submerged cultures, which are more convenient from an industrial point of view, the oxygen transfer to the submerged cells is sufficient, resulting in a drop in BC productivity due to increased viability of cellulose negative strains. Gluconacetobacter strains require oxygen as an essential substrate, so volumetric oxygen transfer coefficient (kLa) is a key limiting factor in the aerobic fermentation for producing BC. Song et al. (2009) investigated the optimum aeration rate for a 50-l spherical type bubble column bioreactor, and it was determined to be 1.0 vvm (30 l/min). Li et al. (2011) studied the oxygen uptake rate (OUR) of G. xylinus KJ1 during BC production process using saccharified food wastes medium in a 50-l modified bubble column bioreactor. The OUR in a 12 h cultivation was 0.21 mg DO/l min, from which the critical dissolved oxygen (DO) concentration was suggested to be maintained above 3.10 ppm to prevent oxygen limitation during BC production. These results indicated that pure oxygen should be supplied during the exponential phase, where DO depletion was observed. Under this condition, production of 7.37 g/l BC was obtained in the 50-l bioreactor with the supplementation of pure oxygen. BC synthesis by Gluconacetobacter sp. requires high oxygen transfer rate at a low shear force. Kim et al. (2012) investigated the effect of soluble polymer (represented by agar) and insoluble BC on the viscosity and kLa in a 50-l up-and-down circulation fermenter. They hypothesized that the drastic reduction of kLa was caused by the soluble and insoluble viscous materials. They also concluded that agar is more influential than BC on kLa, and the BC productivity was increased as the agar concentration was raised to 0.4 %.
Effects of other additives on BC production
Attempts to enhance BC production by adding different additives in the fermentation medium have been made. The possible mechanisms of these various additives to enhance BC were also proposed such as, reduction of the shear force by increasing the viscosity of medium (Bae et al. 2004), inhibition of the crystallization process (Haigler et al. 1980), switch of cell type (Park et al. 2003) and enhanced production rate by interrupting ribbon formation (Zhou et al. 2007b; Lai et al. 2009).
Different chemical compounds including alcohols (Lu et al. 2011), glycerol (Jung et al. 2010b; Mikkelsen et al. 2009), organic acids (Jung et al. 2010b), polysaccharides (Shah et al. 2010; Kim et al. 2012), thin stillage from rice wine distillery (Wu and Liu 2012) and thin stillage from beer culture broth (Ha et al. 2008; Shezad et al. 2010) have been used as additives to the fermentation medium with the aim of increasing BC production. Cheng et al. (2009a) also reported that A. xylinum can produce 8.2 g/l of BC with the addition of 1 % carboxymethyl cellulose (CMC) in flask study, which is 6.3-fold than the result of suspension cell culture. In addition, small pellets were formed inside the reactor instead of large chunks. This was found to be an advantage as the cellulose was also easier to harvest and opened the possibility of continuous BC production. The slight decrease of crystallinity also indicated that CMC may attach to the microfibrils at some point prior or during crystallization, or even after crystallization but in a fashion where some impact on the crystallization process may still occur.
With the same purpose, Lu et al. (2011) investigated the stimulatory effects of six different alcohols, added at different concentrations, during fermentation of G. xylinus 186. The produced effects could be ranked as n-butanol > mannitol > glycerol > ethylene glycol > methanol > n-propanol. However, results showed that n-butanol only improves BC production when added at concentrations fewer than 1.5 % v/v (maximum production of 132.6 mg/100 ml, 56.0 % higher than the control), while mannitol stimulates BC production at any concentration, with a maximum effect at the concentration of 4 % v/v (maximum production of 125.2 mg/100 ml, 47.3 % higher than the control).
More studies have been performed with the addition of polysaccharides, such as agar and acetan, to decrease the shear rate and improve the BC productivity. Song et al. (2009) obtained a maximum BC production of 5.8 g/l when 0.4 % agar was added to saccharified food wastes (SFW) medium, compared with 5.0 g/l in the control culture. Shah et al. (2010) also worked on surface modified reactors (SMRs) and they found that the maximum BC production (5.03 g/l) was obtained in the SMRs with 2 % agar. This value was about 1.7 times higher than the control culture (3.05 g/l). The agar was responsible for the increase in viscosity and cell growth rate, which resulted in the increase of BC production and productivity, maintaining the basic structural characteristics of BC.
Hu and Catchmark (2010b) reported that with the addition of 0.14 mg of 1-Methylcyclopropene (1-MCP) to the culture medium, less biomass was produced and bacterial cellulose yield increased up to 25.4 % over controls.
A second purpose of adding compounds to the fermentative media is the chemical modification of structural and physical properties of the cellulose intended to broaden its practical applications. Huang et al. (2010) demonstrated that the in situ modification with hydroxypropylmethyl cellulose and CMC during fermentation can improve rehydration ability of BC by altering its network structure. Similarly, Lin et al. (2009) proposed the addition of enzymatically modified gelatin to enhance the rehydration abilities and mechanical properties of BC. Outcomes demonstrate that while the rehydration ratio of dried BC is only around 16.5 % and does not increase with longer rehydration times, the rehydration ratio of dried enzymatically modified form (EMG) composites can be 3 (immersed 10 min) to 4 (immersed 420 min) times higher.
Application of BC
Since cellulose exhibits several unique properties, such as high tensile strength and water content, several recent publications have focused on its applications or potential applications in various fields. White and Brown (1989) first evaluated the properties of microbial cellulose as they apply to commercialization. The important features outlined include no delignification required during processing, high water retention ability as if never dried, the capacity of being formed into any shape or size, shape retention, formation from a wide variety of substrates, and properties which can be controlled during synthesis. Based on its wide application, number of BC patents and journals are reported, and the publishing shows an upward trend (Siró and Plackett 2010). The applications of BC are summarized in the following sections and in Table 3.
Acoustic transducer diaphragm
Yamanaka and Watanabe (1994) reported potential and possible applications of BC and its composites in the area of acoustic transducers, which is the first non-food high value application. The exceptional shape retention ability of BC, measured as the Young’s Modulus, coupled with the high internal loss of the material make it ideal for speaker diaphragms. The novel diaphragms demonstrated two distinctive properties: high sonic velocity and low dynamic loss, and have been marketed by Sony Corp as loudspeakers and headphones (Iguchi et al. 2000).
Paper manufacturing
Johnson and Neogi (1989) reported that highly branched, reticulated BC pellets produced from agitation culture are suitable for the production of high-quality paper. They also developed composites containing glass fibers, calcium carbonate, and copper powder. Yamanaka and Watanabe (1994) also illustrated that the addition of disintegrated BC to paper pulp make possible to create a paper with higher tensile strength. The fragments of bacterial cellulose were also found effective for reinforcing pulp papers and improved its folding endurance (Iguchi et al. 2000). With the addition of 15 % BC, the composite paper exhibited around four-fold higher folding endurance when compared to the pure pulp paper. In addition, the Young’s modulus was increased from 2.0 to 3.5 (GPa) by addition of BC. In our study, the mechanical strength of CMC-BC paper was also evaluated. Results demonstrated that the CMC-BC paper sheets exhibited higher tensile strength and Young’s modulus when compared with regular paper (Cheng et al. 2011). Hu et al. (2011) synthesized a novel conductive polyaniline/bacterial cellulose (PANI/BC) nanocomposite membrane in situ by oxidative polymerization of aniline. Gutierrez et al. (2012b) evaluate the conductive properties of TiO2 nanoparticles and TiO2/BC hybrid inorganic/organic fibers. Results indicate that TiO2/BC hybrid fibers respond to applied bias regardless of the sign of the applied voltage (−3, 0 and 3 V).
Filtration
The specific application of BC as a filtration material was examined by Takai (1994). Several polymers, such as polyethylene glycol (PEG), CMC, carboxymethyl chitin, and other cellulose-based polymers, were incorporated into the cellulose by simply adding the materials to the starting medium. Some of these polymers showed very high solute rejection which makes them useful for ultrafiltration as well as pervaporation.
Another study focused on the evaluation of filtration properties of BC, testing its usefulness as a dialysis membrane (Shibazaki et al. 1994). When compared to a commercial dialysis membrane made of regenerated cellulose, the BC film showed a significantly higher permeation rate and a greater molecular weight cut-off. An additional benefit of the material as compared with the regenerated cellulose was that the added mechanical strength allowed the use of a thinner membrane.
Pharmaceutical applications
Wound healing is a dynamic process that involves the complex interaction of various cell types, extracellular matrix (ECM) molecules, and soluble compounds (Eming et al. 2002). Winter (1962) discovered that healing, and specifically re-epithelialization, was accelerated if the wound was kept moist. Because of its unique properties, BC has been shown to be a highly effective wound dressing material (Winter 1962; Alvarez et al. 2004; Legeza et al. 2004; Czaja et al. 2006; Czaja et al. 2007; Stanislaw et al. 2012). Jonas and Farah (1998) worked on efficacy of BC as a temporary skin substitute called “Biofill®”. They discussed some clinical results when applied to burns and other skin injuries. Biofill® showed positive indications of diminished post-surgery discomfort, faster healing, immediate pain relief, reduced infection rate, improved exudates retention, and, most important, reduced treatment time and cost. Meftahi et al. (2010) reported a novel cellulose film coated with cotton gauze which exhibits a 30 % higher water absorbency and wicking ability than native cellulose film, and is more suitable for wound dressings. Dissolvable carboxymethyl cellulose foam (CMCF) dressing was reported to be adopted as a substitute of routine nasal packing (RNP) in functional endoscopic sinus surgery (Szczygielski et al. 2010). The results demonstrated that CMCF dressing is associated with lower levels of localized pain, postoperative bleeding and synechia formation when compared to RNP.
Stanislaw et al. (2012) mentioned that BC is an excellent biomaterial in cosmetics industry due to its high water holding capacity, nontoxicity, and no allergic side effect.
The applicability of BC pellicle as a substrate for mammalian cell culture has also been examined. The first detailed report was presented by Watanabe et al. (1993). They cultured eight kinds of cells on the membrane, which was comparable to that achieved in plastic Petri dishes. The ionic charge, roughness of the membrane, and adsorption of collagen are crucial factors promoting cellular adhesion to the membrane surface. Svensson et al. (2005) reported that the growth of bovine chondrocytes on BC pellicle exhibited that this BC pellicle supported bovine chondrocyte proliferation at about 50 % more than that observed from collagen. Later on, the growth method of human keratinocytes and fibroblasts on BC film was developed (Sanchavanakit et al. 2006). The results demonstrated that the percentage of the living keratinocytes and fibroblast cells after seeding on BC film was comparable to the results of polystyrene culture plate. However, only the keratinocytes cells can spread over the surface of BC film, whereas proliferative fibroblasts form clumps. A possible reason is that the adhesion of fibroblasts to BC film was less than that between themselves. Backdahl et al. (2006) examined the interaction between BC and smooth muscle cells (SMC). SMCs attached on both compact and porous sides of BC. The BC pellicle holds 99 % water and hence leaves room for cell ingrowth and proliferation. The results demonstrated that SMC adhered to and proliferated on the BC film. A 40 μm ingrowth of SMC was observed after 2 weeks of cultivation. Cai and Kim (2010) reported a BC/PEG composite, which exhibits higher capability of forming fibroblast cell adhesion and proliferation than the pure BC.
In addition to serving as a support for cell growth, modified BC film can be applied as an anti-adhesion and anti-proliferative material. Extremina et al. (2010) reported that a cellulose triacetate (CTA) membranes with the antibiotic imipenem (IPM) entrapped (CTA-IPM) were developed. The bacterial adhesion tests showed a statistically significant decrease in the adhesion of Staphylococcus epidermidis to CTA-IPM compared with its adhesion to CTA alone. With this invention, a BC membrane with anti-adhesive and anti-proliferative properties can provide a better simulation of the in vivo clinical situation.
BC was previously reported to induce only low inflammatory and foreign body reactions (Klemm et al. 2001; Helenius et al. 2006). Therefore, BC became a proposed new biosynthetic vascular graft material since traditional methods may cause intimal hyperplasia, poor blood flow and surface thrombogenicity. Fink et al. (2010) used BC to substitute conventional vascular graft, expanded poly(tetrafluoroethylene) (ePTFE) and poly(ethyleneterephtalat) (PET) and the results demonstrated that BC was found to generate longest lag time indicating a slower coagulation process on its surface. Klemm et al. (2001) also reported that a Bacterial Synthesized Cellulose (BASYC®) was designed tubularly during the cultivation with the aim of medical applications. In a microsurgical study, the BC implants were attached in an artificial defect of the carotid artery of rats for 1 year. This long term result showed the incorporation of the BC under formation of neointima and ingrowth of active fibroblasts (Schumann et al. 2009). The tensile strength of BC artificial vascular has also been investigated by Backdahl et al. (2006). The BC rings demonstrated similar tensile results (stress at break and Young’s modulus) when compared to porcine carotid artery (PCA). The orientation of BC fibrils can be further controlled by the curvature of the silicone tube (Putra et al. 2008).
In many biomedical applications, bioabsorbability is highlighted, where the material will degrade over time into a product which can be metabolized by human body. A bioabsorbable BC was demonstrated through the incorporation of cellulose degrading enzymes (Hu and Catchmark 2011a; Hu and Catchmark 2011b). In this case, BC films loaded with multiple cellulases were freeze dried and the degradation behavior was examined. Approximately 97 % of the BC was converted into glucose over a 7-day period in the case where buffer ingredients were also incorporated into the BC film to maintain a local pH in the optimal range for the cellulases used. Further animal studies revealed no adverse reactions associated with the enzymes used (Hu 2011.).
Its unique properties as a very high water-holding capacity, great elasticity, high wet strength, and conformability coupled with its purity, make BC an excellent biomaterial with many applications in the biomedical field (Klemm et al. 2011; Petersen and Gatenholm 2011). Most of the biomedical applications, like artificial skin or wound dressing, require BC to be in a proper shape as a film or membrane, which can be produced only in static cultivations (Park et al. 2009). However, a research on the use of BC pellets (originated in agitated conditions), was conducted by Wu and Lia (2008) as a technique for enzyme immobilization.
In vitro studies with endothelial cells, smooth muscle cells, chondrocytes, and osteoprogenitor cells grown on BC have shown good cell adhesion and migration into the material. Besides, in vivo biocompatibility of BC has been established by Helenius et al. (2006) and later confirmed by Mendes et al. (2009). Cai and Kim (2010) also evaluated the biocompatibility of a BC/Poly (ethylene glycol) composite as wound dressing or tissue-engineering scaffolds. 3T3 fibroblast cells incubated with BC/PEG scaffolds for 48 h were capable of forming cell adhesion and proliferation, showing much better biocompatibility than the pure BC. The potential of BC for in vitro and in vivo tissue regeneration still continues to be explored and shows great promise.
Amin et al. (2012b) used the electron-beam radiation technique to fabricate hydrogels with BC and acrylic acid. BC provides mechanical strength to the hydrogel without limiting its swelling properties, while acrylic acid brings pH-responsiveness. These modifications along with thermal stability, allow hydrogels to serve as versatile materials in many biomedical and pharmaceutical applications such as the delivery of antibodies, antibiotics, enzymes, hormones, anFd contraceptives that act through a variety of routes. They also found that hydrogels showed a reduced swelling at body temperature suggesting that they can be applied for temperature-controlled drug delivery. Other BC applications as pharmaceutical biomaterials have been summarized in Table 3.
Food applications
Okiyama et al. (1992a, b, 1993) suggested several applications for BC in the food industry, such as thickening agents, low-calorie desserts, salads, and fabricated food. A 3 % paste of cellulose was added to a chocolate drink in place of xanthan gum. Viscosity comparisons were nearly identical after mixing, but heat treatment caused a severe drop in viscosity for the xanthan gum drink, while no viscosity decrease was observed for cellulose. The addition of cellulose to ice cream prevents flow after melting as a result of increased shear stress. Similar results were shown when the cellulose paste was added to tofu, pasty condiments, and boiled fish paste. The authors, therefore, concluded that BC is widely applicable in food industry. BC has been determined to be “generally recognized as safe” (GRAS) and accepted by the Food and Drug Administration in 1992. It has important applications in a variety of food formulations, especially when low use levels, lack of flavor interactions, foam stabilization, and stability over wide pH range, temperature, and freeze–thaw conditions are required. BC in combination with other agents such like sucrose and CMC improve the dispersion of the product. Potential applications also include low-calorie additive, thickener, stabilizer, texture modifier, pasty condiments, and ice cream additive.
Its high crystallinity, high water holding capacity, large surface area, elasticity, mechanical strength, and biocompatibility enable BC to be used as a support for cell immobilization (Rezaee et al. 2008a, b). The method for immobilizing wine yeast in BC was first reported by Nguyen et al. (2009). Ton and Le (2011a) proved that the immobilized yeasts on BC exhibited much higher metabolic activity and resistance to unfavorable conditions during wine fermentation in comparison with free yeasts. Later, Ton and Le (2011a) studied the suitability of wine yeasts immobilized on BC to perform a repeated batch fermentation in winemaking. The results showed that during 10 consecutive cycles of the repeated batch fermentation, the sugar uptake rate of the immobilized yeast increased from 1.71 g/l/h (cycle 1) to 3.28 g/l/h (cycle 7) and then reduced to 2.75 g/l/h (cycle 10). They concluded that the application of yeast immobilization in winemaking enhanced economic effectiveness of the production-line because of cost reduction in inoculum preparation and simple separation of yeast at the end of the fermentation.
Recently, Mikkelsen et al. (2011) found that BC composite containing soluble polysaccharide is a useful model for the in vitro fermentation of plant dietary fibers in a nutritional study. George and Siddaramaiah (2012) suggested the use of BC nanocrystals in the fabrication of edible, biodegradable and high-performance gelatin nanocomposite films for food packaging applications. Similarly, Yang et al. (2012a) proposed the use of BC/silver nanoparticles (AgNPs) composite as an antimicrobial material for food packages and water sterilization.
Other applications
Modification and incorporation with other ingredients to make novel BC composites broaden the spectrum of BC applications (Table 4). A graphite film has been prepared by pyrolysis of BC (Yoshino et al. 1996). A highly graphitized film with very high electrical conductivity has been made by pyrolysis at 2,900 °C. Shah and Brown (2005) produced an electricity conducting (or semi-conducting) BC sheet by depositing ions around the microfibrils to provide conducting pathways and then immobilizing electrochronic dyes within the microstructure. The device has the potential to be extended to various applications, such as e-book tablets, e-newspapers, dynamic wall papers, rewritable maps and learning tools. Yoon et al. (2006) also incorporated multiwalled carbon nanotubes (MWCNTs) into BC pellicles to produce high electricity conducting polymeric membranes. Another application is as a membrane inside as amperometric glucose sensor (Ammon et al. 1995). Both in vitro and in vivo testing compared sensors made from BC and wood cellulose. All data showed that membranes made from BC were stable six to seven times of durability longer than those that were made from wood. Although the applications of BC rarely use the pellet type, BC beads (0.5–1.5 mm) have also been used as a substrate for enzyme immobilization (Seo et al. 2009). The immobilized glucoamylase demonstrated its stability against changes in the pH value and temperature.
Seo et al. (2009) reported a non-compartmented microbial fuel cell (NCMFC) and adopted semipermeable cellulose acetate film as a cathode material, which can selectively retain protons and hence maintain the redox potential difference between the anode and cathode. The hydrophilicity of cellulose acetate can also be modified. Matama et al. (2010) reported that an engineered cutinases can improve the degree of substitution of hydroxyl group onto cellulose acetate fibers. An increase on the hydroxyl groups at the fiber surface was 25 % for diacetate and 317 % for triacetate after a 24 h treatment, respectively.
The latest trends in BC applications reside in the formation of new nanocomposites that enhance the versatility of this biomaterial. Different type of micro/nano particles can be suspended in the bacteria culture media during the formation of cellulose fibrils (Grande et al. 2009; Sun et al. 2010; Trovatti et al. 2010; Zhijiang and Guang 2011; Ashori et al. 2012; Yang et al. 2012c). There is also a patent about in situ bioproduction and composition of bacterial cellulose nanocomposites (Laborie and Brown 2008).
There are extensive studies on the use of BC in electronic devices (Nogi and Yano 2008; Feng et al. 2012; Juntaro et al. 2012). Evans et al. (2005) patented a method for the deposition of metals in BC for the construction of fuel cells and other electronic equipment. Recent studies also disclosed the potential of nanocellulose as substrates for flexible optoelectronic and photonic devices. Legnani et al. (2008) prepared an organic light emitting diode (OLED) device using flexible bacterial cellulose sheet deposited with SiO2. The maximum luminance was measured to be 1200 cd/m2. Ummartyotin et al. (2012) also reported the successful fabrication of OLED display using a transparent bacterial cellulose nanocomposite film as substrate.
Conclusion and future prospects
In addition to the traditional applications, BC offers a broad spectrum of applications due to its high purity and special chemical properties. Although the economics of industrial BC production can be improved by the agitated cultivation using genetically stable strains (Toyosaki et al. 1995; Zhou et al. 2007b; Park et al. 2003; Naritomi et al. 2002), applications of the produced BC pellets, which exhibit lower mechanical strength, are limited. Rotating disk reactor has been introduced to restore BC tensile strength and produce BC in a pellicle form (Cheng et al. 2002; Serafica 1997; Krystynowicz et al. 2002), however, the non-continuous process limits its feasibility for industrial production. Therefore, a possible solution is to create a rotating disk biofilm reactor, which can accumulate G. xylinus cells on the disks and continuously produce BC without further inoculation (Zinnanti et al. 2009; Lin and Cheng 2012). The composition of the solid support disk can also be designed to release nutrient compounds satisfying the specific requirement of G. xylinus. Enhanced BC production through genetic manipulation is another possible solution since strains (i.e. E. coli) with comparable shorter doubling time were reported (Zogaj et al. 2001). For the specific needs of BC derivatives, the post-modification of BC, with different conjugation of functional groups, will potentially broaden its applications in tissue engineering, paper manufacturing, electronics, and filtration membranes since its properties of surface roughness, affinity, thermostability and electric conductivity will be altered.
In summary, the versatile BC materials demonstrate a wide variety of bioactivities and applications. In order to meet the growing demand of BC, a robust and feasible industrial production and supply is crucial. Despite of the relatively simple fermentation process, new engineering processes are needed to produce desired BC with specific material properties. Moreover, genetic and biochemical investigations are also necessary to enhance BC production at molecular biological level and commercial purposes.
References
Aloni Y, Delmer DP, Benziman M (1982) Achievement of high rates of in vitro synthesis of 1, 4-beta-d-glucan: activation by cooperative interaction of the Acetobacter xylinum enzyme system with GTP, polyethylene glycol, and a protein factor. Proc Natl Acad Sci USA 79(21):6448–6452
Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE (2004) Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem 23(7):1640–1648
Amin MCIM, Abadi AG, Ahmad N, Katas H, Jamal JA (2012a) Bacterial cellulose film coating as drug delivery system: physicochemical, thermal and drug release properties. Sains Malaysiana 41(5):561–568
Amin MCIM, Abadi AG, Ahmad N, Katas H, Jamal JA (2012b) Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr Polym 88(2):465–473. doi:10.1016/j.carbpol.2011.12.022
Ammon HP, Ege W, Oppermann M, Gopel W, Eisele S (1995) Improvement in the long-term stability of an amperometric glucose sensor system by introducing a cellulose membrane of bacterial origin. Anal Chem 67(2):466–471
Andersson J, Stenhamre H, Backdahl H, Gatenholm P (2010) Behavior of human chondrocytes in engineered porous bacterial cellulose scaffolds. J Biomed Mater Res A 94A(4):1124–1132. doi:10.1002/Jbm.A.32784
Andrade FK, Costa R, Domingues L, Soares R, Gama M (2010a) Improving bacterial cellulose for blood vessel replacement: functionalization with a chimeric protein containing a cellulose-binding module and an adhesion peptide. Acta Biomater 6(10):4034–4041. doi:10.1016/j.actbio.2010.04.023
Andrade FK, Moreira SMG, Domingues L, Gama FMP (2010b) Improving the affinity of fibroblasts for bacterial cellulose using carbohydrate-binding modules fused to RGD. J Biomed Mater Res A 92A(1):9–17. doi:10.1002/Jbm.A.32284
Ashori A, Sheykhnazari S, Tabarsa T, Shakeri A, Golalipour M (2012) Bacterial cellulose/silica nanocomposites: preparation and characterization. Carbohydr Polym 90(1):413–418. doi:10.1016/j.carbpol.2012.05.060
Backdahl H, Helenius G, Bodin A, Nannmark U, Johansson BR, Risberg B, Gatenholm P (2006) Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 27(9):2141–2149. doi:10.1016/j.biomaterials.2005.10.026
Bäckdahl H, Risberg B, Gatenholm P (2011) Observations on bacterial cellulose tube formation for application as vascular graft. Mater Sci Eng C Mater Biol Appl: C 31(1):14–21. doi:10.1016/j.msec.2010.07.010
Bae S, Sugano Y, Shoda M (2004) Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J Biosci Bioeng 97(1):33–38. doi:10.1016/S1389-1723(04)70162-0
Barud HS, Regiani T, Marques RFC, Lustri WR, Messaddeq Y, Ribeiro SJL (2011) Antimicrobial bacterial cellulose-silver nanoparticles composite membranes. J Nanomater. doi: 10.1155/2011/721631
Ben-Hayyim G, Ohad I (1965) Synthesis of cellulose by Acetobacter xylinum: VIII. On the formation and orientation of bacterial cellulose fibrils in the presence of acidic polysaccharides. J Cell Biol 25(2):191–207
Benziman M, Haigler CH, Brown RM, White AR, Cooper KM (1980) Cellulose biogenesis: polymerization and crystallization are coupled processes in Acetobacter xylinum. Proc Natl Acad Sci USA 77(11):6678–6682
Bergey DH, Harrison FC, Breed RS, Hammer BW, Huntoon FM (1925) Bergey’s manual of systematic bacteriology, 2nd edn. Williams & Wilkins, New York
Bhowmick PP, Devegowda D, Ruwandeepika HAD, Fuchs TM, Srikumar S, Karunasagar I, Karunasagar I (2011) gcpA (stm1987) is critical for cellulose production and biofilm formation on polystyrene surface by Salmonella enterica serovar Weltevreden in both high and low nutrient medium. Microb Pathog 50(2):114–122. doi:10.1016/j.micpath.2010.12.002
Bodin A, Bharadwaj S, Wu SF, Gatenholm P, Atala A, Zhang YY (2010) Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 31(34):8889–8901. doi:10.1016/j.biomaterials.2010.07.108
Brackmann C, Zaborowska M, Sundberg J, Gatenholm P, Enejder A (2012) In situ imaging of collagen synthesis by osteoprogenitor cells in microporous bacterial cellulose scaffolds. Tissue Eng Part C Methods 18(3):227–234. doi:10.1089/ten.TEC.2011.0211
Brown AJ (1886) LXII-Further notes on the chemical action of Bacterium aceti. J Chem Soc 51:638–643
Brown RM Jr (1985) Cellulose microfibril assembly and orientation: recent developments. J Cell Sci Suppl 2:13–32
Brown RM (2004) Cellulose structure and biosynthesis: what is in store for the 21st century? J Polym Sci Pol Chem 42(3):487–495. doi:10.1002/Pola.10877
Brown RM Jr, Willison JH, Richardson CL (1976) Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci USA 73(12):4565–4569
Bungay, Henry R, Serafica, Gonzalo C (1991) Production of microbial cellulose. Us Patent US6071727, 2000/06/06
Bureau TE, Brown RM (1987) In vitro synthesis of cellulose II from a cytoplasmic membrane fraction of Acetobacter xylinum. Proc Natl Acad Sci USA 84(20):6985–6989
Cai Z, Kim J (2010) Bacterial cellulose/poly(ethylene glycol) composite: characterization and first evaluation of biocompatibility. Cellulose 17(1):83–91. doi:10.1007/s10570-009-9362-5
Cannon RE, Anderson SM (1991) Biogenesis of bacterial cellulose. Crit Rev Microbiol 17(6):435–447. doi:10.3109/10408419109115207
Carreira P, Mendes JA, Trovatti E, Serafim LS, Freire CS, Silvestre AJ, Neto CP (2011) Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour Technol 102(15):7354–7360. doi:10.1016/j.biortech.2011.04.081
Castro C, Zuluaga R, Putaux J-L, Caro G, Mondragon I, Gañán P (2011) Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr Polym 84(1):96–102. doi:10.1016/j.carbpol.2010.10.072
Chao YP, Sugano Y, Kouda T, Yoshinaga F, Shoda M (1997) Production of bacterial cellulose by Acetobacter xylinum with an air-lift reactor. Biotechnol Tech 11(11):829–832. doi:10.1023/A:1018433526709
Chao YP, Ishida T, Sugano Y, Shoda M (2000) Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol Bioeng 68(3):345–352. doi:10.1002/(Sici)1097-0290(20000505)68:3<345:Aid-Bit13>3.3.Co;2-D
Chao Y, Mitarai M, Sugano Y, Shoda M (2001a) Effect of addition of water-soluble polysaccharides on bacterial cellulose production in a 50-L airlift reactor. Biotechnol Prog 17(4):781–785. doi:10.1021/bp010046b
Chao Y, Sugano Y, Shoda M (2001b) Bacterial cellulose production under oxygen-enriched air at different fructose concentrations in a 50-liter, internal-loop airlift reactor. Appl Microbiol Biotechnol 55(6):673–679
Chawla PR, Bajaj IB, Survase SA, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotech 47(2):107–124
Chen S, Shen W, Yu F, Wang H (2009a) Kinetic and thermodynamic studies of adsorption of Cu2+ and Pb2+ onto amidoximated bacterial cellulose. Polym Bull 63(2):283–297. doi:10.1007/s00289-009-0088-1
Chen S, Zou Y, Yan Z, Shen W, Shi S, Zhang X, Wang H (2009b) Carboxymethylated-bacterial cellulose for copper and lead ion removal. J Hazardous Mater 161(2–3):1355–1359. doi:10.1016/j.jhazmat.2008.04.098
Cheng HP, Wang PM, Chen JW, Wu WT (2002) Cultivation of Acetobacter xylinum for bacterial cellulose production in a modified airlift reactor. Biotechnol Appl Biochem 35(Pt 2):125–132
Cheng KC, Catchmark JM, Demirci A (2009a) Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 16(6):1033–1045
Cheng KC, Catchmark JM, Demirci A (2009b) Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J Biol Eng 3:12
Cheng KC, Catchmark JM, Demirci A (2011) Effects of CMC addition on bacterial cellulose production in a biofilm reactor and its paper sheets analysis. Biomacromolecules 12(3):730–736
Choi J, Park S, Cheng J, Park M, Hyun J (2012) Amphiphilic comb-like polymer for harvest of conductive nano-cellulose. Colloids Surf B Biointerfaces 89:161–166. doi:10.1016/j.colsurfb.2011.09.008
Colvin JR, Leppard GG (1977) The biosynthesis of cellulose by Acetobacter xylinum and Acetobacter aceti genus. Can J Microbiol 23(6):701–709
Cook KE, Colvin JR (1980) Evidence for a beneficial influence of cellulose production on growth of Acetobacter xylinum in liquid-medium. Curr Microbiol 3(4):203–205. doi:10.1007/Bf02602449
Coucheron DH (1991) An Acetobacter xylinum insertion sequence element associated with inactivation of cellulose production. J Bacteriol 173(18):5723–5731
Czaja W, Krystynowicz A, Bielecki S, Brown RM Jr (2006) Microbial cellulose—the natural power to heal wounds. Biomaterials 27(2):145–151. doi:10.1016/j.biomaterials.2005.07.035
Czaja WK, Young DJ, Kawecki M, Brown RM (2007) The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 8(1):1–12. doi:10.1021/Bm060620d
Deinema MH, Zevenhuizen LP (1971) Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol 78(1):42–51
Delmer DP (1999) Cellulose biosynthesis: exciting times for a difficult field of study. Annu Rev Plant Physiol Plant Mol Biol 50:245–276. doi:10.1146/annurev.arplant.50.1.245
Delmer DP, Benziman M, Padan E (1982) Requirement for a membrane potential for cellulose synthesis in intact cells of Acetobacter xylinum. Proc Natl Acad Sci USA 79(17):5282–5286
Deslandes Y, Marchessault RH (1983) Cellulose and other natural polymer systems: biogenesis, structure, and degradation, R. Malcolm Brown, Jr., ed., Plenum, New York, 1982, 519 pp. Price: 9.50. J Polym Sci Polym Lett Ed 21(7):583. doi:10.1002/pol.1983.130210713
DeWulf P, Joris K, Vandamme EJ (1996) Improved cellulose formation by an Acetobacter xylinum mutant limited in (keto)gluconate synthesis. J Chem Technol Biot 67(4):376–380. doi:10.1002/(Sici)1097-4660(199612)67:4<376:Aid-Jctb569>3.0.Co;2-J
El-Saied H, El-Diwany AI, Basta AH, Atwa NA, El-Ghwas DE (2008) Production and characterization of economical bacterial cellulose. BioResources 3:1196–1217
Embuscado ME, Marks JS, Bemiller JN (1994) Bacterial cellulose. 1. Factors affecting the production of cellulose by Acetobacter xylinum. Food Hydrocolloid 8(5):407–418
Eming SA, Smola H, Krieg T (2002) Treatment of chronic wounds: state of the art and future concepts. Cells Tissues Organs 172(2):105–117
Esguerra M, Fink H, Laschke MW, Jeppsson A, Delbro D, Gatenholm P, Menger MD, Risberg B (2010) Intravital fluorescent microscopic evaluation of bacterial cellulose as scaffold for vascular grafts. J Biomed Mater Res A 93A(1):140–149. doi:10.1002/Jbm.A.32516
Evans BR, O’Neill HM, Jansen VM, Woodward J (2005) Metallization of bacterial cellulose for electrical and electronic device manufacture. Us Patent US7803477, 2010/09/28
Extremina CI, Fonseca AF, Granja PL, Fonseca AP (2010) Anti-adhesion and antiproliferative cellulose triacetate membrane for prevention of biomaterial-centred infections associated with Staphylococcus epidermidis. Int J Antimicrob Agents 35(2):164–168. doi:10.1016/j.ijantimicag.2009.09.017
Fang B, Wan YZ, Tang TT, Gao C, Dai KR (2009) Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng Part A 15(5):1091–1098. doi:10.1089/ten.tea.2008.0110
Feng Y, Zhang X, Shen Y, Yoshino K, Feng W (2012) A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr Polym 87(1):644–649. doi:10.1016/j.carbpol.2011.08.039
Fink H, Faxalv L, Molnar GF, Drotz K, Risberg B, Lindahl TL, Sellborn A (2010) Real-time measurements of coagulation on bacterial cellulose and conventional vascular graft materials. Acta Biomater 6(3):1125–1130. doi:10.1016/j.actbio.2009.09.019
Fink H, Hong J, Drotz K, Risberg B, Sanchez J, Selborn A (2011) An study of blood compatibility of vascular grafts made of bacterial cellulose in comparison with conventionally-used graft materials. J Biomed Mater Res A 97A(1):52–58. doi:10.1002/Jbm.A.33031
Finkenstadt VL, Millane RP (1998) Fiber diffraction patterns for general unit cells: the cylindrically projected reciprocal lattice. Acta Crystallogr Sect A: Found Crystallogr 54(Pt 2):240–248
Fujiwara T, Kawabata S, Hamada S (1992) Molecular characterization and expression of the cell-associated glucosyltransferase gene from Streptococcus mutans. Biochem Biophys Res Commun 187(3):1432–1438
George J, Siddaramaiah (2012) High performance edible nanocomposite films containing bacterial cellulose nanocrystals. Carbohydr Polym 87(3):2031–2037. doi:10.1016/j.carbpol.2011.10.019
Glaser L (1958) The synthesis of cellulose in cell-free extracts of Acetobacter xylinum. J Biol Chem 232(2):627–636
Goelzer FDE, Faria-Tischer PCS, Vitorino JC, Sierakowski MR, Tischer CA (2009) Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater Sci Eng, C 29(2):546–551. doi:10.1016/j.msec.2008.10.013
Grande CJ, Torres FG, Gomez CM, Bano MC (2009) Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater 5(5):1605–1615. doi:10.1016/j.actbio.2009.01.022
Griffin AM, Morris VJ, Gasson MJ (1996) Identification, cloning and sequencing the aceA gene involved in acetan biosynthesis in Acetobacter xylinum. FEMS Microbiol Lett 137(1):115–121
Gutierrez J, Fernandes SC, Mondragon I, Tercjak A (2012a) Conductive photoswitchable vanadium oxide nanopaper based on bacterial cellulose. ChemSusChem. doi:10.1002/cssc.201200516
Gutierrez J, Tercjak A, Algar I, Retegi A, Mondragon I (2012b) Conductive properties of TiO2/bacterial cellulose hybrid fibres. J Colloid Interface Sci 377(1):88–93. doi:10.1016/j.jcis.2012.03.075
Ha J, Park J (2012) Improvement of bacterial cellulose production in Acetobacter xylinum using byproduct produced by Gluconacetobacter hansenii. Korean J Chem Eng 29(5):563–566. doi:10.1007/s11814-011-0224-0
Ha J, Shehzad O, Khan S, Lee S, Park J, Khan T, Park J (2008) Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J Chem Eng 25(4):812–815. doi:10.1007/s11814-008-0134-y
Ha JH, Shah N, Ul-Islam M, Khan T, Park JK (2011) Bacterial cellulose production from a single sugar α-linked glucuronic acid-based oligosaccharide. Process Biochem 46(9):1717–1723. doi:10.1016/j.procbio.2011.05.024
Haigler CH (1985) Cellulose chemistry and its applications. In: Zeronian SH, Nevell TP (eds) Ellis Horwood, England, pp 30–83
Haigler CH, Malcolmbrown R, Benziman M (1980) Calcofluor White St Alters the invivo assembly of cellulose microfibrils. Science 210(4472):903–906. doi:10.1126/science.7434003
Helenius G, Backdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B (2006) In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A 76A(2):431–438. doi:10.1002/Jbm.A.30570
Henneberg W (1906) Bergey’s manual of systematic bacteriology. In: Garrity GM (ed) Bergey’s manual of systematic bacteriology. Williams & Wilkins, New York
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58(2):345–352
Hestrin S, Aschner M, Mager J (1947) Synthesis of cellulose by resting cells of Acetobacter-Xylinum. Nature 159(4028):64–65. doi:10.1038/159064a0
Hibbert H, Barsha J (1931) Synthetic cellulose and textile fibers from glucose. J Am Chem Soc 53:3907. doi:10.1021/Ja01361a507
Hofinger M, Bertholdt G, Weuster-Botz D (2011) Microbial production of homogeneously layered cellulose pellicles in a membrane bioreactor. Biotechnol Bioeng 108(9):2237–2240. doi:10.1002/bit.23162
Hong F, Qiu K (2008) An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr Polym 72(3):545–549. doi:10.1016/j.carbpol.2007.09.015
Hong F, Zhu YX, Yang G, Yang XX (2011) Wheat straw acid hydrolysate as a potential cost-effective feedstock for production of bacterial cellulose. J Chem Technol Biot 86(5):675–680. doi:10.1002/jctb.2567
Hong F, Guo X, Zhang S, Han S-f, Yang G, Jönsson LJ (2012) Bacterial cellulose production from cotton-based waste textiles: enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresource Technol 104:503–508. doi:10.1016/j.biortech.2011.11.028
Hornung M, Ludwig M, Gerrard AM, Schmauder HP (2006) Optimizing the production of bacterial cellulose in surface culture: evaluation of substrate mass transfer influences on the bioreaction (Part 1). Eng Life Sci 6(6):537–545. doi:10.1002/elsc.200620162
Hornung M, Ludwig M, Schmauder HP (2007) Optimizing the production of bacterial cellulose in surface culture: a novel aerosol bioreactor working on a fed batch principle (Part 3). Eng Life Sci 7(1):35–41. doi:10.1002/elsc.200620164
Hu Y (2011) A Novel Bioabsorbable bacterial cellulose. PhD dissertation, The Pennsylvania State University, Penn State
Hu Y, Catchmark JM (2010a) Formation and characterization of spherelike bacterial cellulose particles produced by Acetobacter xylinum JCM 9730 strain. Biomacromolecules 11(7):1727–1734. doi:10.1021/Bm100060v
Hu Y, Catchmark JM (2010b) Influence of 1-methylcyclopropene (1-MCP) on the production of bacterial cellulose biosynthesized by Acetobacter xylinum under the agitated culture. Lett Appl Microbiol 51(1):109–113. doi:10.1111/j.1472-765X.2010.02866.x
Hu Y, Catchmark JM (2011a) In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomater 7(7):2835–2845. doi:10.1016/j.actbio.2011.03.028
Hu Y, Catchmark JM (2011b) Integration of cellulases into bacterial cellulose: toward bioabsorbable cellulose composites. J Biomed Mater Res B 97B(1):114–123. doi:10.1002/Jbm.B.31792
Hu W, Chen S, Li X, Shi S, Shen W, Zhang X, Wang H (2009) In situ synthesis of silver chloride nanoparticles into bacterial cellulose membranes. Mater Sci Eng, C 29(4):1216–1219. doi:10.1016/j.msec.2008.09.017
Hu W, Chen S, Yang Z, Liu L, Wang H (2011) Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. J Phys Chem B 115(26):8453–8457. doi:10.1021/jp204422v
Huang HC, Chen LC, Lin SB, Hsu CP, Chen HH (2010) In situ modification of bacterial cellulose network structure by adding interfering substances during fermentation. Bioresour Technol 101(15):6084–6091. doi:10.1016/j.biortech.2010.03.031
Hutchens SA, Leon RV, O’Neill HM, Evans BR (2007) Statistical analysis of optimal culture conditions for Gluconacetobacter hansenii cellulose production. Lett Appl Microbiol 44(2):175–180. doi:10.1111/j.1472-765X.2006.02055.x
Hwang JW, Yang YK, Hwang JK, Pyun YR, Kim YS (1999) Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J Biosci Bioeng 88(2):183–188
Ifuku S, Tsuji M, Morimoto M, Saimoto H, Yano H (2009) Synthesis of silver nanoparticles templated by TEMPO-mediated oxidized bacterial cellulose nanofibers. Biomacromolecules 10(9):2714–2717. doi:10.1021/bm9006979
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci 35(2):261–270. doi:10.1023/A:1004775229149
Ishida T, Mitarai M, Sugano Y, Shoda M (2003) Role of water-soluble polysaccharides in bacterial cellulose production. Biotechnol Bioeng 83(4):474–478. doi:10.1002/bit.10690
Jagannath A, Kalaiselvan A, Manjunatha SS, Raju PS, Bawa AS (2008) The effect of pH, sucrose and ammonium sulphate concentrations on the production of bacterial cellulose (Nata-de-coco) by Acetobacter xylinum. World J Microbiol Biotechnol 24(11):2593–2599. doi:10.1007/s11274-008-9781-8
Jahan F, Kumar V, Rawat G, Saxena RK (2012) Production of microbial cellulose by a bacterium isolated from fruit. Appl Biochem Biotechnol 167(5):1157–1171. doi:10.1007/s12010-012-9595-x
Jahn CE, Selimi DA, Barak JD, Charkowski AO (2011) The Dickeya dadantii biofilm matrix consists of cellulose nanofibres, and is an emergent property dependent upon the type III secretion system and the cellulose synthesis operon. Microbiology 157(10):2733–2744. doi:10.1099/mic.0.051003-0
Johnson DC, Neogi AN (1989) Sheeted products formed from reticulated microbial cellulose. Us Patent US4863565, 1989/09/05
Jonas R, Farah LF (1998) Production and application of microbial cellulose. Polym Degrad Stabil 59(1–3):101–106. doi:10.1016/S0141-3910(97)00197-3
Jung JY, Khan T, Park JK, Chang HN (2007) Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J Chem Eng 24(2):265–271. doi:10.1007/s11814-007-5058-4
Jung H-I, Lee OM, Jeong J-H, Jeon Y-D, Park K-H, Kim H-S, An W-G, Son H-J (2010a) Production and characterization of cellulose by Acetobacter sp. V6 using a cost-effective molasses–corn steep liquor medium. Appl Biochem Biotechnol 162(2):486–497. doi:10.1007/s12010-009-8759-9
Jung HI, Jeong JH, Lee OM, Park GT, Kim KK, Park HC, Lee SM, Kim YG, Son HJ (2010b) Influence of glycerol on production and structural-physical properties of cellulose from Acetobacter sp. V6 cultured in shake flasks. Bioresour Technol 101(10):3602–3608. doi:10.1016/j.biortech.2009.12.111
Juntaro J, Ummartyotin S, Sain M, Manuspiya H (2012) Bacterial cellulose reinforced polyurethane-based resin nanocomposite: a study of how ethanol and processing pressure affect physical, mechanical and dielectric properties. Carbohydr Polym 87(4):2464–2469. doi:10.1016/j.carbpol.2011.11.020
Kang YJ, Chun SJ, Lee SS, Kim BY, Kim JH, Chung H, Lee SY, Kim W (2012) All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano 6(7):6400–6406. doi:10.1021/nn301971r
Kawano S, Yasutake Y, Tajima K, Satoh Y, Yao M, Tanaka I, Munekata M (2005) Crystallization and preliminary crystallographic analysis of the cellulose biosynthesis-related protein CMCax from Acetobacter xylinum. Acta Crystallogr Sect F Struct Biol Crystall Commun 61(Pt 2):252–254. doi:10.1107/S174430910500206X
Khan T, Park J, Kwon J-H (2007) Functional biopolymers produced by biochemical technology considering applications in food engineering. Korean J Chem Eng 24(5):816–826. doi:10.1007/s11814-007-0047-1
Kim S, Li H, Oh I, Kee C, Kim M (2012) Effect of viscosity-inducing factors on oxygen transfer in production culture of bacterial cellulose. Korean J Chem Eng 29(6):792–797. doi:10.1007/s11814-011-0245-8
Kingkaew J, Jatupaiboon N, Sanchavanakit N, Pavasant P, Phisalaphong M (2010) Biocompatibility and growth of human keratinocytes and fibroblasts on biosynthesized cellulose-chitosan film. J Biomater Sci Polym Ed 21(8–9):1009–1021. doi:10.1163/156856209X462763
Kitamura N, Yamaya E. July 1987. Japanese patent application no. 87/168628
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26(9):1561–1603. doi:10.1016/S0079-6700(01)00021-1
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem 50(24):5438–5466. doi:10.1002/anie.201001273
Kohno T, Fujioka Y, Goto T, Morimatsu S, Morita C, Nakano T, Sano K (1998) Contrast-enhancement for the image of human immunodeficiency virus from ultrathin section by immuno electron microscopy. J Virol Methods 72(2):137–143. doi:10.1016/S0166-0934(98)00022-6
Kongruang S (2008) Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. Appl Biochem Biotechnol 148(1–3):245–256. doi:10.1007/s12010-007-8119-6
Kralisch D, Hessler N, Klemm D, Erdmann R, Schmidt W (2010) White biotechnology for cellulose manufacturing—The HoLiR concept. Biotechnol Bioeng 105(4):740–747. doi:10.1002/bit.22579
Krystynowicz A, Czaja W, Wiktorowska-Jezierska A, Goncalves-Miskiewicz M, Turkiewicz M, Bielecki S (2002) Factors affecting the yield and properties of bacterial cellulose. J Ind Microbiol Biotechnol 29(4):189–195. doi:10.1038/sj.jim.7000303
Kuga S, Brown RM Jr (1988) Silver labeling of the reducing ends of bacterial cellulose. Carbohydr Res 180:345–350
Kuo CH, Lee CK (2009) Enhancement of enzymatic saccharification of cellulose by cellulose dissolution pretreatments. Carbohydr Polym 77(1):41–46. doi:10.1016/j.carbpol.2008.12.003
Kuo C-H, Lin P-J, Lee C-K (2010) Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J Chem Technol Biot 85(10):1346–1352. doi:10.1002/jctb.2439
Kurosumi A, Sasaki C, Yamashita Y, Nakamura Y (2009) Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr Polym 76(2):333–335. doi:10.1016/j.carbpol.2008.11.009
Laborie M-P, Brown E (2008) Method of in situ bioproduction and composition of bacterial cellulose nanocomposites. Us Patent US7968646, 2011/06/28
Lai SW, Liao KF, Lai HC, Chou CY, Cheng KC, Lai YM (2009) The prevalence of gallbladder stones is higher among patients with chronic kidney disease in Taiwan. Medicine 88(1):46–51. doi:10.1097/MD.0b013e318194183f
Lapuz MM, Gallardo EG, Palo MA (1967) The nata organism-cultural requirements, characteristics and identity. Philipp J Sci 96:91–108
Legeza VI, Galenko-Yaroshevskii VP, Zinov’ev EV, Paramonov BA, Kreichman GS, Turkovskii II, Gumenyuk ES, Karnovich AG, Khripunov AK (2004) Effects of new wound dressings on healing of thermal burns of the skin in acute radiation disease. B Exp Biol Med+ 138(3):311–315. doi:10.1007/Bf02694188
Legnani C, Vilani C, Calil VL, Barud HS, Quirino WG, Achete CA, Ribeiro SJL, Cremona M (2008) Bacterial cellulose membrane as flexible substrate for organic light emitting devices. Thin Solid Films 517(3):1016–1020. doi:10.1016/j.tsf.2008.06.011
Li H, Kim S-J, Lee Y-W, Kee C, Oh I (2011) Determination of the stoichiometry and critical oxygen tension in the production culture of bacterial cellulose using saccharified food wastes. Korean J Chem Eng 28(12):2306–2311. doi:10.1007/s11814-011-0111-8
Lin SP, Cheng KC (2012) Bacterial cellulose production by Gluconacetobacter xylinum in the rotating PCS semi-continuous bioreactor and its materials property analysis. Paper presented at the 2012 mini symposium frontiers in biotechnology, National Taiwan University, Taipei
Lin FC, Brown RM Jr, Cooper JB, Delmer DP (1985) Synthesis of fibrils in vitro by a solubilized cellulose synthase from Acetobacter xylinum. Science 230(4727):822–825. doi:10.1126/science.230.4727.822
Lin SB, Hsu CP, Chen LC, Chen HH (2009) Adding enzymatically modified gelatin to enhance the rehydration abilities and mechanical properties of bacterial cellulose. Food Hydrocolloid 23(8):2195–2203. doi:10.1016/j.foodhyd.2009.05.011
Lu Z, Zhang Y, Chi Y, Xu N, Yao W, Sun B (2011) Effects of alcohols on bacterial cellulose production by Acetobacter xylinum 186. World J Microbiol Biotechnol 27(10):2281–2285. doi:10.1007/s11274-011-0692-8
Ludwig B (1989) Bergey’s manual of systematic bacteriology. In: Garrity GM (ed) Bergey’s manual of systematic bacteriology. Williams & Wilkins, New York
Malm CJ, Risberg B, Bodin A, Bäckdahl H, Johansson BR, Gatenholm P, Jeppsson A (2012) Small calibre biosynthetic bacterial cellulose blood vessels: 13-months patency in a sheep model. Scand Cardiovasc J 46(1):57–62. doi:10.3109/14017431.2011.623788
Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym 72(1):43–51. doi:10.1016/j.carbpol.2007.07.025
Martinez Neto EE, Dolci JE (2010) Nasal septal perforation closure with bacterial cellulose in rabbits. Braz J Otorhinolaryngol 76(4):442–449
Martinez H, Brackmann C, Enejder A, Gatenholm P (2012) Mechanical stimulation of fibroblasts in micro-channeled bacterial cellulose scaffolds enhances production of oriented collagen fibers. J Biomed Mater Res, Part A 100(4):948–957. doi:10.1002/jbm.a.34035
Masaoka S, Ohe T, Sakota N (1993) Production of cellulose from glucose by Acetobacter xylinum. J Ferment Bioeng 75(1):18–22. doi:10.1016/0922-338x(93)90171-4
Matama T, Araujo R, Gubitz GM, Casal M, Cavaco-Paulo A (2010) Functionalization of cellulose acetate fibers with engineered cutinases. Biotechnol Prog 26(3):636–643. doi:10.1002/btpr.364
Matsuoka M, Tsuchida T, Matsushita K, Adachi O, Yoshinaga F (1996) A synthetic medium for bacterial cellulose production by Acetobacter xylinum subsp sucrofermentans. Biosci Biotech Bioch 60(4):575–579
Meftahi A, Khajavi R, Rashidi A, Sattari M, Yazdanshenas ME, Torabi M (2010) The effects of cotton gauze coating with microbial cellulose. Cellulose 17(1):199–204. doi:10.1007/s10570-009-9377-y
Mendes PN, Rahal SC, Pereira-Junior OC, Fabris VE, Lenharo SL, de Lima-Neto JF, da Cruz Landim-Alvarenga F (2009) In vivo and in vitro evaluation of an Acetobacter xylinum synthesized microbial cellulose membrane intended for guided tissue repair. Acta Vet Scand 51:12. doi:10.1186/1751-0147-51-12
Mikkelsen D, Flanagan BM, Dykes GA, Gidley MJ (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 107(2):576–583. doi:10.1111/j.1365-2672.2009.04226.x
Mikkelsen D, Gidley MJ, Williams BA (2011) In vitro fermentation of bacterial cellulose composites as model dietary fibers. J Agric Food Chem 59(8):4025–4032. doi:10.1021/jf104855e
Mohammadi H, Boughner D, Millon LE, Wan WK (2009) Design and simulation of a poly(vinyl alcohol)-bacterial cellulose nanocomposite mechanical aortic heart valve prosthesis. Proc Inst Mech Eng Part H 223(6):697–711
Moosavi-nasab M, Yousefi A (2011) Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran J Biotechnol 9(2):94–101
Morgan JL, Strumillo J, Zimmer J (2013) Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 493(7431):181–186. doi:10.1038/nature11744
Mormino R, Bungay H (2003) Composites of bacterial cellulose and paper made with a rotating disk bioreactor. Appl Microbiol Biotechnol 62(5–6):503–506. doi:10.1007/s00253-003-1377-5
Muangman P, Opasanon S, Suwanchot S, Thangthed O (2011) Efficiency of microbial cellulose dressing in partial-thickness burn wounds. J Am Col Certif Wound Spec 3(1):16–19. doi:10.1016/j.jcws.2011.04.001
Nakai T, Nishiyama Y, Kuga S, Sugano Y, Shoda M (2002) ORF2 gene involves in the construction of high-order structure of bacterial cellulose. Biochem Biophys Res Commun 295(2):458–462
Nakai T, Sugano Y, Shoda M, Sakakibara H, Oiwa K, Tuzi S, Imai T, Sugiyama J, Takeuchi M, Yamauchi D, Mineyuki Y (2012) Formation of highly twisted ribbons in a carboxymethylcellulase gene-disrupted strain of a cellulose-producing bacterium. J Bacteriol. doi:10.1128/JB.01473-12
Naritomi T, Kouda T, Naritomi M, Yano H, Yoshinaga F (1997) Process for continuously preparing bacterial cellulose. Jp Patent US6132998, 2000/10/17
Naritomi T, Kouda T, Yano H, Yoshinaga F (1998) Effect of ethanol on bacterial cellulose production from fructose in continuous culture. J Ferment Bioeng 85(6):598–603. doi:10.1016/S0922-338x(98)80012-3
Naritomi T, Kouda T, Yano H, Yoshinaga F, Shigematsu T, Moriumura S, Kida K (2002) Influence of broth exchange ratio on bacterial cellulose production by repeated-batch culture. Process Biochem 38(1):41–47. doi:10.1016/S0032-9592(02)00046-8
Nguyen VT, Gidley MJ, Dykes GA (2008) Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol 25(3):471–478. doi:10.1016/j.fm.2008.01.004
Nguyen DN, Ton NMN, Le VVM (2009) Optimization of Saccharomyces cerevisiae immobilization in bacterial cellulose by ‘adsorption-Incubation’ method. Int Food Res J 16(1):59–64
Nishi Y, Uryu M, Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S (1990) The structure and mechanical-properties of sheets prepared from bacterial cellulose. 2. Improvement of the mechanical-properties of sheets and their applicability to diaphragms of electroacoustic transducers. J Mater Sci 25(6):2997–3001. doi:10.1007/Bf00584917
Nogi M, Yano H (2008) Transparent nanocomposites based on cellulose produced by bacteria offer potential innovation in the electronics device industry. Adv Mater 20(10):1849–1852. doi:10.1002/adma.200702559
Noro N, Sugano Y, Shoda M (2004) Utilization of the buffering capacity of corn steep liquor in bacterial cellulose production by Acetobacter xylinum. Appl Microbiol Biotechnol 64(2):199–205. doi:10.1007/s00253-003-1457-6
Ohad I, Danon IO, Hestrin S (1962) Synthesis of cellulose by Acetobacter xylinum. V. Ultrastructure of polymer. J Cell Biol 12:31–46
Oikawa T, Morino T, Ameyama M (1995) Production of cellulose from D-arabitol by Acetobacter xylinum Ku-1. Biosci Biotech Bioch 59(8):1564–1565
Okiyama A, Motoki M, Yamanaka S (1992a) Bacterial cellulose II. Processing of the gelatinous cellulose for food materials. Food Hydrocol 6(5):479–487
Okiyama A, Shirae H, Kano H, Yamanaka S (1992b) Bacterial cellulose I. Two-stage fermentation process for cellulose production by Acetobacter aceti. Food Hydrocol 6(5):471–477
Okiyama A, Motoki M, Yamanaka S (1993) Bacterial cellulose IV. Application to processed foods. Food Hydrocol 6(6):503–511
Okuda K (2002) Structure and phylogeny of cell coverings. J Plant Res 115(4):283–288. doi:10.1007/s10265-002-0034-x
Park JK, Jung JY, Park YH (2003) Cellulose production by Gluconacetobacter hansenii in a medium containing ethanol. Biotechnol Lett 25(24):2055–2059
Park JK, Khan T, Jung JY (2009) Bacterial cellulose. Handbook of hydrocolloids Cambridge, UK
Pertile R, Moreira S, Andrade F, Domingues L, Gama M (2012) Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol Progr 28(2):526–532. doi:10.1002/Btpr.1501
Petersen N, Gatenholm P (2011) Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol 91(5):1277–1286. doi:10.1007/s00253-011-3432-y
Portal O, Clark WA, Levinson DJ (2009) Microbial cellulose wound dressings in the treatment of nonhealing lower extremity ulcers. Wounds 21:1–3
Pourramezan G, Roayaei A, Qezelbash Q (2009) Optimization of culture conditions for bacterial cellulose production by Acetobacter sp. 4B-2. Biotechnology 8(1):150–154
Putra A, Kakugo A, Furukawa H, Gong JP, Osada Y (2008) Tubular bacterial cellulose gel with oriented fibrils on the curved surface. Polymer 49(7):1885–1891. doi:10.1016/j.polymer.2008.02.022
Quero F, Nogi M, Yano H, Abdulsalami K, Holmes SM, Sakakini BH, Eichhorn SJ (2010) Optimization of the mechanical performance of bacterial cellulose/poly(l-lactic) acid composites. Acs Appl Mater Inter 2(1):321–330. doi:10.1021/Am900817f
Rezaee A, Godini H, Bakhtou H (2008a) Microbial cellulose as support material for the immobilization of denitrifying bacteria. Environ Eng Manag J 7(5):589
Rezaee A, Godini H, Dehestani S, Reza Yazdanbakhsh A, Mosavi G, Kazemnejad A (2008b) Biological denitrification by Pseudomonas stutzeri immobilized on microbial cellulose. World J Microbiol Biotechnol 24(11):2397–2402. doi:10.1007/s11274-008-9753-z
Ross P, Aloni Y, Weinhouse C, Michaeli D, Weinberger-Ohana P, Meyer R, Benziman M (1985) An unusual guanyl oligonucleotide regulates cellulose synthesis in Acetobacter xylinum. FEBS Lett 186(2):191–196
Ross P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55(1):35–58
Ruka DR, Simon GP, Dean KM (2012) Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr Polym 89(2):613–622. doi:10.1016/j.carbpol.2012.03.059
Sanchavanakit N, Sangrungraungroj W, Kaomongkolgit R, Banaprasert T, Pavasant P, Phisalaphong M (2006) Growth of human keratinocytes and fibroblasts on bacterial cellulose film. Biotechnol Progr 22(4):1194–1199. doi:10.1021/Bp060035o
Saska S, Barud HS, Gaspar AM, Marchetto R, Ribeiro SJ, Messaddeq Y (2011) Bacterial cellulose-hydroxyapatite nanocomposites for bone regeneration. Int J Biomater 2011:175362. doi:10.1155/2011/175362
Saska S, Scarel-Caminaga RM, Teixeira LN, Franchi LP, Dos Santos RA, Gaspar AM, de Oliveira PT, Rosa AL, Takahashi CS, Messaddeq Y, Ribeiro SJ, Marchetto R (2012) Characterization and in vitro evaluation of bacterial cellulose membranes functionalized with osteogenic growth peptide for bone tissue engineering. J Mater Sci Mater Med 23(9):2253–2266. doi:10.1007/s10856-012-4676-5
Saxena IM, Kudlicka K, Okuda K, Brown RM Jr (1994) Characterization of genes in the cellulose-synthesizing operon (acs operon) of Acetobacter xylinum: implications for cellulose crystallization. J Bacteriol 176(18):5735–5752
Schramm M, Hestrin S (1954) Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol 11:123–129
Schumann D, Wippermann J, Klemm D, Kramer F, Koth D, Kosmehl H, Wahlers T, Salehi-Gelani S (2009) Artificial vascular implants from bacterial cellulose: preliminary results of small arterial substitutes. Cellulose 16(5):877–885. doi:10.1007/s10570-008-9264-y
Seo HN, Lee WJ, Hwang TS, Park DH (2009) Electricity generation coupled with wastewater treatment using a microbial fuel cell composed of a modified cathode with a ceramic membrane and cellulose acetate film. J Microbiol Biotechn 19(9):1019–1027. doi:10.4014/Jmb.0812.663
Serafica GC (1997) Production of bacterial cellulose using a rotating disk film bioreactor by Acetobacter xylinum. Troy, NY
Serafica G, Bungay H (1996) Production of bacterial cellulose using a rotating disk film bioreactor. Abstr Pap Am Chem S 211:148-BIOT
Serafica G, Mormino R, Bungay H (2002) Inclusion of solid particles in bacterial cellulose. Appl Microbiol Biotechnol 58(6):756–760. doi:10.1007/s00253-002-0978-8
Shah J, Brown RM (2005) Towards electronic paper displays made from microbial cellulose. Appl Microbiol Biotechnol 66(4):352–355. doi:10.1007/s00253-004-1756-6
Shah N, Ha J, Park J (2010) Effect of reactor surface on production of bacterial cellulose and water soluble oligosaccharides by Gluconacetobacter hansenii PJK. Biotechnol Bioproc E 15(1):110–118. doi:10.1007/s12257-009-3064-6
Shezad O, Khan S, Khan T, Park JK (2010) Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr Polym 82(1):173–180. doi:10.1016/j.carbpol.2010.04.052
Shi Q, Li Y, Sun J, Zhang H, Chen L, Chen B, Yang H, Wang Z (2012) The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 33(28):6644–6649. doi:10.1016/j.biomaterials.2012.05.071
Shibazaki H, Kuga S, Onabe F (1994) Jpn TAPPI J 48:1621–1630
Shoda M, Sugano Y (2005) Recent advances in bacterial cellulose production. Biotechnol Bioproc E 10(1):1–8. doi:10.1007/Bf02931175
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17(3):459–494. doi:10.1007/s10570-010-9405-y
Sneath PHA (1958) International code of nomenclature of bacteria and viruses. ASM Press, Herndon
Solano C, Garcia B, Valle J, Berasain C, Ghigo JM, Gamazo C, Lasa I (2002) Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol Microbiol 43(3):793–808
Solway DR, Clark WA, Levinson DJ (2011) A parallel open-label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. Int Wound J 8(1):69–73. doi:10.1111/j.1742-481X.2010.00750.x
Song H-J, Li H, Seo J-H, Kim M-J, Kim S-J (2009) Pilot-scale production of bacterial cellulose by a spherical type bubble column bioreactor using saccharified food wastes. Korean J Chem Eng 26(1):141–146. doi:10.1007/s11814-009-0022-0
Songsurang K, Pakdeebumrung J, Praphairaksit N, Muangsin N (2011) Sustained release of amoxicillin from ethyl cellulose-coated amoxicillin/chitosan-cyclodextrin-based tablets. AAPS PharmSciTech 12(1):35–45. doi:10.1208/s12249-010-9555-0
Standal R, Iversen TG, Coucheron DH, Fjaervik E, Blatny JM, Valla S (1994) A new gene required for cellulose production and a gene encoding cellulolytic activity in Acetobacter xylinum are colocalized with the bcs operon. J Bacteriol 176(3):665–672
Stanislaw B, Halina K, Alina K, Katarzyna K, Marek Ko, Manu de G (2012) Wound dressings and cosmetic materials from bacterial nanocellulose. In: Bacterial nanocellulose. Perspectives in nanotechnology. CRC Press, New York, pp 157–174. doi:10.1201/b12936-910.1201/b12936-9
Stoica-Guzun A, Stroescu M, Jinga S, Jipa I, Dobre T, Dobre L (2012) Ultrasound influence upon calcium carbonate precipitation on bacterial cellulose membranes. Ultrason Sonochem 19(4):909–915. doi:10.1016/j.ultsonch.2011.12.002
Sun D, Yang J, Wang X (2010) Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2(2):287–292
Sunagawa N, Fujiwara T, Yoda T, Kawano S, Satoh Y, Yao M, Tajima K, Dairi T (2013) Cellulose complementing factor (Ccp) is a new member of the cellulose synthase complex (terminal complex) in Acetobacter xylinum. J Biosci Bioeng. doi:10.1016/j.jbiosc.2012.12.021
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M, Gatenholm P (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26(4):419–431. doi:10.1016/j.biomaterials.2004.02.049
Swissa M, Aloni Y, Weinhouse H, Benizman M (1980) Intermediatry steps in Acetobacter xylinum cellulose synthesis: studies with whole cells and cell-free preparations of the wild type and a celluloseless mutant. J Bacteriol 143(3):1142–1150
Szczygielski K, Rapiejko P, Wojdas A, Jurkiewicz D (2010) Use of CMC foam sinus dressing in FESS. Eur Arch Otorhinolaryngol 267(4):537–540. doi:10.1007/s00405-009-1117-2
Takai M (1994) Bacterial cellulose composites. In: Gilbert RD (ed) Cellulose polymer blends composites. Hanser, Munich, pp 233–240
Tarr HLA, Hibbert H (1931) Polysacharide synthesis by the action of Acetobacter xylinum on carbohydrates and related compounds. Canad J Res 4:372–388
Ton NMN, Le VVM (2011a) Application of immobilized yeast in bacterial cellulose to the repeated batch fermentation in wine-making. Int Food Res J 18(3):983–987
Ton NMN, Le VVM (2011b) Influence of initial pH and sulfur dioxide content in must on wine fermentation by immobilized yeast in bacterial cellulose. Int Food Res J 17:743–749
Toyosaki H, Naritomi T, Seto A, Matsuoka M, Tsuchida T, Yoshinaga F (1995) Screening of bacterial cellulose-producing acetobacter strains suitable for agitated culture. Biosci Biotech Bioch 59(8):1498–1502
Trovatti E, Oliveira L, Freire CSR, Silvestre AJD, Pascoal Neto C, Cruz Pinto JJC, Gandini A (2010) Novel bacterial cellulose–acrylic resin nanocomposites. Compos Sci Technol 70(7):1148–1153. doi:10.1016/j.compscitech.2010.02.031
Trovatti E, Freire CS, Pinto PC, Almeida IF, Costa P, Silvestre AJ, Neto CP, Rosado C (2012) Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: in vitro diffusion studies. Int J Pharm 435(1):83–87. doi:10.1016/j.ijpharm.2012.01.002
Tse ML, Chung KM, Dong L, Thomas BK, Fu LB, Cheng KC, Lu C, Tam HY (2010) Observation of symmetrical reflection sidebands in a silica suspended-core fiber Bragg grating. Opt Express 18(16):17373–17381. doi:10.1364/OE.18.017373
Ummartyotin S, Juntaro J, Sain M, Manuspiya H (2012) Development of transparent bacterial cellulose nanocomposite film as substrate for flexible organic light emitting diode (OLED) display. Ind Crops Prod 35(1):92–97. doi:10.1016/j.indcrop.2011.06.025
Valla S, Kjosbakken J (1981) Isolation and characterization of a new extracellular polysaccharide from a cellulose-negative strain of Acetobacter xylinum. Can J Microbiol 27(6):599–603
Valla S, Ertesvag H, Tonouchi N, Fjaervik E (2009) Microbial production of biopolymers and polymer precursors: applications and perspectives. Caister Acedemic Press, Norwich
Vitta S, Drillon M, Derory A (2010) Magnetically responsive bacterial cellulose: synthesis and magnetic studies. J Appl Phys 108(5):053905–053907. doi:10.1063/1.3476058
Wang W, Zhang TJ, Zhang DW, Li HY, Ma YR, Qi LM, Zhou YL, Zhang XX (2011) Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles-bacteria cellulose nanofibers nanocomposite. Talanta 84(1):71–77. doi:10.1016/j.talanta.2010.12.015
Wang J, Wan Y, Huang Y (2012) Immobilisation of heparin on bacterial cellulose-chitosan nano-fibres surfaces via the cross-linking technique. IET Nanobiotechnol 6(2):52–57. doi:10.1049/iet-nbt.2011.0038
Watanabe K, Eto Y, Takano S, Nakamori S, Shibai H, Yamanaka S (1993) A new bacterial cellulose substrate for mammalian cell culture. A new bacterial cellulose substrate. Cytotechnology 13(2):107–114
Wei B, Yang G, Hong F (2011) Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr Polym 84(1):533–538. doi:10.1016/j.carbpol.2010.12.017
White AR, Brown RM (1981) Enzymatic hydrolysis of cellulose: visual characterization of the process. Proc Natl Acad Sci USA 78(2):1047–1051
White DG, Brown RM Jr (1989) Prospects for the commercialization of the biosynthesis of microbial cellulose. In: Schuerech C (ed) Cellulose and wood-chemistry and technology. Wiley, New York, p 573
Williams WS, Cannon RE (1989) Alternative environmental roles for cellulose produced by Acetobacter xylinum. Appl Environ Microbiol 55(10):2448–2452
Winter GD (1962) Formation of scab and rate of epithelization of superficial wounds in skin of young domestic pig. Nature 193(4812):293. doi:10.1038/193293a0
Winter HT, Cerclier C, Delorme N, Bizot H, Quemener B, Cathala B (2010) Improved colloidal stability of bacterial cellulose nanocrystal suspensions for the elaboration of spin-coated cellulose-based model surfaces. Biomacromolecules. doi:10.1021/bm100953f
Wippermann J, Schumann D, Klemm D, Kosmehl H, Salehi-Gelani S, Wahlers T (2009) Preliminary results of small arterial substitute performed with a new cylindrical biomaterial composed of bacterial cellulose. Eur J Vasc Endovasc Surg 37(5):592–596. doi:10.1016/j.ejvs.2009.01.007
Wong HC, Fear AL, Calhoon RD, Eichinger GH, Mayer R, Amikam D, Benziman M, Gelfand DH, Meade JH, Emerick AW et al (1990) Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc Natl Acad Sci USA 87(20):8130–8134
Wu SC, Lia YK (2008) Application of bacterial cellulose pellets in enzyme immobilization. J Mol Catal B Enzym 54(3–4):103–108. doi:10.1016/j.molcatb.2007.12.021
Wu JM, Liu RH (2012) Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr Polym 90(1):116–121. doi:10.1016/j.carbpol.2012.05.003
Wu SQ, Li MY, Fang BS, Tong H (2012) Reinforcement of vulnerable historic silk fabrics with bacterial cellulose film and its light aging behavior. Carbohydr Polym 88(2):496–501. doi:10.1016/j.carbpol.2011.12.033
Yamanaka S, Watanabe K (1994) Applications of bacterial cellulose in cellulosic polymers. In: Gilbert R (ed) Hanser Publishers Inc, Cincinnati
Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S, Nishi Y, Uryu M (1989) The structure and mechanical-properties of sheets prepared from bacterial cellulose. J Mater Sci 24(9):3141–3145. doi:10.1007/Bf01139032
Yan Z, Chen S, Wang H, Wang B, Jiang J (2008) Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr Polym 74(3):659–665. doi:10.1016/j.carbpol.2008.04.028
Yang J, Yu J, Fan J, Sun D, Tang W, Yang X (2011) Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J Hazard Mater 189(1–2):377–383. doi:10.1016/j.jhazmat.2011.02.048
Yang G, Xie J, Deng Y, Bian Y, Hong F (2012a) Hydrothermal synthesis of bacterial cellulose/AgNPs composite: a “green” route for antibacterial application. Carbohydr Polym 87(4):2482–2487. doi:10.1016/j.carbpol.2011.11.017
Yang G, Xie J, Hong F, Cao Z, Yang X (2012b) Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: effect of fermentation carbon sources of bacterial cellulose. Carbohydr Polym 87(1):839–845. doi:10.1016/j.carbpol.2011.08.079
Yang Z, Chen S, Hu W, Yin N, Zhang W, Xiang C, Wang H (2012c) Flexible luminescent CdSe/bacterial cellulose nanocomoposite membranes. Carbohydr Polym 88(1):173–178. doi:10.1016/j.carbpol.2011.11.080
Yao W, Wu X, Zhu J, Sun B, Zhang YY, Miller C (2011) Bacterial cellulose membrane—a new support carrier for yeast immobilization for ethanol fermentation. Process Biochem 46(10):2054–2058. doi:10.1016/j.procbio.2011.07.006
Yoon SH, Jin HJ, Kook MC, Pyun YR (2006) Electrically conductive bacterial cellulose by incorporation of carbon nanotubes. Biomacromolecules 7(4):1280–1284. doi:10.1021/bm050597g
Yoshino T, Asakura T, Toda K (1996) Cellulose production by Acetobacter pasteurianus on silicone membrane. J Ferment Bioeng 81(1):32–36. doi:10.1016/0922-338x(96)83116-3
Yu HC, Chen LJ, Cheng KC, Li YX, Yeh CH, Cheng JT (2012) Silymarin inhibits cervical cancer cell through an increase of phosphatase and tensin homolog. Phytother Res 26(5):709–715. doi:10.1002/ptr.3618
Zaborowska M, Bodin A, Bäckdahl H, Popp J, Goldstein A, Gatenholm P (2010) Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater 6(7):2540–2547. doi:10.1016/j.actbio.2010.01.004
Zahedmanesh H, Mackle JN, Sellborn A, Drotz K, Bodin A, Gatenholm P, Lally C (2011) Bacterial cellulose as a potential vascular graft: mechanical characterization and constitutive model development. J Biomed Mater Res B 97B(1):105–113. doi:10.1002/Jbm.B.31791
Zeng X, Small DP, Wan W (2011) Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr Polym 85(3):506–513. doi:10.1016/j.carbpol.2011.02.034
Zhang W, Chen S, Hu W, Zhou B, Yang Z, Yin N, Wang H (2011) Facile fabrication of flexible magnetic nanohybrid membrane with amphiphobic surface based on bacterial cellulose. Carbohydr Polym 86(4):1760–1767. doi:10.1016/j.carbpol.2011.07.015
Zhijiang C, Guang Y (2011) Optical nanocomposites prepared by incorporating bacterial cellulose nanofibrils into poly(3-hydroxybutyrate). Mater Lett 65(2):182–184. doi:10.1016/j.matlet.2010.09.055
Zhou LL, Sun DP, Hu LY, Li YW, Yang JZ (2007a) Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. J Ind Microbiol Biotechnol 34(7):483–489. doi:10.1007/s10295-007-0218-4
Zhou WB, Zhu DW, Tan LF, Liao SJ, Hu ZH, Hamilton D (2007b) Extraction and retrieval of potassium from water hyacinth (Eichhornia crassipes). Bioresour Technol 98(1):226–231. doi:10.1016/j.biortech.2005.11.011
Zimmermann KA, LeBlanc JM, Sheets KT, Fox RW, Gatenholm P (2011) Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater Sci Eng, C 31(1):43–49. doi:10.1016/j.msec.2009.10.007
Zinnanti WJ, Lazovic J, Griffin K, Skvorak KJ, Paul HS, Homanics GE, Bewley MC, Cheng KC, Lanoue KF, Flanagan JM (2009) Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain 132(Pt 4):903–918. doi:10.1093/brain/awp024
Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U (2001) The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39(6):1452–1463
Zuo KW, Cheng HP, Wu SC, Wu WT (2006) A hybrid model combining hydrodynamic and biological effects for production of bacterial cellulose with a pilot scale airlift reactor. Biochem Eng J 29(1–2):81–90. doi:10.1016/j.bej.2005.02.020
Author information
Authors and Affiliations
Corresponding author
Additional information
Shin-Ping Lin and Iris Loira Calvar contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, SP., Loira Calvar, I., Catchmark, J.M. et al. Biosynthesis, production and applications of bacterial cellulose. Cellulose 20, 2191–2219 (2013). https://doi.org/10.1007/s10570-013-9994-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9994-3