Abstract
This study demonstrated the feasibility of a biological denitrification process using immobilized Pseudomonas stutzeri. The microbial cellulose (MC) from Acetobacter xylinum was used as the support material for immobilization of the bacterium. Nitrate removal took place mainly in the anoxic system. The effects of various operating conditions such as the initial nitrate concentration, pH, and carbon source on biological denitrification were demonstrated experimentally. The system demonstrated a high capacity for reducing nitrate concentrations under optimum conditions. The denitrification rate increased up to a maximal value of 1.6 kg NO3-N m−3 day−1 with increasing nitrate loading rate. Because of its porosity and purity, MC may be considered as appropriate supports for adsorbed immobilized cells. The simplicity of immobilization and high efficiency in operation are the main advantages of such systems. To date, the immobilization of microorganisms onto MC has not been carried out. The results of this research shows that a pilot bioreactor containing P. stutzeri immobilized on MC exhibited efficient denitrification with a relatively low retention time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrate contamination of groundwater sources is one of the consequences of anthropogenic activities. Increased levels of nitrate can create the potential for eutrophication and toxic algal blooms in the receiving waters (Sawant et al. 2007). Ingestion of water with a high nitrogen concentration (nitrate and nitrite) may cause diseases such as methemoglobinemia (Fan and Steinberg 1996) or stomach cancer (Yang et al. 2007). To address this problem, specific rules have been established globally. The European Union and the US Environmental Protection Agency, set the 5.6 mg (NO3-N)/l and 10 mg (NO3-N)/l respectively (Aslan and Cakici 2007). The removal of nitrate from wastewater and drinking water sources has become one of the main requirments throughout the world. Conventional methods for nitrogen removal include biological, chemical, and physical processes. Recently, biological processes have been found to be the most effective for nitrogen treatment because of ease of implementation, efficiency, moderate cost and the possibility of combining carbon removal with nitrogen removal. Biological denitrification enables the transformation of different forms of nitrogenous compounds through a wide spectrum of bacteria into harmless nitrogen gas with accompanying carbon removal (Bernat and Wojnowska-Baryla 2007).
The investigation of the immobilization of microbial cells has received increasing interest in the field of biological wastewater treatment because of advantages such as the long retention time of biomass in the system, manipulation of growth rate independent of washout, necessary protection from high concentrations of recalcitrant organics that are toxic to free cells, ease of use in a continuous reactor and the ability to scale up the process. Biofilm technology has been shown to be one of the most advanced methods for removing nitrate and nitrite in a selective low-cost way by dissimilatory reduction (Lazarova and Manem 1995; Vrtovsek et al. 2006). When a denitrifier is immobilized in a matrix, O2 is consumed near the surface and an anoxic core may exist. Biological denitrification is inhibited when the dissolved O2 is above 0.2 mg/l (Dawson and Murphy 1972). With decreasing O2 flux from the bulk solution into the immobilized denitrifier, the volume of the anoxic core increases, resulting in an increase in the denitrification rate (Yamagiwa et al. 1997). Different natural materials (agar, agarose, collagen, alginates and chitosan) and synthetic polymer materials (polyacrylamide, polyurethane, polyethylene glycol and polyvinyl alcohol) have been applied as immobilization techniques (Wang et al. 1995; Manohar and Karegoudar 1998). Among the various matrices that are available, microbial cellulose (MC) has been chosen for its ease of use, low cost, low toxicity and high operational stability (Son et al. 2003; Bäckdahl et al. 2006; Svensson et al. 2005). The MC synthesized by A. xylinum is identical to that made by plants with respect to molecular structure. Secreted polysaccharide, however, is free of lignin, pectin and hemicellulose as well as other biogenic products, which are associated with plant cellulose. This cellulose has high crystalline, high water absorption capacity, and mechanical strength in the wet state, ultra fine network structure, and availability in an initial wet state (Astley et al. 2001; George et al. 2005). Because of these features, there is an increasing interest in the development of new applications (Klemm et al. 2001; Bäckdahl et al. 2006; Svensson et al. 2005). In the present study, a bacterium isolated from the wastewater to be treated was immobilized onto MC using the adsorption method and the feasibility of using synthetic solution in an anoxic system was investigated.
Materials and methods
Denitrifying bacteria isolation and identification
The samples were obtained from an up-flow anaerobic sludge blanket reactor (UASB Tehran, Iran). The effluent was inoculated into 500 ml basal salt medium (BSM) (per liter of distilled water) containing: K2HPO4, 0.9 g; KH2PO4, 0.45 g; NH4Cl, 0.45 g; MgSO4, 0.2 g; CaCl2 · 2H2O, 0.02 g; FeCl3, 0.005 g; and a trace elements solution of 1 ml that contained: H3BO3, 400 mg/l; ZnSO4 · 7H2O, 400 mg/l; CoCl2, 50 mg/l; NiCl2 · 6H2O, 200 mg/l; Na2MoO4·2H2O, 300 mg/l; CuSO4 · 5H2O, 10 mg/l and MnSO4 · H2O, 500 mg/l. The inoculated media were incubated and constantly shaken (120 rev/min) at room temperature and anaerobic conditions. Serial dilutions were then made and plated onto the BSM agar medium, and the solid media were incubated at 30°C. To obtain pure cultures, single colonies were picked and then streaked on to slant solid media. The cultures were examined microscopically for purity. Initial identification schemes were performed with biochemical tests as suggested by the Bergey’s Manual of Systematic Bacteriology (Krieg and Holt 1984). For the final and specific identification, 16S rRNA sequencing was performed with the following protocols. Bacterial cultures grown in the LB medium at 35°C for 24 h were used for DNA extraction. Following centrifugation (1500g) of 1 ml of the culture media, the bacterial pellet was suspended in 16 μl of cell lysis solution and incubated at 80°C for 5 min. Then, RNase A (3 μl) was added and the solution incubated at 37°C for 20 min. The resulting suspension was subjected to phenol-chloroform extraction and following centrifugation (1500g) the purified DNA sample was collected. Polymerase chain reaction (PCR) with specific primers for the 16S rRNA gene (5′-GCGAGGAAATGAAGCTG-3′, 3′-AAGGTGATCGACGAGGTC-5′) was performed, which was followed by electrophoresis on agarose gel.
Microbial cellulose production
In this study, A. xylinum (ATCC 23768) was used. It was grown in SH medium at 28°C under static culture conditions. The SH medium was composed of 2% (w/v) glucose, 0.5% (w/v) yeast extract, 0.5% peptone, 0.27% (w/v) Na2HPo4 and 0.115% (w/v) citric acid. Preinoculum for all experiments was prepared by transferring a single A. xylinum colony grown on SH agar into a 50-ml Erlenmeyer flask filled with liquid SH medium. After 5 days of cultivation at 28°C, the cellulose pellicle formed on the surface of the culture broth. Ten milliliters of the cell suspension was introduced into a 500-ml Erlenmeyer flask containing 100 ml of fresh SH medium. The culture was carried out statically for 72 h and the cell suspension derived from the synthesized cellulose pellicle was used as the inoculum for further cultures. The stationary cultures were grown in Erlenmeyer flasks filled with different volumes of the medium. After cultivation, the cellulose sheets were removed and rinsed with distilled water and cleaned of bacterial and medium residues using 2% sodium dodecyl sulfate and 4% NaOH solutions in a boiling-water bath. The MC was cut into 5–10 mm pieces and used for cell immobilization and bioreactor media. The surface morphology of A. xylinum cellulose was studied by scanning microscopy (XL30 Philips model). The specific surface area of the MC was determined using the multiple BET method (Micromeritics, Gemini) with nitrogen gas.
Cell immobilization
The cell suspension of Pseudomonas stutzeri (OD650nm: 0.5) and the MC sheets were shaken together for 2 h on a rotary shaker (120 rev/min) and subsequently washed twice with BSM to remove free cells. To determine the effectiveness of the immobilized cell mass, the method of Chen et al. (1998) was modified and a biomass estimation method based on the determination of cell protein content in the MC was established. Briefly, around 1 g of MC was placed on a clear glass plate and was cut into several fine pieces with a sharp surgical knife. The crushed cellulose pieces were collected in a test tube. A solution of SDS was added (10%) and sonication treatment was performed for 20 min to extract cell protein in an ice-water bath. After centrifugation, the cell protein of the MC was measured according to the Bradford method; thereafter, the biomass of the beads sample was estimated from the standard curve of the number of bacteria versus protein concentration.
Denitrification activity
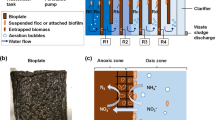
The bioreactor used for testing the reduction of nitrate consisted of a plexiglass cylinder [90 cm × 8 cm (ID)], which was packed with P. stutzeri immobilized on MC (up to 60 cm) and closed at the top with a rubber stopper and at the other end with glass wool. Inlet and outlet points were set at 2 cm from the bottom and top of the column, respectively. A headspace of around 30 cm3 was allowed in the column. The immobilized material was suspended with synthetic wastewater containing (/l): 0.1 g MgSO4 · 7H2O, 1 g KH2PO4, 2.5 g K2HPO4, 0.17 g CaCl2 · 2H2O and 5 g NaCl. The column was initially filled with 10 l of synthetic solution, and after complete removal of nitrate, the continuous process was started by running the effluent through the reactor at 100–200 mg NO3-N concentration and 1.5–3 l/h flow rates. The reactor operated under anoxic conditions with heterotrophic denitrification. The denitrification rate of the continuous bioreactor with immobilized cells was determined using the following equation:
where R is the wastewater flow rate, [NO3-N]input and [NO3-N]output are the influent and effluent NO3 concentrations (g NO3-N/l) respectively, and V is the reactor volume. All experiments were conducted at a temperature of 25°C and a pH of 7.0.
Analytical methods
Samples of influent and effluent were collected and filtered through membranes of 0.45 μm pore size. The filtrates were analysed for NO3-N, NO2-N and COD. The analyses were performed according to standard methods (APHA 2005). All data reported in our study refer to steady state conditions. Scanning electron microscopy (SEM) was used to identify P. stutzeri development on the MC surface. All experiments were conducted in triplicate and average values are presented in subsequent section.
Statistical analysis
Analysis of variance (ANOVA) of the effect of different carbon sources and pH on nitrate reduction was carried out using IRRISTAT version 3/93. If significance was determined (P < 0.05), Fisher’s protected last-significant difference was used to determine differences among treatment means.
Results and discussion
Characterization of denitrifying isolates
Denitrifying enrichment cultures were readily established with UASB plant effluent. Initially, some bacteria grew on the BSM medium after incubation. The colonies were chosen for additional analysis. Because all of the characteristics studied were found to be identical for the isolates, only one representative isolate (P. stutzeri) is discussed below. The bacterium was able to remove nitrate under anoxic conditions. Growth on solid media was fast and resulted in yellow colonies after 18 h. The bacterium showed these characteristics: gram-negative, small cells, motile, catalase- and oxidase-positive and yellow colonies with a regular outline on the agar plate. In 16S rDNA sequence analysis, more than 98% similarity was observed between the sequences of the isolate bacterium and the sequences of P. stutzeri from the database. The results of the biochemical tests along with the phylogenic identification of the bacterium using 16S rDNA sequence analysis showed that the bacterium was P. stutzeri. Kariminiaae-Hamedaani et al. (2004) reported denitrification activity of P. stutzeri isolated from the Ariake Sea that confirmed the feasibility of the application of the isolate strain in a packed bioreactor.
Immobilization of Pseudomonas stutzeri
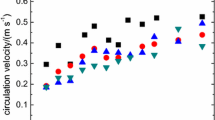
The immobilization of viable cells is a versatile tool that increases the stability of a microbial system, allowing its application under extreme environmental conditions as well as its reuse and the development of continuous bioprocesses. The amount of cells immobilized on MC was measured as a function of total protein of the cell immobilized. Data show that the amount of cells immobilized on the surface depended on the time of immobilization (Fig. 1. The MC provided a high performance support for the immobilization of the bacterium (Fig. 2). It can be used to improve the performance and stability of biological treatment systems designed for bioremediation of wastewater and water contaminated with different biologically degradable compounds while protecting the cells against harsh external conditions, such as pH and organic solvents. The MC immobilized with the denitrifiers (0.3 g/l) dry weight of P. stutzeri and the suspended growth of the bacterium were checked for denitrification. After every 12 h interval, nitrate reduction was evaluated. Comparable denitrification efficiency could be achieved by immobilized (74.9%) and free cells (25.1%).
Denitrification performance
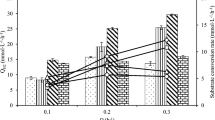
The bioreactor exhibited a high nitrate removal rate with immobilized P. stutzeri cells. The advantage of this system is the use of MC as an advance adsorbent. The carrier material used for immobilization in other systems has not characterization of MC (Klemm et al. 2001). Saliling et al. (2007) reported that a denitrification rate for wood chips and wheat straw as alternative biofilter media at 1.36 kg NO3-N/m3.days was achieved using an anoxic filter. With our bioreactor, the denitrification rate was 1.6 kg NO3-N/m3.days. For some denitrification systems, a source of organic carbon is necessary to provide the energy required for reduction of the nitrate ion (Peng et al. 2007). Nitrate removal of the bacterium was obtained using various carbon sources under stationary culture conditions. No significant denitrification was observed when methanol was used as the sole carbon source (Fig. 3). At this figure, nitrate concentration and carbon sources are dependent and independent variables respectively. Faster nitrate removal rates were observed with succinate in comparison to ethanol (P < 0.05). Among the reactor systems reported in the literature, immobilized Pseudomonas butanovora cells exhibited the highest denitrification rate with ethanol as the organic carbon (Kesseri et al. 2002). In addition, the use of ethanol as the carbon source, which is relatively cheap, makes the process more promising. Nitrate removal efficiency of immobilized P. stutzeri was checked with different COD:NO3-N ratios. The average denitrification activity for ethanol-C:nitrate-N ratios of 3:1 and 1.5:1 was 1.6 ± 0.02 and 1.3 ± 0.08 kg NO3-N/m3.days, respectively. Mohseni-Bandpi et al. (1999[01]) reported that a nitrate removal efficiency of nearly 100% (100 mg/l NO3-N) was achieved with HRTs of 9 and 8.8 h using a bench-scale anoxic filter and the RBC systems. With our bioreactor, the nitrate removal efficiency was 100% (200 mg/l NO3-N). Nitrite accumulated (23 mg/l) during the initial period but was reduced (<1 mg/l) later to N2O or N2 as evidenced by gas production and the treated effluent COD reaching the permissible limit. The optimum pH for most environmental strains of denitrifying bacteria was reported to be between 7 and 8. pH values lower than 6 or higher than 9 may cause significant decay or even stop the denitrification process for many denitrification systems (Oh et al. 2000). Faster nitrate removal rates were observed with pH 7.2 in comparison to 5 and 9 (P < 0.05). At Fig. 4, nitrate concentration and pH are dependent and independent variables respectively. The denitrification process was restricted when the pH was lower than 7.0. (Fig. 4) Obviously, low initial nitrate concentration favored nitrate removal. The nitrate could be completely removed for an initial concentration of 10 mg N/l in 8 h, but for the 50 mg N/l nitrate removal was only 60% at 8 h. Further, inhibition of denitrification occurred at concentrations greater than 300 mg N/l. The higher initial presence of nitrate also led to more severe nitrite accumulation and higher peak concentrations. Although the nitrate removal rate was found to have decreased with initial nitrate loading, the actual amount of nitrate removal increased, which indicates that the denitrification rate increased with initial nitrate loading (Fig. 5). Denitrification rates for the different NO3-N loading values are shown in Fig. 5. The maximum nitrate load for elevated nitrogen elimination was by 1.61 kg NO3-N/(m3 day). This fact might be explained by the increase of electron acceptor when the nitrate concentration increased. Immobilized bacterial bioreactors are ideal for small manufacturers and commercial laboratories that generally have neither the space nor the existing conventional free cell treatment plants (Chibata and Wingard 1983).
Conclusions
Based on the experimental results of this study, it can be concluded that P. stutzeri immobilized on MC can be used effectively for nitrate removal. It was found that the reactor performed better nitrate removal at a neutral pH. Although a low initial nitrate concentration favored nitrate removal, the denitrification rate increased with nitrate loading. Accounting for the high denitrification rate and better performance in a wider range of conditions, this process has the potential to be applied to nitrate removal at contaminated sites.
References
APHA, AWW, WPCF (2005) Standard methods for the examination of water and wastewater, 21th edn. American Public Health Association, Washington
Aslan S, Cakici H (2007) Biological denitrification of drinking water in a slow sand filter. J Hazard Mater 148:253–258. doi:10.1016/j.jhazmat.2007.02.012
Astley OM, Chanliaud E, Donald AM, Gidley MJ (2001) Structure of Acetobacter cellulose composites in the Hydrated State. Int J Biol Macromol 29:193–202. doi:10.1016/S0141-8130(01)00167-2
Bäckdahl H, Helenius G, Bodin A, Nannmark U (2006) Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 27:2141–2149. doi:10.1016/j.biomaterials.2005.10.026
Bernat K, Wojnowska-Baryla I (2007) Carbon source in aerobic denitrification. Biochem Eng J 36:116–122. doi:10.1016/j.bej.2007.02.007
Chen KC, Lee SC, Chin SC, Houng JY (1998) Simultaneous carbon-nitrogen removal in wastewater using phosphorylated PVA-immobilized microorganisms. Enzyme Microb Technol 23:311–320. doi:10.1016/S0141-0229(98)00054-4
Chibata I, Wingard LB (1983) Immobilized microbial cells. In: Wingard LB et al (eds) Applied biochemistry and bioengineering, vol 4. Academic Press, New York
Dawson RN, Murphy KL (1972). The temperature dependency of biological denitrification. Water Res 6:71–83. doi:10.1016/0043-1354(72)90174-1
Fan AM, Steinberg VE (1996) Health implications on nitrate and nitrite in drinking water: an update on methaemoglobinemia occurrence and reproductive and development toxicity. Regul Toxicol Pharmacol 23:35–43. doi:10.1006/rtph.1996.0006
George, j, Ramana KV, Sabapathy SN, Bawa AS (2005) Physico-mechanical properties of chemically treated bacterial (Acetobacter xylinum) cellulose membrane. World J Microb Biot 21:1323–1327. doi:10.1007/s11274-005-3574-0
Kariminiaae-Hamedaani HR, Kanda K, Kato F (2004) Denitrification activity of the bacterium Pseudomonas sp ASM-2-3 isolated from the Ariake Sea tideland. J Biosci Bioeng 97:1–88
Kesseri P, Kiss I, Bihari Z, Polyak B (2002) Investigation of the denitrification activity of immobilized Pseudomonas butanovora cells in the presence of different organic substrates. Water Res 36:1565–1571. doi:10.1016/S0043-1354(01)00364-5
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26:1561–1603. doi:10.1016/S0079-6700(01)00021-1
Krieg NR, Holt JG (1984) Bergey’s manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore
Lazarova V, Manem J (1995). Biofilm characterization and activity analysis in water and wastewater treatment. Water Res 29:2227–2245. doi:10.1016/0043-1354(95)00054-O
Manohar S, Karegoudar TB (1998) Degradation of naphthalene by cells of Pseudomonas sp. strain NGK 1 immobilized in alginate, agar and polyacrylamide. Appl Microbiol Biotechnol 49:785–792
Mohseni-Bandpi A, Elliott DJ, Momeny-Mazdeh A (1999) Denitrification of groundwater using acetic acid as a carbon source. Water Sci Technol 40:53–59. doi:10.1016/S0273-1223(99)00430-8
Oh SE, Kim KS, Choi HC (2000) Kinetics and physiological characteristics of autotrophic denitrification by denitrification sulphur bacteria. Water Sci Technol 42:59–63
Peng YZ, Yong MA, Wang SY (2007) DenitrificationTT potential enhancement by addition of external carbon sources in a pre-TTdenitrificationTT process. J Environ Sci 19:284–289. doi:10.1016/S1001-0742(07)60046-1
Saliling WJB, Westerman PW, Losordo TM (2007) Wood chips and wheat straw as alternative biofilter media for denitrification reactors treating aquaculture and other wastewaters with high nitrate concentrations. Aquac Eng 37:222–233. doi:10.1016/j.aquaeng.2007.06.003
Sawant SS, Prabhudessai L, Venkat K (2007) Eutrophication status of marine environment of Mumbai and Jawaharlal Nehru ports. Environ Monit Assess 127:283–291
Son HJ, Kim HG, Kim KK (2003). Increased production of bacterial cellulose by Acetobacter sp. V6 in synthetic media under shaking culture conditions. Bioresour Technol 86:215–219. doi:10.1016/S0960-8524(02)00176-1
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26:419–431. doi:10.1016/j.biomaterials.2004.02.049
Vrtovsek J, Ros M, Milenko R (2006) Denitrification of ground water in the biofilm reactor with a specific biomass support material. Acta Chim Slov 53:396–400
Wang J, Hou W, Qian Y (1995) Immobilization of microbial cells using polyvinyl alcohol (PVA)—polyacrylamide gels. Biotechnol Tech 9:203–208
Yamagiwa K, Kozawa T, Nakamura A, Ohkawa A (1997) Immobilization of denitrifier within a matrix with low oxygen permeability. Biotechnol Tech 11:95–98. doi:10.1023/A:1018468506352
Yang CY, Wu DC, Chang CC (2007). Nitrate in drinking water and risk of death from colon cancer in Taiwan. Environ Int 33:649–653. doi:10.1016/j.envint.2007.01.009
Acknowledgment
The authors would like to thank Eng. Rezaee for his kind assistance with the SEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaee, A., Godini, H., Dehestani, S. et al. Biological denitrification by Pseudomonas stutzeri immobilized on microbial cellulose. World J Microbiol Biotechnol 24, 2397–2402 (2008). https://doi.org/10.1007/s11274-008-9753-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9753-z