Abstract
A novel N-halamine precursor with tertiary amino group (5,5-dimethylhydantoinyl-3-ylethyl)-dimethylamine (DEADH), was synthesized and then covalently bonded onto cotton fabrics modified by 3-chloropropyltrimethoxysilane to form quaternarized N-halamine precursor grafted cotton fabrics which could be transferred to N-halamine structure upon exposure to dilute sodium hypochlorite solution. The grafted cotton fabrics were characterized by FT-IR, X-ray photoelectron spectroscopy, and field emission scanning electron microscope. The antimicrobial test showed that the cotton fabrics grafted with the quaternarized N-halamine were capable of 7-log inactivation of Staphylococcus aureus and Escherichia coli O157:H7 within 1 min of contact time. Very interestingly, it was found that the grafting process and following chlorination had almost no adverse effect on the tensile strength of cotton fabrics. Furthermore, the antimicrobial cotton fabrics exhibited good washing durability and stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial contamination of polymeric materials plays an important role in the transmission of infectious diseases (Binder et al. 1999; Larson and Olmsted 2001; Neely and Maley 2000). Among these materials, textile materials, particularly natural fibers, with the favorable conditions such as humidity, warmth, and nutrients, are prone to microbial growth (Ren et al. 2009). Use of contaminated medical textiles is an important contributor to cross-infection. However, cotton does possess many functional groups, such as hydroxyl groups, which make it easily modified to render it antimicrobial. In that sense, quaternary ammonium salts (Colak and Tew 2008; Lin et al. 2003; Tiller et al. 2005; Wynne et al. 2011; Fulmer and Wynne 2011; Klibanov 2007; Waschinski et al. 2008; Eren et al. 2008), metal ions (El-Shishtawy et al. 2011; Takano et al. 2011), and N-halamines (Sun and Sun 2001; Liu et al. 2010; Makal et al. 2006; Cao and Sun 2009; Dong et al. 2011; Wang et al. 2011; Luo et al. 2011; Zhao et al. 2011), have been immobilized onto surfaces of cotton materials to reduce the risk of spreading pathogenic microorganisms.
As one of the most effective biocides, N-halamine materials have created a great deal of interest in recent years for several reasons including their durability, recharge ability, and high antimicrobial efficacy against a broad spectrum of microorganisms. Among various N-halamine compounds, heterocyclic structures such as hydantoins, imidazolidinones, triazines, and oxazolidinones have been studied extensively to achieve higher stability in a wide range of temperatures and pH values as compared to noncyclic structures (Worley et al. 1999). Moreover, antimicrobial activity rates increase from amine halamine to amide halamine to imide halamine, whereas their stability decreases in that order (Qian and Sun 2004). 5,5-dimethylhydantoin, a commercially available cyclic N-halamine precursor containing both amide and imide groups, can react with halogenated siloxanes or epoxides through its imide group to form hydantoinyl siloxanes or epoxides which can be grafted onto the cotton fibers. It was reported that a powerful antimicrobial cotton fabric material was successfully prepared by covalently bonding a hydantoinyl siloxane onto cotton fabric and then chlorinating the grafted one (Ren et al. 2008a). However, the hydantoinyl siloxane was water-insoluble and the organic solvent was used to prepare the grafting solution. To avoid the use of organic solvents in grafting process, water-soluble N-halamine precursors are desirable.
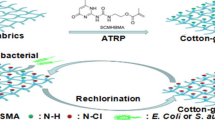
In this study, a novel water-soluble N-halamine precursor with tertiary amino group, (5,5-dimethylhydantoinyl-3-ylethyl)-dimethylamine (DEADH), was synthesized by a dehydrochlorination reaction between 5,5-dimethylhydantoin and (2-chloroethyl)dimethylamine in ethanol. DEADH can not be directly grafted onto untreated cotton fabrics since it does not have coupling groups. To solve this issue, we grafted hydrolyzed 3-chloropropyltrimethoxysilane onto cotton fabric first and then bonded DEADH onto the modified cotton fabric through a quaternarization between DEADH and the grafted hydrolyzed 3-chloropropyltrimethoxysilane. After a convenient chlorination process, DEADH grafted on cotton fabric was converted to amide N-halamine structure. The whole procedure was shown in Fig. 1. Since DEADH was water-soluble and 3-chloropropyltrimethoxysilane could be hydrolyzed in water, no organic solvents were needed in the grafting process. Moreover, the coexistence of quaternary ammonium group and N-halamine group in the surface of the as-prepared antimicrobial cotton fabric might lead to an enhancement of antimicrobial efficacy.
Experimental
Chemicals and reagents
Fabric swatches (Bleached 100 % Cotton Print Cloth) were obtained from Shanghai Luyang Window Decoration Co., Ltd. Gram-positive bacteria Staphylococcus aureus (S. aureus, ATCC 6538P) and gram-negative bacterial Escherichia coli (O157:H7 ATCC 11229) were purchased from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences. The Trypticase soy agar used was from Sinophram Chemical Reagent. Co., Ltd. 5,5-Dimethylhydantoin was obtained from Shanghai Yinghan Chemical Co., Ltd. Other chemicals and solvents were purchased from Aladdin Chemical Company.
Instruments
The NMR spectra were obtained using Bruker Avance 400. The IR spectra were obtained using Mattson PK-60000 infrared spectrometer. Elementary analysis was performed on Elementar Vario El III elementalanalyser. The X-ray photoelectron spectroscopy (XPS) spectra were obtained using Perkin Elmer PHI 5000 ESCT System. Field emission scanning electron microscope (FE-SEM) pictures were obtained using Hitachi S4800 scanning electron microscope.
Synthesis of (5,5-dimethylhydantoinyl-3-ylethyl)-dimethylamine (DEADH)
12.83 g (0.11 mol) of 99 % thionyl chloride was added to a 250 mL flask in the ice bath and then 8.91 g (0.10 mol) of 2-aminoethanol was slowly added under stirring. The mixture was heated to 35–50 °C while being stirred for 1 h, then 150 mL of ethanol was added and heated to its boiling. After cooled to ambient temperature, the intermediate compound (2-chloroethyl)dimethylamine hydrochloride was collected by filtration and purified by recrystallization from ethanol (Burckhalter et al. 1950). The obtained intermediate was mixed with 10.24 g (0.08 mol) of 5,5-dimethylhydantoin and 12.76 g (0.18 mol) of sodium ethoxide in 300 mL of ethanol and stirred at 60 °C for 12 h. Finally, the light yellow DEADH product was obtained after filtration of the produced NaCl and then removal of solvent. Yield was 83.24 %, calculated from the mass percentage of DEADH in the crude product obtained by an ion association titration method (Sakai 2001). For characterization of DEADH, 2.00 g of crude DEADH product was dissolved in dichloromethane and purified by column chromatography, 1.62 g of white solid product was obtained, which exhibited the following properties: elemental analysis: found (calc) C9H17O2N3: N% 21.04 (21.09); C% 54.49 (54.27); H% 8.56 (8.60). IR (KBr): 3,259, 1,779, 1,704, 1,451, 712 cm−1; 1H–NMR(D2O–d2): δ:1.41(6H, –CH3), 2.24(6H, N–CH3),2.56(2H, CH2–N(CH3)2), 3.60(2H, N–CH2–C).
Grafting procedure
10.00 g of cotton fabrics were soaked in 6.00 wt% of hydrolyzed 3-chloropropyltrimethoxysilane aqueous solution at ambient temperature for 15 min, and then the wet cotton fabrics were cured at 95 °C for 1 h. After the first curing process, the fabrics grafted with hydrolyzed 3-chloropropyltrimethoxysilane were immersed in 6.00 wt% of (5,5-dimethylhydantoinyl-3-ylethyl)-dimethylamine (DEADH) aqueous solution without agitation for 1 h. After immersion, the wet cotton fabrics were dried at 95 °C for 1 h, and further cured at 180 °C for 3 min. After the second curing process, the fabrics grafted with DEADH were soaked in a 0.5 % detergent solution for 15 min, washed with water, and dried in an oven at 70 °C for 2 h.
Chlorination procedure and analytical titration
The grafted cotton fabrics were immersed in a 0.1 % NaOCl aqueous solution with pH adjusted to 7 for 45 min. The chlorinated cotton fabrics were then rinsed three times in water to remove unreacted free chlorine and then dried at 45 °C for 2 h. The percentage of oxidative chlorine (Cl+), which is covalently bonded to the amide nitrogen atom of DEADH in the grafted cotton fabrics was determined by a modified iodometric/thiosulfate titration procedure (Liang et al. 2006a). In this procedure, a small chlorinated cotton fabric sample (about 0.40 g) was suspended in a solution of 45.0 mL ethanol and 5.0 mL 0.1 N acetic acid. After addition of 0.30 g of KI, the solution was titrated with 0.10 N of sodium thiosulfate solution until the orange color disappeared at the end point. The weight percentage of oxidative chlorine in the cotton fabrics could then be calculated using the following equation.
Where Cl+% is the weight percent of oxidative chlorine on the sample, N and V are the normality (equiv/L) and volume (L) of the Na2S2O3 consumed in the titration, respectively, and W is the weight of the cotton sample (g).
Evaluation of fabric mechanical properties
The tensile strength of cotton fabric was measured according to GB/T3923-1997 ‘‘Textiles-Tensile properties of fabrics-Part 1: Determination of breaking force and elongation at breaking force-Strip method”. The size of testing samples was 250 × 50 mm2. Each sample was tested at least 3 times and average value was obtained.
Evaluation of antibacterial properties of textiles
2.54 cm2 of square cotton fabrics, some untreated to serve as controls, others treated with DEADH, but unchlorinated, to serve as a second type of controls, and others treated with DEADH, but chlorinated, served as samples for antimicrobial efficacy testing. Dried fabrics were challenged with either S. aureus ATCC 6538P or E. coli O157:H7 ATCC 11229 using a modified AATCC 100-199 Test Method. 25 μL of bacterial suspensions buffered to pH 7 were placed in the center of a pair of cotton fabrics held in a place by a sterile weight to ensure good contact of the fabrics. After contact times of 1 and 5 min, the various fabrics were placed in sterile conical centrifuge tubes containing 9.5 mL of phosphate buffer and 0.5 mL of sterile 0.05 M sodium thiosulfate to quench any oxidative free chlorine which might have been present, and vortexed for 250 s to remove bacteria. Then the swatches were removed, and serial dilutions of the quenched solutions were plated on Trypticase soy agar. The plates were incubated at 37 °C for 24 h and then counted for viable CFU of bacteria.
Evaluation of washing durability and stability
In this study, we adopted a standard washing test according to AATCC Test Method 61-2007 to measure the washing durability of both unchlorinated and chlorinated DEADH treated cotton fabrics (Ren et al. 2008b). Stainless steel canisters containing 150 mL of 0.15 % AATCC detergent water solution and 50 stainless steel balls and fabrics (1 in. × 2 in.) were fixed in a Launder-Ometer (Darong Textile Instrument Co., Ltd., Zhejiang, China) and rotated at 42 rpm and 49 °C for 45 min. After 1, 2, 5, 10 washing cycles, samples were taken out and rinsed three times with distilled water and then dried at ambient temperature. Each cycle of washing in this method is equivalent to five machine washings. For unchlorinated DEADH treated cotton fabrics, one set of samples were chlorinated after 1, 2, 5, and 10 washing cycles, respectively. The percentages of oxidative chlorine (Cl+) were measured by the modified iodometric/thiosulfate titration procedure to assess the durability of DEADH moieties bonded on cotton fabrics. For chlorinated DEADH treated cotton fabrics, the percentages of Cl+ after 0, 1, 2, 5, and 10 washing cycles were measured to assess the stability of amide N–Cl bonds during washing. Also, chlorinated DEADH treated cotton fabrics were rechlorinated after each washing cycle and then the percentages of Cl+ were measured to assess the durability of DEADH moieties on chlorinated cotton fabrics.
For stability test, 10.00 g of chlorinated DEADH treated cotton fabrics was stored at room temperature and then the percentages of Cl+ were measured after a period of time to assess the stability of N-halamine antimicrobial moieties of chlorinated DEADH treated cotton fabrics during storage.
Results and discussions
Synthesis of DEADH
5,5-dimethylhydantoin is an important N-halamine precursor containing both cyclic amide and imide groups. Its active imide group can react with some organic halides to form the derivatives of 5,5-dimethylhydantoin which can be grafted on the surfaces of materials and its amide group can be easily transferred to N-halamine functional group upon exposure to dilute sodium hypochlorite solution (Ren et al. 2008a; Liang et al. 2006b; Liang et al. 2007a). In this study, (2-chloroethyl)dimethylamine was used to react with 5,5-dimethylhydantoin to form (5,5-dimethylhydantoinyl-3-ylethyl)-dimethylamine (DEADH), a water-soluble hydantoinyl tertiary amine. It was found that ethanol is suitable solvent for this reaction. After reaction was over, the solvent could be collected by evaporation for reuse. The structure of DEADH was confirmed by 1H-NMR spectrum, shown in Fig. 2.
Grafting DEADH onto the surface of cotton fabrics
3-Chloropropyltrimethoxysilane is a useful coupling agent which can react with tertiary amines or N-halamine precursors containing imide groups to form siloxane quaternary ammonium salts or siloxane N-halamine precursors. So far, many siloxane quaternary ammonium salts and siloxane N-halamine precursors have been synthesized and grafted onto cotton fabrics (Shao et al. 2004; Liang et al. 2007b). However, the syntheses of such kinds of siloxanes required higher reaction temperature, longer reaction time, and high boiling solvent such as DMF. After reaction, the evaporation of DMF consumed more energy and took longer time. To overcome these disadvantages, we adopted an innovative grafting process to bond DEADH onto the surface of cotton fabrics. 3-Chloropropyltrimethoxysilane was hydrolyzed in water and then grafted onto cotton fabrics through a dehydration reaction. The chloropropyl groups on the surface of grafted cotton fabrics can react with tertiary amino groups of DEADH to form the quaternarized N-halamine precursor grafted cotton fabrics. In this paper, the immersion-dry-cure process was used to covalently bond DEADH onto the surface of hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabrics. It was found that the key parameters for grafting were curing temperature and time. After immersed in 6 % DEADH aqueous solution for 1 h, the wet cotton fabrics were dried at 95 °C for 1 h, and further cured at different temperatures for various times.
After curing, 0.10 % NaOCl aqueous solution was used to chlorinate the treated cotton fabrics to form antimicrobial quaternarized N-halamine on the cellulose. The mass-on of DEADH grafted onto cellulose can be estimated from oxidative chlorine percentage (Cl+%) in the cotton fabrics. Higher Cl+% indicates more DEADH grafted on the cotton fabrics. Figure 3 shows the effect of curing time on Cl+% in the cotton fabrics under 170 and 180 °C. It was found that longer curing time leads to higher Cl+% in the cotton fabrics under the same curing temperature. As shown in Fig. 4, higher curing temperature leads to a higher Cl+% in the cotton fabrics. However, too high curing temperature may lead to deterioration of cotton fabrics. Considering this factor, we chose 180 °C as optimistic curing temperature and 3 min as curing time.
Characterization of treated cotton fabrics
FTIR spectra were recorded and used to conform the effective grafting of DEADH onto cellulose. As shown in Fig. 5, Compared to untreated cotton fabric and hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabric, the DEADH treated one has two strong peaks at 1,712 and 1,769 cm−1 which are ascribed to the C=O stretching vibration on the hydantoinyl ring of DEADH (Wei et al. 2010; Cerkez et al. 2012). It clearly demonstrated that DEADH should have been covalently bonded onto cellulose because non-grafted water-soluble DEADH could be easily washed off from the surface of cotton fabric after curing.
The successful grafting of hydrolyzed 3-chloropropyltrimethoxysilane and then DEADH onto the surface of cotton fabrics can be ascertained by comparing the XPS spectra before and after the treatment of them. Figure 6 shows the XPS wide scans of untreated cotton fabric, hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabric, and DEADH treated one. In the case of untreated cotton fabric (a), the appearances of a C 1 s signal at a binding energy of 284 eV and an O 1 s signal at 532 eV verify the existence of carbon and oxygen in the untreated cotton fabric. In the case of hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabric (b), except C 1 s peak and O 1 s peak, the appearances of a Cl 2p signal at a binding energy of 197 eV, a Si 2 s signal at a binding energy of 154 eV, and a Si 2p signal at a binding energy of 103 eV conform the existence of chlorine and silicon which are characteristic elements of hydrolyzed 3-chloropropyltrimethoxysilane. It means that hydrolyzed 3-chloropropyltrimethoxysilane has been successfully grafted onto the surface of cotton fabric. In the case of the DEADH treated cotton fabric (c), a new peak at about 400 eV corresponds to N 1 s signal. From the inset of Fig. 6, it was found that a weak peak at 402 eV, corresponding to the nitrogen element of quaternary ammonium salt, almost merges with the peak at 400 eV which is ascribed to the nitrogen element of amide and imide groups in the hydantionyl ring of DEADH (Cheng et al. 2005; Yao et al. 2008; Tamura et al. 2012). It demonstrates that DEADH has been covalently bonded onto the cellulose and a quaternization reaction has occurred during curing process.
Surface morphologies of cotton specimens were investigated by the scanning electron microscopy (SEM). Figure 7 reveals the SEM images of untreated cotton fabric, hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabric, and DEADH treated cotton fabric. It seems that the curing process might cause slight damage on the cotton fabrics.
Antimicrobial efficacy evaluation
The antimicrobial efficacies for untreated cotton fabric, hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabric, DEADH treated cotton fabric, and chlorinated DEADH treated cotton fabric against Gram-positive S. aureus and Gram-negative E. coli O157:H7 are presented in Table 1. As can be seen from Table 1, the chlorinated DEADH treated cotton fabric provided more than 7 log reduction of both S. aureus and E. coli O157:H7 within a contact time of 1 min. The super powerful antimicrobial efficacy of the chlorinated DEADH treated cotton fabric is probably due to synergistic enhancement of quaternary ammonium salt and N-halamine functional groups. For untreated cotton fabric, the log reductions of S. aureus and E. coli are 0.22 and 0.58 with a contact time of 1 min, 0.19 and 0.67 with a contact time of 5 min, respectively. For hydrolyzed 3-chloropropyltrimethoxysilane treated cotton fabric, the log reductions of S. aureus and E. coli are 0.64 and 0.61 with a contact time of 1 min, 0.74 and 0.79 with a contact time of 5 min, respectively. These small log reductions are possibly due to the adsorption of bacteria on the surface of cotton fabrics (Kang et al. 2013). For DEADH treated cotton fabric with quaternary ammonium salt groups on the surface, the log reductions of S. aureus and E. coli are 0.61 and 1.45 with a contact time of 1 min, 0.86 and 1.95 with a contact time of 5 min, respectively. It means that quaternary ammonium salt groups have much weaker antimicrobial efficacy than N-halamine groups.
Tensile strength
Figure 8 shows the tensile strength results of weft and warp yarns received from untreated cotton fabric, DEADH treated cotton fabric, and chlorinated DEADH treated cotton fabric. Statistically significant difference was not found on the tensile strength values after curing and chlorination processes. According to these results, it can be concluded that the curing and chlorination processes of the cotton fabrics did not cause any significant damage to the structure of the yarns.
Washing durability and stability
The greatest challenge to antimicrobial textiles is the durability of the antimicrobial moieties. For N-halamine grafted textiles, the washing durability mainly depends on the stabilities of covalent bonds between N-halamine molecules and the textile and N-halamine functional groups (N–Cl) during washing process. In this study, we adopted a standard washing test to measure the washing durability of DEADH treated cotton fabrics. The results were shown in Table 2. For unchlorinated DEADH treated cotton fabrics, they were chlorinated after 1, 2, 5, and 10 washing cycles. The percentages of oxidative chlorine (Cl+) were 0.27, 0.22, 0.17, and 0.11 %, respectively. Based on the original 0.33 % of Cl+, it can be calculated that the Cl+% of DEADH treated cotton fabrics after 1, 2, 5, and 10 washing cycles decreased by 18.19, 33.34, 48.49, and 66.67 % which was caused by the departure of the whole DEADH moieties from cotton fabrics due to the disconnection of covalent bonds between DEADH moieties and cotton fabrics. For chlorinated DEADH treated cotton fabrics, the percentages of Cl+ after 1, 2, 5, and 10 washing cycles were 0.12, 0.05, 0.03, and 0.01 %, respectively. However, the percentages of Cl+ reached 0.27, 0.25, 0.18, and 0.14 % by rechlorination after 1, 2, 5, and 10 washing cycles. It clearly demonstrates that the decrease of Cl+% on chlorinated DEADH treated cotton fabrics during washing is caused by both the conversion of N–Cl to N–H groups and the departure of the whole DEADH moieties from cotton fabrics. Also, after 10 washing cycles, the lost percentages of the whole DEADH moieties for the chlorinated and unchlorinated DEADH treated cotton fabrics are 57.57 and 66.67 % according to the differences of Cl+% between original Cl+% and the Cl+% after 10 cycles. It means that the chlorinated DEADH treated cotton fabrics are more resistant to washing than the unchlorinated DEADH treated cotton fabrics, probably due to the increase of the surface hydrophobicity through the transformation of N–H to N–Cl groups (Cerkez et al. 2011).
N–X(X=Cl, Br, I) bonds in N-halamine compounds may change to N–H bonds during storage due to their interaction with microorganisms, moistures, etc. Figure 9 shows the stability of N–Cl bonds in chlorinated DEADH treated cotton fabric under room temperature. After 5 days storage, the Cl+% of chlorinated DEADH treated cotton fabric decreased by 7.14 % from initial 0.28 to 0.26 %. From 5 to 30 days storage, the Cl+% of chlorinated DEADH treated cotton fabric kept almost constant. It means that the amide N–Cl bonds in chlorinated DEADH treated cotton fabric are very stable under room temperature. Also, as mentioned above, the lost Cl+ during storage can be easily regained by rechlorination. In fact, most textiles need regular washing and the diluted bleach solution (NaOCl solution) can be added to washing machine to run in situ rechlorination for N-halamine textiles after a period of time.
Conclusions
DEADH, a new water-soluble hydantoinyl derivative, was synthesized by a dehydrochlorination reaction between 5,5-dimethylhydantoin and (2-chloroethyl)- dimethylamine. In order to avoid the time-consuming and energy-consuming synthesis of a quaternarized hydantoinyl siloxane between DEADH and 3-chloropropyltrimethoxysilane to form a quaternarized N-halamine precursor containing a coupling group for grafting, we developed a useful method to covalently bond DEADH onto the surface of cotton fabrics by the aid of 3-chloropropyltrimethoxysilane. DEADH is water-soluble and 3-chloropropyltrimethoxysilane can be hydrolyzed in water, so the two-step immersion-dry-cure process does not require any organic solvents. Very interestingly, the as-prepared antimicrobial cotton fabrics exhibited super strong capacity to kill harmful micro-organisms. Washing durability test indicated that 43.43 % of DEADH moieties remained on the surface of chlorinated DEADH treated cotton fabrics after 10 washing cycles (equivalent to 50 machine washings) and 0.14 % of Cl+ could be reached by rechlorination which could still quickly kill bacteria. More importantly, the N-halamine moieties of the as-prepared antimicrobial cotton fabrics are very stable under storage condition due to the amide N-halamine structure and the tensile strength of them are almost the same as the untreated ones. With above-mentioned advantages, the as-prepared antimicrobial cotton fabrics will have potential applications in sports and leisure clothing, home furnishings, hotel industry product, and particularly medical and healthcare textiles.
References
Binder S, Levitt AM, Sacks JJ, Hughes JM (1999) Emerging infectious diseases: public health issues for the 21st century. Science 284:1311–1313
Burckhalter JH, Stephens VC, Hall LA (1950) Antihistaminics: the N-2-dimethylaminoethyl derivatives of carbazole and diphenylamine. J Am Pharm Assoc 39:271–273
Cao Z, Sun Y (2009) Polymeric N-halamine latex emulsions for use in antimicrobial paints. ACS Appl Mater Inter 1:494–504
Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2011) N-halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir 27:4091–4097
Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2012) Epoxide tethering of polymeric N-halamine moieties. Cellulose 19:959–966
Cheng Z, Zhu X, Shi ZL, Neoh KG, Kang ET (2005) Polymer microspheres with permanent antibacterial surface from surface-initiated atom transfer radical polymerization. Ind Eng Chem Res 44:7098–7104
Colak S, Tew GN (2008) Synthesis and solution properties of norbornene based polybetaines. Macromolecules 41:8436–8440
Dong A, Lan S, Huang J, Wang T, Zhao T, Xiao L, Chen Y (2011) Modifying Fe3O4-functionalized nanoparticles with N-halamine and their magnetic/antibacterial properties. ACS Appl Mater Inter 3:4228–4235
El-Shishtawy RM, Asiri AM, Abdelwahed NA, Al-Otaibi MM (2011) In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 18:75–82
Eren T, Som A, Rennie JR, Nelson CF, Urgina Y, Nüsslein K, Tew GN (2008) Antibacterial and hemolytic activities of quaternary pyridinium functionalized polynorbornenes. Macromol Chem Phys 209:516–524
Fulmer PA, Wynne JH (2011) Development of broad-spectrum antimicrobial latex paint surfaces employing active amphiphilic compounds. ACS Appl Mater Interfaces 3:2878–2884
Kang ZZ, Zhang B, Jiao YC, Xu YH, He QZ, Liang J (2013) High-efficacy antimicrobial cellulose grafted by a novel quaternarized N-halamine. Cellulose 20:885–893
Klibanov AM (2007) Permanently microbicidal materials coatings. J Mater Chem 17:2479–2482
Larson E, Olmsted RN (2001) Research priorities project, year 2000: the lone ranger rides again. Am J Infect Control 29:69–72
Liang J, Chen Y, Barnes K, Wu R, Worley SD, Huang TS (2006a) N-halamine/quat siloxane copolymers for use in biocidal coatings. Biomaterials 27:2495–2501
Liang J, Owens JR, Huang TS, Worley SD (2006b) Biocidal hydantoinylsiloxane polymers. IV. N-halamine siloxane-functionalized silica gel. J Appl Polym Sci 101:3448–3454
Liang J, Chen Y, Ren X, Wu R, Barnes K, Worley SD, Huang TS (2007a) Fabric treated with antimicrobial N-halamine epoxides. Ind Eng Chem Res 46:6425–6429
Liang J, Barnes K, Akdag A, Worley SD, Lee J, Broughton RM, Huang TS (2007b) Improved antimicrobial siloxane. Ind Eng Chem Res 46:1861–1866
Lin J, Qiu S, Lewis K, Klibanov AM (2003) Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine. Biotechnol Bioeng 83:168–172
Liu S, Zhao N, Rudenja S (2010) Surface interpenetrating networks of poly (ethylene terephthalate) and polyamides for effective biocidal properties. Macromol Chem Phys 211:286–296
Luo J, Porteous N, Sun Y (2011) Rechargeable biofilm-controlling tubing materials for use in dental unit water lines. ACS Appl Mater Inter 3:2895–2903
Makal U, Wood L, Ohman DE, Wynne KJ (2006) Polyurethane biocidal polymeric surface modifiers. Biomaterials 27:1316–1326
Neely AN, Maley MP (2000) Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol 38:724–726
Qian L, Sun G (2004) Durable and regenerable antimicrobial textiles: improving efficacy and durability of biocidal functions. J Appl Polym Sci 91:2588–2593
Ren X, Kou L, Liang J, Worley SD, Tzou YM, Huang TS (2008a) Antimicrobial efficacy and light stability of N-halamine siloxanes bound to cotton. Cellulose 15:593–598
Ren X, Kou L, Kocer HB, Zhu C, Worley SD, Broughton RM, Huang TS (2008b) Antimicrobial coating of an N-halamine biocidal monomer on cotton fibers via admicellar polymerization. Colloid Surf A 317:711–716
Ren X, Kocer HB, Worley SD, Broughton RM, Huang TS (2009) Rechargeable biocidal cellulose: synthesis and application of 3-(2,3-dihydroxypropyl)-5,5-dimethylimidazolidine-2,4-dione. Carbohydr Polym 75:683–687
Sakai T (2001) Stepwise Determination of Quaternary Ammonium Salts and Aromatic Amines in Pharmaceuticals by Ion Association Titration. Anal Sci 17:1379–1382
Shao H, Meng WD, Qing FL (2004) Synthesis and surface antimicrobial activity of a novel perfluorooctylated quaternary ammonium silane coupling agent. J Fluor Chem 125:721–724
Sun Y, Sun G (2001) Durable and refreshable polymeric N-halamine biocides containing 3-(4′-vinylbenzyl)-5,5-dimethylhydantoin. J Polym Sci Polym Chem 39:3348–3355
Takano S, Tamegai H, Itoh T, Ogata S, Fujimori H, Ogawa S, Wakatsuki Y (2011) ROMP polymer-based antimicrobial films repeatedly chargeable with silver ions. React Funct Polym 71:195–203
Tamura A, Nishi M, Kobayashi J, Nagase K, Yajima H, Yamato M, Okano T (2012) Simultaneous Enhancement of Cell Proliferation and Thermally Induced Harvest Efficiency Based on Temperature-Responsive Cationic Copolymer-Grafted Microcarriers. Biomacromolecules 13:1765–1773
Tiller JC, Sprich C, Hartmann L (2005) Amphiphilic conetworks as regenerative controlled releasing antimicrobial coatings. J Control Release 103:355–367
Wang D, Xu W, Sun G, Chiou BS (2011) Radical graft polymerization of an allyl monomer onto hydrophilic polymers and their antibacterial nanofibrous membranes. ACS Appl Mater Inter 3:2838–2844
Waschinski CJ, Zimmermann J, Salz U, Hutzler R, Sadowski G, Tiller JC (2008) Design of contact-active antimicrobial acrylate-based materials using biocidal macromers. Adv Mater 20:104–108
Wei X, Wang Z, Zhang Z, Wang J, Wang S (2010) Surface modification of commercial aromatic polyamide reverse osmosis membranes by graft polymerization of 3-allyl-5, 5-dimethylhydantoin. J Memb Sci 351:222–233
Worley SD, Eknoian MW, Li Y (1999) Monomeric and polymeric surface active biocidal N-halo-oxazolidinones. Patent WO9929678
Wynne JH, Fulmer PA, McCluskey DM, Mackey NM, Buchanan JP (2011) Synthesis and development of a multifunctional self-decontaminating polyurethane coating. ACS Appl Mater Interfaces 3:2005–2011
Yao C, Li X, Neoh KG, Shi Z, Kang ET (2008) Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J Memb Sci 320:259–267
Zhao N, Zhanel GG, Liu S (2011) Regenerability of antibacterial activity of interpenetrating polymeric N-halamine and poly (ethylene terephthalate). J Appl Polym Sci 120:611–622
Acknowledgments
The authors would like to acknowledge the financial support from Shanghai Pujiang Talent Project (11PJ1407600), the Research and Innovation Project of Municipal Education Commission of Shanghai (12YZ085), the Shanghai Natural Science Foundation (10ZR1407700). This work is supported by PCSIRT (RT1269), the Program of Shanghai Normal University (DZL124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, B., Jiao, Y., Kang, Z. et al. Durable antimicrobial cotton fabrics containing stable quaternarized N-halamine groups. Cellulose 20, 3067–3077 (2013). https://doi.org/10.1007/s10570-013-0031-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0031-3