Abstract
Three heterocyclic N-halamine structures containing amine, amide, or both functional groups were immobilized onto cotton fabric through epoxide tethering. The coatings were rendered biocidal upon exposure to dilute household bleach solution. The coatings exhibited superior biocidal functionality with complete inactivation of about 6 logs of Staphylococcus aureus and Escherichia coli O157:H7 within 2–10 min contact time depending on the structure. Moreover, the coatings were quite stable against repeated laundering so that recharging was not even necessary after 50 washing cycles. Stability of the coatings against ultraviolet light exposure was studied with a comparison of the amide- and amine-containing N-halamines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With a growing concern about multidrug resistance microorganisms such as Methicillin-resistant Staphylococcus aureus and Vancomysin-resistant Enterococcus, antimicrobial treatment of materials has gained great importance. The increasing number of healthcare-associated infections requires the prevention of biofilm formation on surfaces to reduce the risk of spreading pathogenic microorganisms (Gagliotti et al. 2011). Therefore, quaternary ammonium salts (Colak and Tew 2008; Klibanov 2007; Sauvet et al. 2000), metal ions (El-Shishtawy et al. 2011), N-halamines (Kenawy et al. 2007; Sun and Sun 2002; Goddard and Hotchkiss 2008; Worley et al. 2003; Zhao et al. 2011), and a variety of materials have been used as biocidal coatings.
N-halamine compounds are among the most effective biocidal agents used to inactive a broad spectrum of microorganisms including bacteria, viruses, fungi, and yeasts. In general, N-halamines may contain amine, amide, or imide functionality as the active site. The stabilities of these different functionalities to hydrolysis increase from imide to amide to amine due to increased stability of the nitrogen–halogen bond caused by the presence of electron-donating substituents adjacent to the nitrogen atom (Akdag et al. 2006). The mechanism of action for the N-halamines involves a direct contact in which the oxidative halogen is transferred to the biological cell membrane leading to oxidation of thiol groups resulting in the destruction of the cell structure (Denyer and Stewart 1998). The disinfection rate of N-halamines increases from amine to imide as in relation to the nitrogen–halogen dissociation constants (Qian and Sun 2004). N-halamines are unique in that once the oxidative halogen is consumed, it can be simply recharged upon exposure to a halogen source such as household bleach (Fig. 1).
Research and development work in these laboratories has produced various novel N-halamine moieties which have been incorporated onto many surfaces including cellulose (Kocer et al. 2011b), polyester (Ren et al. 2008a), nylon (Lin et al. 2001), polyurethane (Worley et al. 2003), and polyacrylonitrile (Ren et al. 2009). Electrostatic attractions (Cerkez et al. 2011), grafting (Sun and Sun 2003), or coupling agents such as alkoxy silanes (Ren et al. 2008b) and epoxides (Liang et al. 2007) have been employed to functionalize material surfaces. In a recent study (Kocer et al. 2011a), we reported the attachment of a new epoxide-containing copolymer, poly(3-chloro-2-hydroxypropylmethacrylate-co-glycidyl methacrylate) (P), onto cotton fabric which was treated with the potassium salt of 5,5-dimethylhydantoin (DMH-K) to produce an N-halamine precursor on the surface. The coating was rendered biocidal upon exposure to dilute household bleach, and it exhibited remarkable biocidal efficacies and washing stabilities.
Since the previously synthesized P-coated cotton fabric exhibited promising stabilities, the work has been extended to other N-halamine structures having different functional groups. In this study, 7,7,9,9-tetramethyl-1,3,8 triazaspiro[4.5]decane-2,4-dione potassium salt (TTDD-K), 2,2,5,5-tetramethylimidazolidinone sodium salt (TMIO-Na), and DMH-K were attached to the backbone of polymer P on cotton fabric with the purpose of comparing the stabilities and biocidal efficacies of these aforementioned structures (Fig. 2).
Experimental
Materials and instrumentation
All starting chemicals were purchased from Aldrich Chemical Company (Milwaukee, WI) or TCI America (Boston, MA) and used as is unless otherwise noted. Desized, scoured, and bleached (100 %) cotton (Style 400 Cotton Print Cloth) was obtained from Testfabrics. Inc. (West Pittson, PA). Clorox® brand (Clorox, Inc., Oakland, CA) household bleach was used for chlorination. Bacteria cultures of S. aureus ATCC 6538 and Escherichia coli O157:H7 ATCC 43895 were purchased from American Type Culture Collection (Rockville, MD), and Trypticase soy agar was obtained from Difco Laboratories (Detroit, MI).
NMR spectra recorded with 16 (1H) and 1,024 (13C) scans were obtained using a Bruker 400 MHz spectrometer. ATR-IR data recorded with 64 scans at 4 cm−1 resolution were obtained with a Nicolet 6700 FT-IR spectrometer with an ATR (Attenuated Total Reflectance) accessory utilizing a diamond crystal. UV/Vis spectra were collected with a Shimadzu UV/Vis Spectrophotometer (Shimadzu Scientific Instruments Inc., Durham, NC).
Synthesis
The copolymer P was synthesized with a yield of 75 % by following a previous procedure (Kocer et al. 2011a), and it was characterized by NMR spectra as shown in the supplementary material section (Figure SP. 1). The intrinsic viscosity of the copolymer was measured to be 0.81 dl/g (in dimethylsulfoxide, 25 °C)
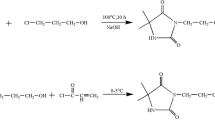
The potassium salt of DMH (Fig. 2) was produced by dissolving an equal molar amount (50 mmol) of DMH and KOH in 200 ml EtOH. After the solution was refluxed for 10 min, EtOH was removed by evaporation, and a white solid was obtained with a yield of 96 %. 1H NMR (DMSO-d6, 400 MHz) 1.07 (s, 6H), 6.41 (s, 1H), 13C NMR (DMSO-d6, 400 MHz) δ 25.55, 59.34, 172.60, 193.20.
The potassium salt of TTDD (Fig. 2) was produced by dissolving an equal molar amount (50 mmol) of TTDD and KOH in 200 ml of EtOH. The solution was refluxed for 10 min, and then the product was recovered with a yield of 95 % as white crystals by evaporation of the solvent. 1H NMR (D2O, 400 MHz) 1.10 (s, 6H), 1.23 (s, 6H), 1.54 (s, 4H); 13C NMR (D2O, 400 MHz) δ 27.23, 33.78, 42.58, 48.65, 64.32, 173.90, 196.49.
In order to produce the sodium salt of TMIO (Fig. 2), 25 mmol TMIO were dissolved in anhydrous dimethylformamide. To this solution, an equal molar amount of NaH was added while the solution was being held in an ice bath. The solution was stirred at room temperature for 5 h. Then the solvent was removed at reduced pressure, and a white solid product was obtained with a yield of 80 %. 1H NMR (D2O, 400 MHz) 1.19 (s, 6H), 1.28 (s, 6H). 13C NMR (D2O, 400 MHz) δ 26.00, 29.56, 60.44, 72.26, 181.37.
Coating and chlorination procedure
The synthesized copolymer P was dissolved in acetone at a specified concentration, and the cotton fabric was soaked in that coating solution for 10 min. The fabrics were then padded onto a laboratory wringer (Birch Brothers Southern, Waxhaw, NC), and cured at 165 °C for 1 h. After the curing process, the swatches were washed with 0.5 % detergent water for 10 min. Finally, the copolymer-coated cotton fabrics were soaked into 0.5 M DMH-K, TTDD-K, or TMIO-Na solutions in EtOH, EtOH, and DMF, respectively, at 80 °C for 15 min. A final washing step with 0.5 % detergent water solution for 10 min was conducted to remove any unattached salts, followed by several water rinses. Overall, this treatment protocol caused reasonable tensile breaking load loss (20–25 %) in the weft (lengthwise) direction (Figure S.P.8). In addition, the procedure can be optimized by reducing the curing temperature and/or time in order to reduce this loss further.
The coated swatches were halogenated with a 10 wt% aqueous solution of household bleach at pH 7 for 1 h, followed by rinsing with distilled water. Then the fabrics were dried at 45 °C for 1 h to remove occluded chlorine from the surfaces. A modified iodometric/thiosulfate titration was used to determine oxidative Cl+% content on the swatches. The weight percentage of chlorine was calculated according to equation 1.
In this equation Cl+% is the weight percent of oxidative chlorine on the samples, N and V are the normality (equiv/L) and volume (L) of the Na2S2O3 (titrant), respectively, and W is the weight of the cotton sample in g.
Stability testing
UVA light (type A, 315–400 nm) stability of the coatings was evaluated using an accelerated weathering tester (The Q-panel Company, Cleveland, OH, USA). Briefly, the chlorinated and unchlorinated swatches were stored in the tester for a certain exposure time, and then chlorine loadings remaining and restored upon rechlorination after the exposure were determined by the analytical titration method mentioned above.
Wash fastness was evaluated using American Association of Textile Chemists and Colorists (AATCC) test method 61-1996 and a laboratory model Lauder-Ometer. In brief, 2.54 cm × 5.08 cm swatches were agitated with stainless steel balls in a rotating canister (42 rpm) filled with 150 mL of 0.15 wt% AATCC detergent water solution at 49 °C. Three different sets of experiments were conducted: the remaining chlorine loadings were measured after the washing test to address the halogen stability (X column, Table 1), the swatches were recharged to evaluate how much of the coating was washed away from the surface (Y column, Table 1), and the unchlorinated swatches were chlorinated after the washing test (Z column, Table 1).
Antimicrobial efficacy testing
The cotton swatches (both chlorinated and unchlorinated) were challenged with S. aureus (ATCC 6538) and E. coli O157:H7 (ATCC 43895) for 2–10 min contact times. A “sandwich test” was conducted for antimicrobial assessment of the coated fabrics. Briefly, a bacterial suspension was prepared in 100 mM phosphate buffer (pH 7), and then 25 μL of the prepared suspension was placed in between two swatches (2.54 cm × 2.54 cm). A sterile weight was placed on top of the swatches to ensure sufficient contact with the bacteria. After specified contact times, oxidative chlorine was neutralized by quenching the swatches with 5.0 mL of 0.02 N sodium thiosulfate solution through vortexing for 2 min. Serial dilutions were prepared using 100 mM phosphate buffer solution (pH 7) and plated on Trypticase soy agar plates which were incubated at 37 °C for 24 h. Finally, the viable bacteria were enumerated for antimicrobial assessment.
Results and discussion
Characterization of the coatings
The coatings and subsequent treatments were characterized by ATR-IR spectroscopy. After the cotton fabric was coated with the copolymer P, an additional band at 1,728 cm−1 formed as an indication of the copolymer immobilization on the surface (Fig. 3). This band corresponds to the ester carbonyl stretching mode present in the copolymer structure, and it is consistent with the observation of a previous study (Kocer et al. 2011a). ATR-IR characterizations of the cotton fabrics after the N-halamine treatments are shown in Fig. 4. When the coated cotton fabric was treated with DMH-K, the amide and imide carbonyl stretching modes of the hydantoin ring appeared at 1,701 and 1,764 cm−1, respectively (Fig. 4a). Similarly, the amide and the imide carbonyl stretching modes of the TTDD-K treated fabric were obtained at 1,712 and 1,774 cm−1 (Fig. 4b). The vibrational band for the amide carbonyl stretching mode of the TMIO ring was observed at 1,735 cm−1 (Fig. 4c).
Upon exposure to bleach, both the imide and amide carbonyl stretching modes of the hydantoin and TTDD rings shifted to higher wavenumber (Figure SP.9). This behavior has also been observed for some of the previous N-halamine coatings and is primarily due to reduced hydrogen bonding after the chlorination process (Kocer et al. 2011b). On the other hand, minimal change was observed for the TMIO treated fabrics upon bleach treatment.
Washing stabilities
The cotton fabrics coated with 3 wt% of the copolymer P were treated with the heterocyclic N-halamines and then subjected to repeated laundering. As can be seen in Table 1, DMH-K and TMIO-Na treated fabrics had the same initial chlorine loading which was almost half of the TTDD-K treated swatches. Because TTDD-K has two nitrogen binding sites for the oxidative halogen, the oxidative halogen loading capacity was twice that of DMH-K and TMIO-Na.
In general, all three treatments provided remarkable washing stabilities as compared to previously studied N-halamine coatings (Kocer et al. 2008). Even after 50 times of laundering, all of the coatings retained sufficient oxidative halogen for effective biocidal activities. Therefore, rebleaching after the washings may not be necessary. Even though they have different functional groups as the active sites, there were minor differences observed among the stabilities of the three treatments.
As expected, due to the presence of the amine functionality, the TTDD-K and TMIO-Na treated fabrics exhibited superior halogen stabilities as compared to the DMH-K treatment. On the other hand, the TMIO-Na treated fabrics provided the least durable coatings (Y and Z columns). A possible reason for this unexpected durability result might be dissolution or degradation of the copolymer P from the fabric surface during the treatment procedure.
UVA light stabilities
The chlorinated and unchlorinated cotton swatches (3 % copolymer coated and 0.5 M N-halamine treated) were exposed to UVA light for times up to 96 h, and the results are summarized in Table 2. The oxidative halogen was lost gradually upon exposure to UVA light in all of the treatments. However, this loss was lower for the DMH treatment which involves an amide functionality. Rechlorinations performed subsequent to 24 h exposures revealed that the halogen loss was not only because of N–Cl bond dissociation, but also because of a slight photodecomposition taking place in the coatings. The magnitude of this decomposition at the end of 96 h exposure was in increasing order of DMH-treated < TTDD-treated < TMIO-treated fabrics. On the other hand, no decomposition was observed for the unchlorinated samples in any of the treatments.
Figure 5 shows UV/Vis spectra of the chlorinated and unchlorinated DMH and TMIO compounds (0.02 mol/L in methanol). TMIO exhibited a broader absorption band with maximal absorption at 255 nm as compared to DMH. Upon chlorination, the absorption band and molar absorptivity of both compounds increased significantly resulting in dissociation of the N–Cl bonds (Chen and Sun 2006). In the UVA region, the unchlorinated DMH and TMIO showed almost no absorption which explains the stability of the unchlorinated swatches against UVA light as shown in Table 2. On the other hand, the chlorinated TMIO exhibited a more intense and broader absorption band in the range of 315–400 nm (UVA region) as opposed to the chlorinated DMH compound. This is the reason for faster oxidative halogen loss induced by UVA light exposure in the TMIO treated swatches as compared to the DMH treated fabrics. Moreover, this broader absorption might also have an effect on increased magnitude of the photolytic decomposition shown in Table 2. The exact mechanism of this decomposition is under study.
Bicodial efficacies
The cotton swatches were challenged with about 6 log of S. aureus and E. coli O157:H7 for antimicrobial assessment. The cotton fabric coated with 3 wt% copolymer P was treated with 0.5 M DMH-K, TMIO-Na and TTDD-K salt solution according to the procedure explained in the experimental section. Antimicrobial efficacy is quite dependent on oxidative halogen loading on surfaces (Kocer et al. 2011a). Therefore, a fourth set of experiments was performed by treating 1.5 % copolymer P coated cotton fabric with 0.5 M TTDD-K solution so as to have similar chlorine loading as for the other two N-halamine treatments. As can be seen in Table 3, the control samples which were not chlorinated did not provide significant bacteria reduction even for the longest contact time. The limited reductions that they exhibited are due to adhesion of bacteria rather than to inactivation. On the other hand, the chlorinated swatches effectively inactivated all of the Gram-negative and Gram-positive bacteria within brief contact times. The rates of bacteria inactivation depended on the chemical composition of the N-halamine structures. N-halamines having amide functionality are known to show a superior biocidal activity as compared to N-halamines containing amine groups because of their weaker N–Cl bond strength (Qian and Sun 2004). Therefore, the TMIO and TTDD treated fabrics exhibited a lower inactivation as compared to the DMH treatment due to the presence of a hindered amine group in their chemical structures. Similarly, in spite of having about the same chlorine loadings, the TTDD treatment provided a more rapid complete inactivation of E. coli O157:H7 as compared to the TMIO treatment, since TTDD has dual (amide and hindered amine) functional groups. As expected, increasing chlorine loading of the TTDD treated fabrics resulted in a more rapid complete inactivation of the Gram-negative bacteria. In general, all three treatments exhibited remarkable biocidal efficacies as compared to other types of biocides such as quaternary ammonium salts and silver.
Conclusions
Heterocyclic N-halamine structures containing oxidatable amide or amine functional groups were anchored to cotton fabric through epoxide bonding to address the stabilities and durabilities of the structures. The coatings exhibited potent antimicrobial activities against S. aureus and E. coli O157:H7 with around 6 log inactivation within 2–10 min contact time. It was observed that the amide-containing N-halamine treatment provided more rapid inactivation of Gram-negative and Gram-positive bacteria as compared to the amine N-halamine treatment. Even though the oxidative halogen was more stable toward hydrolysis in the amine-treated swatches, the amide-treated swatches provided a better wash fastness. In general, all of the treatments exhibited superior durability against repeated laundering such that bleaching should not be necessary after each washing cycle. On the other hand, a photolytic decomposition induced by UVA light exposure was observed for all of the chlorinated fabrics. The amide-containing N-halamine was least prone to this decomposition among the treatments.
References
Akdag A, Okur S, McKee ML, Worley SD (2006) The stabilities of N–Cl bonds in biocidal materials. J Chem Theory Comput 2(3):879–884
Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2011) N-halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir 27(7):4091–4097
Chen Z, Sun Y (2006) N-halamine-based antimicrobial additives for polymers: preparation, characterization, and antimicrobial activity. Ind Eng Chem Res 45(8):2634–2640
Colak S, Tew GN (2008) Synthesis and solution properties of norbornene based polybetaines. Macromolecules 41(22):8436–8440
Denyer SP, Stewart G (1998) Mechanisms of action of disinfectants. Int Biodeterior Biodegradation 41(3–4):261–268
El-Shishtawy RM, Asiri AM, Abdelwahed NAM, Al-Otaibi MM (2011) In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 18(1):1–8
Gagliotti C, Balode A, Baquero F, Degener J, Grundmann H, Gür D, Jarlier V, Kahlmeter G, Monen J, Monnet DL (2011) Escherichia coli and Staphylococcus aureus: bad news and good news from the European Antimicrobial Resistance Surveillance Network (EARS-Net, formely EARSS), 2002 to 2009. Euro Surveill 16:1–5
Goddard JM, Hotchkiss JH (2008) Rechargeable antimicrobial surface modification of polyethylene. J Food Protection&# 174; 71(10):2042–2047
Kenawy ER, Worley SD, Broughton R (2007) The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules 8(5):1359–1384
Klibanov AM (2007) Permanently microbicidal materials coatings. J Mater Chem 17(24):2479–2482
Kocer HB, Akdag A, Ren X, Broughton RM, Worley SD, Huang TS (2008) Effect of alkyl derivatization on several properties of N-halamine antimicrobial siloxane coatings. Ind Eng Chem Res 47(20):7558–7563
Kocer HB, Cerkez I, Worley SD, Broughton RM, Huang TS (2011a) Polymeric antimicrobial N-halamine epoxides. ACS Appl Mater Interfaces 3(8):3189–3194
Kocer HB, Worley SD, Broughton RM, Huang TS (2011b) A novel N-halamine acrylamide monomer and its copolymers for antimicrobial coatings. React Funct Polym 71(5):561–568
Liang J, Chen Y, Ren X, Barnes K, Worley SD, Broughton RM, Cho U, Kocer H, Huang TS (2007) Fabric treated with antimicrobial N-halamine epoxides. Ind Eng Chem Res 46(20):6425–6429
Lin J, Winkelmann C, Worley SD, Broughton RM, Williams JF (2001) Antimicrobial treatment of nylon. J Appl Polym Sci 81(4):943–947
Qian L, Sun G (2004) Durable and regenerable antimicrobial textiles: improving efficacy and durability of biocidal functions. J Appl Polym Sci 91(4):2588–2593
Ren X, Kocer HB, Kou L, Worley SD, Broughton RM, Tzou YM, Huang TS (2008a) Antimicrobial polyester. J Appl Polym Sci 109(5):2756–2761
Ren X, Kou L, Liang J, Worley SD, Tzou YM, Huang TS (2008b) Antimicrobial efficacy and light stability of N-halamine siloxanes bound to cotton. Cellulose 15(4):593–598
Ren X, Akdag A, Zhu C, Kou L, Worley SD, Huang TS (2009) Electrospun polyacrylonitrile nanofibrous biomaterials. J Biomed Mater Res Part A 91(2):385–390
Sauvet G, Dupond S, Kazmierski K, Chojnowski J (2000) Biocidal polymers active by contact. V. Synthesis of polysiloxanes with biocidal activity. J Appl Polym Sci 75(8):1005–1012
Sun Y, Sun G (2002) Synthesis, characterization, and antibacterial activities of novel N-halamine polymer beads prepared by suspension copolymerization. Macromolecules 35(23):8909–8912
Sun Y, Sun G (2003) Novel refreshable N halamine polymeric biocides: grafting hydantoin containing monomers onto high performance fibers by a continuous process. J Appl Polym Sci 88(4):1032–1039
Worley SD, Li F, Wu R, Kim J, Wei CI, Williams JF, Owens JR, Wander JD, Bargmeyer AM, Shirtliff ME (2003) A novel N-halamine monomer for preparing biocidal polyurethane coatings. Surf Coat Int Part B Coat Trans 86(4):273–277
Zhao N, Zhanel GG, Liu S (2011) Regenerability of antibacterial activity of interpenetrating polymeric N-halamine and poly(ethylene terephthalate). J Appl Polym Sci 120(1):611–622
Acknowledgments
This work was supported by US Air Force through Grant FA8650-07-1-5908.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cerkez, I., Kocer, H.B., Worley, S.D. et al. Epoxide tethering of polymeric N-halamine moieties. Cellulose 19, 959–966 (2012). https://doi.org/10.1007/s10570-012-9699-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9699-z