Abstract
Oxidative stress caused by reactive oxygen species (ROS) induces DNA base modifications and DNA strand breaks. In this study, the protective effect of baicalein against H2O2-induced DNA damage was investigated in V79-4 Chinese hamster fibroblast cells. H2O2 treatment increased the levels of intracellular ROS and DNA double-strand breaks (DSBs) and decreased the level of Ku70 protein and the phosphorylation (activation) of DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which are involved in the repair of DSBs by nonhomologous end joining. Baicalein effectively scavenged intracellular ROS induced by H2O2, reduced DSBs, and rescued Ku70 protein level and phosphorylation of DNA-PKcs. In cellular response to DNA base damage, 8-oxoguanine DNA glycosylase 1 (OGG1) plays a vital role in the removal of 8-oxoguanine (8-OxoG), which is formed mainly by oxidative stress. Baicalein significantly decreased the levels of 8-OxoG induced by H2O2, and this correlated with increases in OGG1 promoter activity and OGG1 mRNA and protein expression. The phosphorylated form of Akt kinase, which is a regulator of OGG1, was sharply decreased by H2O2, but was prevented by baicalein. A specific Akt inhibitor abolished the cytoprotective effects of baicalein, suggesting that OGG1 induction by baicalein involves the Akt pathway. In conclusion, baicalein exerted protective effects against DNA damage induced by oxidative stress by activating DNA repair systems and scavenging ROS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DNA is sensitive to many endogenous and exogenous factors, which may damage it at rates of up to 1 × 106 lesions per cell per day. Reactive oxygen species (ROS) can lead to single-stranded and double-stranded breaks in DNA, as well as modification of purine and pyrimidine bases and oxidation of 2′-deoxyribose (Powell et al. 2005). Oxidative DNA modifications and mutagenic lesions contribute to the pathogenesis of many diseases, including cancer (Ribeiro et al. 2008).
H2O2 originates during partial reduction of O2 by light, radiation, ferrous iron, or enzymes. This nonradical ROS under physiological conditions is more stable than its radical relatives with a half-life of 1 ms, which is 1,000 times longer than that of O ·−2 (Reth 2002). This extended stability allows H2O2 to engage in the Fenton reaction yielding OH·. Thus, H2O2 damages molecules via the more potent OH·. OH· interacts with all biological molecules and causes subsequent cellular damages such as DNA damage, lipid peroxidation, protein damage, and membrane destruction (Foyer et al. 1997).
Deficiency in the repair of DNA double-strand breaks (DSBs) may cause the accumulation of genomic rearrangements or mutations that ultimately lead to tumorigenesis (van Gent et al. 2001). Proteins of the nonhomologous end joining (NHEJ) repair pathway assemble at the ends of broken DNA to form a nuclear serine/threonine kinase complex (Smith and Jackson 1999). This complex consists of a heterodimer of Ku70 and Ku80 and a DNA-dependent protein kinase catalytic subunit (DNA-PKcs). After the initial loading of the Ku70/Ku80 heterodimer onto the DNA ends, DNA-PKcs is recruited, forming a synapse which protects the DNA from exonuclease activity and ensures the juxtaposition of the DNA ends (van Gent and van der Burg 2007). The presence of Ku70/Ku80 and DNA-PKcs at the DNA ends is not fixed, but rather constitutes a dynamic equilibrium of DNA-bound and DNA-free proteins (Mari et al. 2006). The Ku70/Ku80 heterodimer binds to DSBs without DNA sequence specificity (Jin et al. 1997). The association of the DNA end-bound Ku70/Ku80 heterodimer with DNA-PKcs activates its kinase activity, which suggests that one major role for Ku is to function as the DNA-binding subunit of this enzyme (Gottlieb and Jackson 1993). Further, DNA-PKcs can phosphorylate various substrates in vitro and may serve as a sensor of DSBs in the context of cellular DSB repair (Jin et al. 1997).
8-Oxoguanine (8-OxoG) is a major base lesion formed through oxidative damage to DNA (Fortini et al. 2003). Large amounts of 8-OxoG are produced in mammalian cells, either as a by-product of normal oxidative metabolism or as a result of exogenous ROS (Zhang et al. 2009). During replication, 8-OxoG can mispair with adenine, giving rise to G:C to T:A transversion mutations, which may compromise essential genes and result in cell death (Hyun et al. 2000, 2003). Oxidative base damage in DNA is corrected mainly by base excision repair (BER), although certain types of lesions can also be repaired by the nucleotide excision repair and mismatch repair pathways (Coppedè et al. 2007). The BER process involves the recognition of damaged bases by specific DNA glycosylases, the hydrolysis of the glycosidic bonds between bases and deoxyribose, and the excision of the affected DNA strand by an apurine/apyrimidine endonuclease at the resulting abasic site, thereby creating a DNA single-strand break (Boiteux et al. 1990). In mammals, 8-oxoguanine DNA glycosylase 1 (OGG1) initiates the removal of 8-OxoG via the BER pathway, thus preserving genomic integrity and genetic function. Recently, antioxidant compounds such as luteolin, quercetin, rosmarinic acid, butin, and 7,8-dihydroxyflavone have been shown to protect DNA against oxidative damage and to increase the repair of damaged DNA, suggesting the possibility that these compounds could be used therapeutically to protect against oxidative stress-induced DNA damage, which is associated with many pathological conditions (Zhang et al. 2009; Silva et al. 2008; Kang et al. 2009).
Baicalein (5,6,7-trihydroxyflavone) is a flavonoid compound derived from the roots of Scutellaria baicalensis. Our recent work showed that baicalein ameliorated mitochondrial oxidative stress by induction of manganese superoxide dismutase (Lee et al. 2011). Baicalein also protected cellular components against oxidative damage by scavenging ROS and inhibiting apoptosis (Kang et al. 2011) and attenuated oxidative stress-induced expression of matrix metalloproteinase-1 by regulating the mitogen-activated protein kinase pathway in human keratinocytes (Kim et al. 2012). In the present study, we investigated whether baicalein could prevent oxidative DNA damage by activating NHEJ and BER repair and examined its possible protective mechanisms.
Materials and methods
Reagents
Baicalein, 2,7-dichlorodihydrofluorescein diacetate (DCF-DA), and [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The OGG1 promoter–luciferase construct was a generous gift from Dr. Ho Jin You (Chosun University, Gwangju, Republic of Korea). The OGG1 antibody was purchased from A.G. Scientific Incorporation (San Diego, CA, USA), and the Ku70, phospho-DNA-PKcs, phospho-Akt, total Akt, and β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The phospho-histone H2A.X antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA), and the Akt inhibitor IV was purchased from Calbiochem (San Diego, CA, USA).
Cell culture
Murray et al. (2004) has reported that many genes were induced by oxidative stress in lung fibroblast cells, which are sensitive to oxidative stress. Therefore, we used the Chinese hamster lung fibroblast (V79-4) in this study. V79-4 cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured in Dulbecco’s modified Eagle’s medium containing 10 % heat-inactivated fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml). Cells were maintained at 37 °C in an incubator with a humidified atmosphere of 5 % CO2. Cell doubling time of V79-4 is approximately 12 h and 60–70 % confluent cells after seeding were used in this study.
Measurement of intracellular ROS
The DCF-DA method was used to measure the levels of intracellular ROS (Rosenkranz et al. 1992). Previously, we reported that baicalein induced manganese superoxide dismutase activity in a time-dependent manner, showing maximum induction at 24 h of baicalein treatment (Lee et al. 2011). So, we detected intracellular ROS at 24 h after H2O2 treatment. Cells were seeded in a 96-well plate at 1.0 × 104 cells per well and, 16 h after plating, were treated with baicalein at the concentration of 1, 5, and 10 μg/ml for 1 h. H2O2 was then added to the medium to a final concentration of 1 mM, and cells were incubated for an additional 24 h at 37 °C. After the addition of 25 μM DCF-DA for 10 min, the fluorescence of 2,7-dichlorofluorescein was detected using a Perkin-Elmer LS-5B spectrofluorometer. The scavenging effect of ROS generation (in percent) was calculated as [(fluorescence value of H2O2-treated cells alone) − (fluorescence value of H2O2-treated cells with baicalein treatment))/(fluorescence value of H2O2-treated cells alone)] × 100. For detection of intracellular ROS using a flow cytometer, cells were treated with DCF-DA for 10 min and trypsinized. The fluorescent cells were measured using flow cytometry (BD Biosciences, San Jose, CA, USA).
Comet assay
A neutral comet assay was performed to assess DNA double-strand breakage (Olive et al. 1991). Cells were seeded in a six-well plate at 1.0 × 105 cells per well and treated with baicalein and H2O2, as described above. The cell pellet (1 × 104 cells) was mixed with 100 μl of 1 % low-melting agarose at 39 °C and the mixture was pipetted onto a slide precoated with 1 % normal-melting agarose, covered with a cover glass, and allowed to gel for 20 min. After solidification of the agarose, the slide was immersed in lysis solution (30 mM ethylenediaminetetraacetic acid [EDTA], 0.5 % sodium dodecyl sulfate [SDS], pH 8.3) for 4 h at 43 °C. After removing the cover glass, the slides were rinsed three times in TBE buffer (90 mM Tris, 90 mM boric acid, 2 mM EDTA, pH 8.5) and stored in TBE buffer overnight at 4 °C. The slides were then placed in a gel electrophoresis apparatus containing TBE buffer. An electrical field was applied (250 mA, 20 V) for 25 min at room temperature to draw negatively charged DNA toward the anode. After electrophoresis, the slides were washed three times for 5 min at 4 °C in distilled and deionized water and then stained with 75 μl of ethidium bromide (20 μg/ml). The slides were then observed under a fluorescence microscope and analyzed using an image analysis software (Kinetic Imaging, Komet 5.5, UK). The percentage of total fluorescence in the tail and the tail length of 50 cells per slide were recorded.
Western blot analysis
Cells were seeded in 1 × 106 cells per dish and treated with baicalein and H2O2, as described above. Cells were harvested and then lysed on ice for 30 min in 100 μl of lysis buffer [120 mM NaCl, 40 mM Tris (pH 8), 0.1 % NP-40] and centrifuged at 13,000×g for 15 min. Lysate supernatants were collected and the protein concentrations were determined. Aliquots of the lysates (40 μg of protein) were boiled for 5 min and electrophoresed on 10 % SDS polyacrylamide gels. Gels were transferred onto nitrocellulose membranes for blotting (Bio-Rad, Hercules, CA, USA), and the membranes were incubated with the appropriate primary antibodies. The membranes were further incubated with secondary immunoglobulin G–horseradish peroxidase conjugates and then exposed to X-ray film. Protein bands were detected using an enhanced chemiluminescence Western blotting detection kit (Amersham, Buckinghamshire, UK).
Detection of 8-OxoG
Cellular DNA was isolated using DNAzol reagent (Life Technologies, Grand Island, NY, USA) and quantified using a spectrophotometer. The amount of 8-hydroxy-2-deoxyguanosine (8-OhdG; a nucleoside of 8-OxoG) in the DNA was determined using the Bioxytech 8-OHdG enzyme-linked immunosorbent assay (ELISA) kit from OXIS Health Products (Portland, OR, USA) according to the manufacturer’s instructions. The amount of 8-OhdG was also estimated in a fluorescent binding assay (Struthers et al. 1998). Avidin binds with high specificity to 8-OhdG. Cells were fixed and permeabilized with ice-cold methanol for 15 min and avidin-conjugated tetramethylrhodamine isothiocyanate (TRITC, fluorescent dye) were incubated for 1 h at room temperature. 8-OhdG was visualized under a fluorescence microscope. The detected 8-OhdG level was considered as the 8-OxoG level.
Transient transfection and OGG1 promoter luciferase assay
Cells were transiently transfected with a reporter plasmid harboring the OGG1 promoter using the transfection reagent DOTAP according to the manufacturer’s instructions (Roche, Mannheim, Germany). After overnight transfection, cells were treated with baicalein for 1 h, 1 mM H2O2 was then added to the medium for 24 h, and then lysed with reporter lysis buffer (Promega, Madison, WI, USA). The lysate supernatant was then mixed with the luciferase assay reagent and the mixture was placed in a luminometer to measure the light produced.
Reverse transcriptase polymerase chain reaction
Total RNA was isolated from cells using TRIzol (Invitrogen, Grand Island, NY, USA). Complementary DNA was synthesized by the reverse transcription process. One microliter of oligo-dT primer (5′-TTTTTTTTTTTTTTTTTT-3′) was added to 5 μl of total RNA, which was the fixed quantity. These products were incubated at 72 °C for 5 min, and then cooled down in ice. Reverse transcription was performed using the following protocol: 42 °C for 60 min and 72 °C for 15 min, after adding the reverse transcriptase, dNTP, RNase inhibitor, and reaction buffer. Polymerase chain reaction of OGG1 and β-actin was conducted using the following protocol: 94 °C for 2 min; followed by 35 cycles of 94 °C for 20 s, 58 °C for 30 s, and 72 °C for 1 min; followed by a final extension at 72 °C for 5 min. The primer pairs (Bionics, Seoul, Republic of Korea) were as follows: mouse OGG1 sense (5′-GCAGAGCCCTGCTCACTGGA-3′) and mouse OGG1 antisense (5′-CGAGGATGGCTTTGGCACTG-3′); mouse β-actin sense (5′-GTGGGCCGCCCTAGGCACCAGG-3′) and mouse β-actin antisense (5′-GGAGGAAGAGGA TGCGGCAGTG-3′). Amplified products were resolved by 1 % agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light.
Cell viability
Cells were seeded in a 96-well plate at a concentration of 1.0 × 104 cells per well and were pretreated with 1 μM of Akt inhibitor IV (a specific inhibitor of Akt phosphorylation) for 1 h, followed by treatment with baicalein (10 μg/ml) for 1 h, and then 1 mM H2O2 for 24 h. MTT (50 μl of a 2-mg/ml stock solution) was then added to each well to yield a total reaction volume of 200 μl. After incubating for 4 h, the plate was centrifuged at 800×g for 5 min and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 μl dimethyl sulfoxide and the A 540 was read on a scanning multiwell spectrophotometer (Carmichael et al. 1987).
TUNEL assay
The terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP nick end labeling (TUNEL) assay was performed using an APO-BrdU™ TUNEL Assay Kit (Invitrogen) according to the manufacturer’s instructions (Li and Darzynkiewicz 1995). Cells were seeded on a 24-well culture plate at 5 × 104 cells per well and treated with baicalein and H2O2 as described above. After 24 h of treatment, the plated cells were fixed with 1 % paraformaldehyde for 15 min at 4 °C and then with 70 % ethanol for 18 h at −20 °C. After fixation, cells were washed twice with wash buffer and incubated with the 5-bromo-2′-deoxyuridine-5-triphosphate DNA-labeling reagent mixture for 1 h at 37 °C. Cells were then washed twice with rinse buffer and incubated with an Alexa Fluor 488-labeled anti-5-bromo-2′-deoxyuridine (BrdU) antibody for 30 min at room temperature in the dark. After fluorescent BrdU labeling, cells were incubated with propidium iodide for 30 min at room temperature in the dark. The stained cells were then observed under a fluorescence microscope (Olympus IX 70, Olympus Optical Co., Japan). The percentage of apoptotic cells was assessed by counting three random fields in three wells per condition.
Statistical analysis
All measurements were made in triplicate (n = 3), and all values are represented as the mean ± standard error. The results were subjected to an analysis of the variance using Tukey’s test for analysis of the differences. Statistical significance was set at p < 0.05.
Results
Baicalein shows intracellular ROS scavenging activity
Previously, our data showed that 1 mM H2O2 was determined as the concentration of 50 % growth inhibition, which was assessed at 24 h after H2O2 treatment using the MTT test (Lee et al. 2011), and 1 mM H2O2 was determined as the optimal dose in this study. The intracellular ROS scavenging activity of baicalein after H2O2 treatment was determined using a DCF-DA assay. Fluorescence spectrometry data revealed that the intracellular ROS scavenging activity of baicalein in H2O2-treated cells was 24 % at 1 μg/ml, 45 % at 5 μg/ml, and 67 % at 10 μg/ml compared with 0 % of scavenging activity in only H2O2-treated cells (Fig. 1a). In our cell culture system, concentrations of baicalein higher than 10 μg/ml were toxic to the cells (data not shown). The intracellular ROS scavenging activity of baicalein determined by this assay was consistent with our previous results (Kang et al. 2011). Therefore, 10 μg/ml was chosen as the optimal concentration of baicalein for further cell culture studies. The fluorescence intensity of DCF was also measured using a flow cytometer. The H2O2-treated cells showed two cell populations in Fig. 1b. These two populations indicate a high level of ROS and a low level of ROS populations, suggesting that the cells are differently affected by H2O2 treatment. Two densities of ROS cell populations are not a specific finding in V79-4 cells only. Several papers reported that two densities of ROS cell populations were also found in various cells such as cancer stem cells, neuronal cells, immune cells, or fibroblast cells (Diehn et al. 2009; Li et al. 2010a; Salmon et al., 2004; Lam et al. 2011; Tsatmali et al. 2006). The DCF fluorescence intensity in cells treated with H2O2 and 10 μg/ml baicalein was decreased more than three fold compared with that in cells treated with H2O2 alone (Fig. 1b).
Baicalein is an effective scavenger of intracellular ROS. a Cells were treated with baicalein at the concentration of 1, 5, and 10 μg/ml for 1 h and then incubated with 1 mM H2O2 for an additional 24 h. After the addition of 25 μM DCF-DA for 10 min, the intracellular ROS generated were detected by spectrofluorometry. *p < 0.05, significantly different from H2O2-treated cells with no baicalein treatment. b The intracellular ROS were detected by flow cytometry and quantified. FI indicates the fluorescence intensity of DCF. *p < 0.05, significantly different from control cells; **p < 0.05, significantly different from H2O2-treated cells
Baicalein reduces the induction of DNA strand breaks by oxidative stress
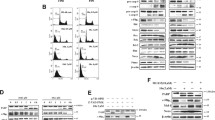
The interaction between ROS and DNA can lead to several types of oxidative DNA damage, including strand breaks and base modifications (Inoue and Kawanishi 1995). The protective effect of baicalein against the formation of DNA strand breaks induced by H2O2 treatment was determined using the neutral comet assay (Olive et al. 1991). H2O2 treatment induced greater DNA tail lengths and increased the mean percentage of DNA in the tails to 47 %. Baicalein treatment decreased the percentage of DNA in the tails to 32 % (Fig. 2a). H2O2 strongly induced the phosphorylation of the histone variant H2A.X (a marker of DSBs), whereas baicalein significantly decreased H2O2-induced phospho-histone H2A.X, as shown in Fig. 2b. The results in Fig. 2a, b are consistent with our previous results (Kang et al. 2011). H2O2 treatment also sharply decreased intracellular levels Ku70 protein and phosphorylated DNA-PKcs (components of the NHEJ repair complex); however, baicalein significantly restored their levels, as shown in Fig. 2c.
Baicalein suppresses the induction of DNA strand breaks by oxidative stress. a The percentage of cellular DNA damage was detected by comet assay. Representative images are shown (magnification, ×400). *p < 0.05, significantly different from control cells; **p < 0.05, significantly different from H2O2-treated cells. Cell lysates were electrophoresed, and phospho-histone H2A.X (b), as well as Ku70 and phospho-DNA-PKcs (c), was detected using specific antibodies
Baicalein inhibits oxidative stress-induced DNA base modification
The levels of 8-OxoG incorporated into DNA, a hallmark of oxidative stress and DNA base damage, were assessed by an ELISA assay using specific antibodies against 8-OhdG (a nucleoside of 8-OxoG). Levels of 8-OhdG were significantly higher in H2O2-treated cells than in control cells. Baicalein decreased the levels of 8-OhdG detected in H2O2-treated cells by nearly half, though not completely to baseline levels (Fig. 3a). 8-OhdG in cellular DNA was also visualized using avidin, which binds 8-OhdG with high specificity (Struthers et al. 1998), conjugated to a TRITC dye. Condensed staining intensity of 8-OhdG was observed in H2O2-treated cells. However, baicalein decreased the intensity of 8-OhdG staining (Fig. 3b). These results indicate that baicalein can decrease the levels of 8-OhdG in cellular DNA induced by oxidative stress.
The effect of baicalein on 8-OxoG levels. a The amount of 8-OxoG in DNA was determined as the 8-OhdG amount using the Bioxytech 8-OHdG ELISA kit. *p < 0.05, significantly different from control cells; **p < 0.05, significantly different from H2O2-treated cells. b The binding of avidin-TRITC, which reflects the 8-OxoG levels, was visualized under a fluorescence microscope. Transmission images are provided to confirm the presence of cells
Baicalein prevents the inhibition of OGG1 protein and mRNA expression by H2O2
A transcriptional reporter vector, in which the promoter region of OGG1 was coupled to a luciferase gene, was used to assess the effects of ROS and baicalein on promoter activity. H2O2 treatment decreased the transcriptional activity of the OGG1 promoter. Baicalein treatment increased the OGG1 promoter activity to more than two fold in unstressed cells and restored promoter activity in cells stressed with H2O2 (Fig. 4a). The expression of OGG1 mRNA was reduced in H2O2-treated cells compared with that in control cells. Baicalein restored the levels of OGG1 mRNA (Fig. 4b) and partially restored OGG1 protein level (Fig. 4c) in H2O2-treated cells. These data suggest that baicalein increases the transcriptional activity of the OGG1 promoter, and hence, the levels of OGG1 mRNA and protein.
Effects of baicalein on the transcriptional activity, mRNA expression, and protein expression of OGG1. a After overnight transfection with the OGG1 promoter–luciferase vector, cells were treated with baicalein for 24 h and cell lysates were mixed with a luciferase substrate. Luciferase activity was measured with a luminometer. *p < 0.05, significantly different from control cells; **p < 0.05, significantly different from H2O2-treated cells. b OGG1 mRNA levels were determined by reverse transcriptase polymerase chain reaction. c Western blot analysis was performed using an anti-OGG1 antibody
Activation of the Akt signaling pathway by baicalein
Akt kinase, also known as protein kinase B, is a major signaling enzyme involved in cell survival during oxidative stress, and recently, it was reported that the phosphoinositide 3-kinase (PI3K)/Akt pathway regulates OGG1 expression (Kang et al. 2009; Ueta et al. 2008). Baicalein pretreatment in H2O2-treated cells increased the level of phosphorylated Akt (the active form of Akt) compared with the level of phosphorylated Akt in cells treated with H2O2 alone (Fig. 5a). Furthermore, Akt inhibitor IV abolished the cytoprotective effect of baicalein against H2O2-induced death (Fig. 5b), suggesting the involvement of Akt signaling in the mechanism by which baicalein promotes DNA damage repair.
Effects of baicalein on Akt phosphorylation and cell viability. a Cell lysate proteins were separated by electrophoresis and transferred to Western blots. Total Akt and phospho-Akt were detected separately using specific antibodies. b Cells were treated with 1 μM Akt inhibitor IV for 1 h, treated with baicalein for 1 h, and then 1 mM H2O2 was added for 24 h. Cell viability was then assessed by the MTT assay. *p < 0.05, significantly different from control cells; **p < 0.05, significantly different from H2O2-treated cells; ***p < 0.05, significantly different from H2O2-treated cells with baicalein
Baicalein inhibits apoptotic DNA fragmentation induced by oxidative stress
The process of apoptosis involves the activation of nucleases that degrade the nuclear DNA into fragments of approximately 200 bp in length (Singh 2000). The effects of baicalein on DNA fragmentation induced by H2O2 treatment was examined using a TUNEL assay, in which BrdU incorporated at the ends of apoptotic DNA fragments is represented by green fluorescence from a labeled antibody to BrdU. Apoptotic nuclei can thus be distinguished from intact nuclei counterstained with propidium iodide (red fluorescence). H2O2 treatment increased the fraction of cells showing DNA fragmentation, from a baseline level of ∼3 % to nearly 60 %, whereas baicalein partially suppressed this effect (Fig. 6). These data suggest that baicalein protects DNA against the fragmentation induced by oxidative stress.
The effect of baicalein on the induction of apoptotic DNA fragmentation by oxidative stress. Cells were treated with baicalein for 1 h and then with 1 mM H2O2 for an additional 24 h. Apoptotic cells were detected by fluorescent TUNEL staining (green) and counterstaining of nuclei with propidium iodide (red). Representative images of each group are shown. White arrows indicate TUNEL-positive cells with fragmented DNA (apoptotic cells; magnification, ×400). The percentage of apoptotic cells was determined by manual scoring of multiple microscopic fields for each treatment group. *p < 0.05, significantly different from control cells; **p < 0.05, significantly different from H2O2-treated cells
Discussion
Natural flavonoids are emerging as potent therapeutic drugs for use in the treatment of free radical-mediated diseases, and researchers have made numerous efforts to search for novel antioxidants. Baicalein inhibits certain types of lipoxygenases (Deschamps et al. 2006), inhibits inflammation (Hsieh et al. 2007), and inhibits CYP2C9, an enzyme of the cytochrome P450 system, which is responsible for drug metabolism (Si et al. 2009). Additionally, our previous results indicated that baicalein attenuated mitochondrial or cellular oxidative stress by scavenging ROS and inducing manganese superoxide dismutase (Lee et al. 2011; Kang et al. 2011; Kim et al. 2012).
Although many studies have reported the protective effect of flavonoids including baicalein against oxidative stress-induced cell damage, few have examined the effects on cellular repair mechanisms mediated by flavonoids. Therefore, in this study, we evaluated the effects of baicalein on cellular DNA repair responses to oxidative stress. Baicalein has free radical scavenging and antioxidant effects due to the trihydroxy structure of its A ring (Gao et al. 1999) and its lipophilic characteristics (Saija et al. 1995). In our study, baicalein effectively reduced ROS and suppressed DNA breakage in response to oxidative stress via the induction of Ku70 and DNA-PKcs, demonstrating the ability of baicalein to promote the repair of DSBs. Additionally, baicalein promoted the removal of oxidized DNA bases via the restoration of OGG1 transcription and translation.
ROS induced oxidative DNA damage, including strand breaks and base and nucleotide modifications (Inoue and Kawanishi 1995; Nguyen et al. 1992). Understanding the mechanisms for processing oxidative DNA damage is important in elucidating the pathogenesis of oxidative stress-mediated diseases. Homologous recombination (HR) and NHEJ processes are the representative responses to DSBs. While the HR repair response uses a homologous template to direct DNA repair synthesis, the NHEJ process is referred to as nonhomologous because the broken ends are directly ligated without the use of a homologous template (Burma et al. 2006). Loss of damaged nucleotides at the break site can lead to small deletions, and the joining of nonmatching termini can result in chromosomal rearrangements including large deletions and translocations. NHEJ may, therefore, introduce mutations during repair (Lieber et al. 2004). Nevertheless, the NHEJ process occurs more frequently in higher eukaryotes than in lower eukaryotes or prokaryotes (Wang et al. 2003). Moreover, the NHEJ process is required for the joining of hairpin-capped double-strand breaks (Jung and Alt 2004). This mechanism of response to DNA DSBs occurs most often in higher eukaryotes (Wang et al. 2003). Among many modified DNA bases generated by oxidative stress, 8-OxoG is the most abundant product, and it seems to play a major role in mutagenesis and carcinogenesis (Fortini et al. 2003). We have previously shown that OGG1-deficient human leukemia cells could be forced to undergo apoptosis by the incorporation of 8-OhdG (Hyun et al. 2000, 2003). The expression of OGG1 suppresses oxidative stress-derived DNA damage and results in enhanced cell survival (Kannan et al. 2006).
The Akt pathway plays a crucial role in controlling transcription, cell cycle progression, and apoptosis (Brazil and Hemmings 2001). Furthermore, DNA-PKcs colocalizes and associates with Akt kinase at the plasma membrane (Feng et al. 2004). In vitro analysis using purified DNA-PK and recombinant Akt protein revealed that DNA-PK directly phosphorylates and thus activates Akt kinase (Feng et al. 2004; Dragoi et al. 2005). In previous studies, we and others have demonstrated that OGG1 is induced via the activation of the PI3K/Akt pathway (Kang et al. 2009; Ueta et al. 2008). However, those results contrast with a study in a rat model of diabetes type 1, which indicated that ROS induced by hyperglycemia activated Akt kinase and was associated with the downregulation of OGG1 rather than induction (Simone et al. 2008). The reasons for this apparent discrepancy are unclear. Also, Akt kinase phosphorylates pro-apoptotic proteins, such as Bad, caspase-9, and the forkhead transcription factors, and their phosphorylation by Akt kinase abolishes their pro-apoptotic activities (Datta et al. 1999; Blume-Jensen and Hunter 2001). Therefore, the reduced apoptosis in H2O2-treated cells with baicalein treatment might involve the activation of Akt kinase by baicalein. However, baicalein suppresses or activates different cell types; baicalein suppresses or does not change Akt activation in human melanoma cells (Li et al. 2010b), microglial cells (Hwang et al. 2008), and vascular smooth muscle cells (Peng et al. 2008); and baicalein increases Akt activation in various cancer cell lines (Chao et al. 2007; Wang et al. 2011), human neuroblastoma cells (Zhang et al. 2012), and adipocytes (Pu et al. 2012). Further studies need to elucidate the possible application of baicalein on DNA damage-associated disease models.
In conclusion, baicalein increased the BER and NHEJ capacity of cells subjected to oxidative stress, thereby reducing DNA damage and rescuing cells from oxidative stress-induced apoptosis.
Abbreviations
- ROS:
-
Reactive oxygen species
- DSBs:
-
Double-strand breaks
- NHEJ:
-
Nonhomologous end joining
- HR:
-
Homologous recombination
- BER:
-
Base excision repair
- OGG1:
-
8-Oxoguanine DNA glycosylase 1
- DNA-PKcs:
-
DNA-dependent protein kinase catalytic subunit
- PI3K:
-
Phosphoinositide 3-kinase
- 8-OhdG:
-
8-Hydroxy-2-deoxyguanosine
- 8-OxoG:
-
8-Oxoguanine
- DCF-DA:
-
2,7-Dichlorodihydrofluorescein diacetate
- MTT:
-
[3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide
- TRITC:
-
Tetramethylrhodamine isothiocyanate
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated deoxyUTP nick end labeling
References
Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;41:355–65.
Boiteux S, O’Connor TR, Lederer F, Gouyette A, Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990;265:3916–22.
Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64.
Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst). 2006;5:1042–8.
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–42.
Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–48.
Coppedè F, Mancuso M, Lo Gerfo A, Manca ML, Petrozzi L, Migliore L, et al. A Ser326Cys polymorphism in the DNA repair gene hOGG1 is not associated with sporadic Alzheimer’s disease. Neurosci Lett. 2007;414:282–5.
Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27.
Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14:4295–301.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3.
Dragoi AM, Fu X, Ivanov S, Zhang P, Sheng L, Wu D, et al. DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA. EMBO J. 2005;24:779–89.
Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–96.
Fortini P, Pascucci B, Parlanti E, D’Errico M, Simonelli V, Dogliotti E. 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat Res. 2003;531:127–39.
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant. 1997;100:241–54.
Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472:643–50.
Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–42.
Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS. Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin Mol Allergy. 2007;5:1–10.
Hwang KY, Oh YT, Yoon H, Lee J, Kim H, Choe W, et al. Baicalein suppresses hypoxia-induced HIF-1α protein accumulation and activation through inhibition of reactive oxygen species and PI 3-kinase/Akt pathway in BV2 murine microglial cells. Neurosci Lett. 2008;444:264–9.
Hyun JW, Choi JY, Zeng HH, Lee YS, Kim HS, Yoon SH, et al. Leukemic cell line, KG-1 has a functional loss of hOGG1 enzyme due to a point mutation and 8-hydroxydeoxyguanosine can kill KG-1. Oncogene. 2000;19:4476–9.
Hyun JW, Jung YC, Kim HS, Choi EY, Kim JE, Yoon BH, et al. 8-Hydroxydeoxyguanosine causes death of human leukemia cells deficient in 8-oxoguanine glycosylase 1 activity by inducing apoptosis. Mol Cancer Res. 2003;1:290–9.
Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–8.
Jin S, Inoue S, Weaver DT. Functions of the DNA dependent protein kinase. Cancer Surv. 1997;29:221–61.
Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311.
Kang KA, Lee JH, Chae S, Zhang R, Piao MJ, Kim HS, et al. Butin decreases oxidative stress-induced 8-hydroxy-2′-deoxyguanosine levels via activation of oxoguanine glycosylase 1. Chem Biol Interact. 2009;181:338–42.
Kang KA, Zhang R, Piao MJ, Chae S, Kim HS, Park JH, et al. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol Ind Health. 2011;28:1–10.
Kannan S, Pang H, Foster DC, Rao Z, Wu M. Human 8-oxoguanine DNA glycosylase increases resistance to hyperoxic cytotoxicity in lung epithelial cells and involvement with altered MAPK activity. Cell Death Differ. 2006;13:311–23.
Kim KC, Lee JS, Hyun JW. Baicalein attenuates oxidative stress-induced expression of matrix metalloproteinase-1 by regulating the ERK/JNK/AP-1 pathway in human keratinocytes. Biomol Ther. 2012;20:57–61.
Lam GY, Fattouh R, Muise AM, Grinstein S, Higgins DE, Brumell JH. Listeriolysin O suppresses phospholipase C-mediated activation of the microbicidal NADPH oxidase to promote Listeria monocytogenes infection. Cell Host Microbe. 2011;10:627–34.
Lee IK, Kang KA, Zhang R, Kim BJ, Kang SS, Hyun JW. Mitochondria protection of baicalein against oxidative damage via induction of manganese superoxide dismutase. Environ Toxicol Pharmacol. 2011;31:233–41.
Li B, Wang CZ, He TC, Yuan CS, Du W. Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2010a;289:62–70.
Li X, Darzynkiewicz Z. Labelling DNA strand breaks with BrdUTP. Detection of apoptosis and cell proliferation. Cell Prolif. 1995;28:571–9.
Li X, Guo L, Sun Y, Zhou J, Gu Y, Li Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int J Mol Med. 2010b;25:923–7.
Lieber MR, Ma Y, Pannicke U, Schwarz K. The mechanism of vertebrate nonhomologous DNA end joining and its role in V(D)J recombination. DNA Repair. 2004;3:817–26.
Mari PO, Florea BI, Persengiev SP, Verkaik NS, Bruggenwirth HT, Modesti M, et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci USA. 2006;103:18597–602.
Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell. 2004;15:2361–74.
Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992;89:3030–4.
Olive PL, Wlodek D, Banath JP. DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res. 1991;51:4671–6.
Peng CY, Pan SL, Huang YW, Guh JH, Chang YL, Teng CM. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-κB activity in vascular smooth-muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:579–88.
Powell CL, Swenberg JA, Rusyn I. Expression of base excision DNA repair genes as a biomarker of oxidative DNA damage. Cancer Lett. 2005;229:1–11.
Pu P, Wang XA, Salim M, Zhu LH, Wang L, Chen KJ, et al. Baicalein, a natural product, selectively activating AMPKα(2) and ameliorates metabolic disorder in diet-induced mice. Mol Cell Endocrinol. 2012;362:128–38.
Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–34.
Ribeiro ML, Priolli DG, Miranda DD, Arcari DP, Pedrazzoli Jr J, Martinez CA. Analysis of oxidative DNA damage in patients with colorectal cancer. Clin Colorectal Cancer. 2008;7:267–72.
Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J Immunol Methods. 1992;156:39–45.
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19:481–6.
Salmon TB, Evert BA, Song B, Doetsch PW. Biological consequences of oxidative stress-induced DNA damage in Saccharomyces cerevisiae. Nucleic Acids Res. 2004;32:3712–23.
Si D, Wang Y, Zhou YH, Guo Y, Wang J, Zhou H, et al. Mechanism of CYP2C9 inhibition by flavones and flavonols. Drug Metab Dispos. 2009;37:629–34.
Silva JP, Gomes AC, Coutinho OP. Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur J Pharmacol. 2008;601:50–60.
Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626–36.
Singh NP. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res. 2000;455:111–27.
Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–34.
Struthers L, Patel R, Clark J, Thomas S. Direct detection of 8-oxodeoxyguanosine and 8-oxoguanine by avidin and its analogues. Anal Biochem. 1998;255:20–31.
Tsatmali M, Walcott EC, Makarenkova H, Crossin KL. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. 2006;33:345–57.
Ueta E, Sasabe E, Yang Z, Osaki T, Yamamoto T. Enhancement of apoptotic damage of squamous cell carcinoma cells by inhibition of the mitochondrial DNA repairing system. Cancer Sci. 2008;99:2230–7.
van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196–206.
van Gent DC, van der Burg M. Non-homologous end-joining, a sticky affair. Oncogene. 2007;26:7731–40.
Wang H, Perrault AR, Takeda Y, Qin W, Wang H, Iliakis G. Biochemical evidence for Ku-independent backup pathways of NHEJ. Nucleic Acids Res. 2003;31:5377–88.
Wang W, Wang F, Yang YJ, Hu ZL, Long LH, Fu H, et al. The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br J Pharmacol. 2011;162:1364–79.
Zhang R, Kang KA, Piao MJ, Ko DO, Wang ZH, Chang WY, et al. Preventive effect of 7,8-dihydroxyflavone against oxidative stress induced genotoxicity. Biol Pharm Bull. 2009;32:166–71.
Zhang Z, Cui W, Li G, Yuan S, Xu D, Hoi MP, et al. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J Agric Food Chem. 2012;60:8171–82.
Acknowledgments
This work was funded by the Ministry of Education, Science and Technology (MEST) of the Republic of Korea (2012R1A1A2005350).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ki Cheon Kim and In Kyung Lee contributed equally to this study.
Rights and permissions
About this article
Cite this article
Kim, K.C., Lee, I.K., Kang, K.A. et al. Baicalein (5,6,7-trihydroxyflavone) reduces oxidative stress-induced DNA damage by upregulating the DNA repair system. Cell Biol Toxicol 28, 421–433 (2012). https://doi.org/10.1007/s10565-012-9233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-012-9233-y