Abstract
6, 4′-Dihydroxy-7-methoxyflavanone (DMF) has been shown to possess anti-inflammatory, anti-oxidative, and neuroprotective activities. However, its effect on oxidative stress-induced aging remains undemonstrated. This study aimed at investigating the anti-senescence effect of DMF on hydrogen peroxide (H2O2)-induced premature senescence, and associated molecular mechanisms in human dermal fibroblasts (HDFs). The cells were DMF pretreated with small interfering RNA (siRNAs) of control or sirtuin 1 (SIRT1) before H2O2 exposure, and western blot analysis, senescence-associated β-galactosidase (SA-β-gal) activity, cell counting, gene silencing, and SIRT1 activity assay were performed. Pretreatment with DMF inhibited H2O2-induced senescence phenotypes, which showed decreased SA-β-gal activity and increased cell growth in comparison with H2O2-treated HDFs. Meanwhile, the decreases in ac-p53, p21Cip1/WAF1, and p16Ink4a and the increases in pRb and cyclin D1 were observed. DMF was also found to induce SIRT1 expression and activity level concentration- and time-dependently. Moreover, SIRT1 inhibition abrogated DMF senescence prevention. Additionally, Akt and ERK were activated with different kinetics after H2O2 exposure, and Akt activity inhibition attenuated SA-β-gal activity augmentation. We also found that DMF inhibited H2O2-induced Akt phosphorylation. This study indicates that DMF effectively protects against oxidative stress-induced premature senescence through SIRT1 expression up-regulation and Akt pathway inhibition in HDFs. These results suggest that DMF can be a potential therapeutic molecule for age-related diseases, or a protective agent against the aging process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with a number of diseases, including dementia, atherosclerosis, osteoarthritis, infection, and cancer [1]. These diseases are associated with inflammation and oxidative stress, likely to promote premature senescent cells formation [2, 3].
Premature senescence is described as a shortened inherent replicative lifespan in stress-exposed cells [2, 4]. Accumulating evidence supports the relationship between oxidative stress and aging, by showing that hydrogen peroxide (H2O2) induces cellular premature senescence [5,6,7]. Senescent cells normally exhibit permanent arrest, large flat morphology, and up-regulate senescence-associated β-galactosidase (SA-β-gal) activity [6]. Senescence is also associated with various altered protein expressions. The tumor suppressor p53 is an important transcription activator, and directly up-regulates the cyclin-dependent kinase (CDK) 2 inhibitor p21, a critical negative cell growth regulator, to induce cellular senescence [8, 9]. Caveolin-1 is a major caveolae membrane component and mediates cell cycle arrest through the p53/p21-dependent pathway [10]. Retinoblastoma protein (Rb) is another tumor suppressor shown to play a crucial role in senescence induction, and it is inactivated by cell cycle-dependent phosphorylation regulators such as cyclin D1 [11, 12]. In senescent human dermal fibroblasts (HDFs), p21 and p16 overexpression results in Rb hypophosphorylation [13]. Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase involved in stress resistance and anti-senescence, through negative regulation of p53-mediated pathways [14]. Previous studies have shown SIRT1 inhibition promotes p53 acetylation at K373/382 by down-regulating p21 protein expression [14,15,16]. Akt is a well-known oxidative stress mediator [17]. The Akt pathway has been suggested to enhance p53 translation and protein stability, and promote p53 accumulation and downstream senescence [18].
Dalbergia odorifera T. Chen. (Leguminosa) possesses a wide range of biological activities, including anti-osteoporotic, anti-inflammatory, anti-oxidant, and neuroprotective effects, in diverse cell types [19,20,21]. In a previous study, 6,4′-Dihydroxy-7-methoxyflavanone (DMF), a naturally occurring flavonoid, isolates from the heartwood of Dalbergia odorifera (D. odorifera), and exhibits protective effects against glutamate-induced oxidative injury in HT22 cells [22]. However, the anti-aging effect of DMF remains unknown. This study therefore aimed at examining the anti-senescence effect of DMF and the associated mechanism, and whether this effect could protect H2O2- exposed HDFs against senescence.
Materials and methods
Preparation of DMF
DMF (no. NNMBP012) was obtained from the Standardized Material Bank, Wonkwang University (Republic of Korea) (Fig. 1a). DMF (> 98%) was isolated from D. odorifera as previously described [22]. DMF was dissolved in DMSO before used and the final vehicle content was less than 0.01% in each experiment.
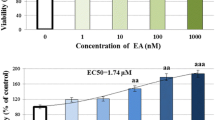
The chemical structure of DMF (a) and its effect on viability (b). b HDFs were incubated for 12 h with different concentrations of DMF (20–320 μM). Cell viability was determined by MTT assay. All the data are presented as the means ± S. D. of 3 three independent experiments. *P < 0.05 versus control
Reagents
H2O2 and 5-Bromo-4-chloro-3-indolyl-β-D-galactosidase (X-Gal) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-acetylated-p53 (-ac-p53), -p16INk4α, -sirtuin 1 (-SIRT1), -phospho-Rb (-pRb), -Akt, -phospho-Akt (-p-Akt), -extracellular signal-regulated kinase (-ERK), -phospho-ERK (-p-ERK), -p38, -phospho-p38 (-p-p38), -c-Jun N-terminal kinase (JNK), and -phospho-JNK (-p-JNK) antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-p21Cip1/WAF1, -cyclin D1, and -β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). LY294002 and PD98059 were purchased from Caliches (San Diego, CA, USA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Primary Human dermal fibroblasts (HDFs) were purchased from ATCC (ATCC PCS-201-010). Cells were maintained in low-glucose Dulbecco’s Modified Eagle’s medium (DMEM; GIBCO BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO BRL) and 1% penicillin/streptomycin (GIBCO BRL) at 37 °C in a humidified incubator with 5% CO2. Cells at early passages (between 9 and 18 passages) were used in all experiments.
Drug treatment
The HDFs were seeded in 60 mm dishes or 6-well plates and cultured for 24 h. Then the cells were treated with different concentrations of DMF (20, 40, 80 μM) for 12 h. The DMF containing medium was then replaced with a new medium containing 200 μM H2O2 for 2 h. After 2 h, the cells were cultured in fresh medium for 72 h after removal of H2O2. The HDFs were maintained in complete medium (DMEM, 10% FBS, 1% penicillin/streptomycin) in all the experimental procedure.
Senescence-associated β-galactosidase (SA-β-gal) staining
SA-β-gal activity assay was performed at pH 6.0 as previously described [23]. Briefly, all the cells were washed twice with PBS, fixed in 3% formaldehyde for 5 min, and then stained at 37 °C with SA-β-gal staining solution (1 mg/ml X-gal, 40 mM citric acid/ sodium phosphate, pH 6.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2). After 24 h, the stained cells were photographed and counted.
Western blot analysis
Western blot analysis was performed as Cheng et al. [24]. Briefly, all the cells were harvested, and lysed with RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2.5% deoxycholic acid, 1% NP-40, 10 mM EDTA) containing a protease inhibitor mixture (0.1 mM phenylmethylsulfonyl fluoride, 1 μg/ml chymostatin, 5 μg/ml aprotinin, and 5 μg/ml pepstatin A). Protein concentration was determined using a Bio-Rad protein assay kit. Lysates (50 μg/well) were separated by 12% or 14% SDS-PAGE and electro-transferred to PVDF membranes. Each membrane was incubated with primary antibody at 4 °C overnight, followed by 2 h in secondary antibody. Protein bands were visualized using the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ).
Cell viability and cell proliferation by MTT assay
Cell viability and cell proliferation were performed as Lee et al. and Yu et al. described, respectively [25, 26]. Briefly, Cells (4 × 104/well) were seeded in 96-well plates and treated with DMF and H2O2 as described in figure legends (Figs. 1b and 2d). Then 20 μl of MTT (3-[4, 5-dimethythiazol-2-yl]-2,5diphenyltetrazolium bromide, a tetrazolium salt) test solution was added and incubated for 4 h at 37 °C in a humidified incubator with 5% CO2. The absorbance was measured at a wavelength of 490 nm using a SpectraMax M3 instrument (Molecular Devices, California, USA).
DMF protected cells against H2O2-induced premature senescence. a–c HDFs were pretreated with DMF (20–80 µM) for 12 h, incubated in 200 µM H2O2 for 2 h, and then incubated in fresh medium for 72 h. The expression of ac-p53, p53, p21Cip1/WAF1, p16Ink4α, pRb, Rb, cyclin D1, and β-actin were measured by Western blotting and quantified by densitometry based on immunoblot images (a). SA-β-gal staining was performed (b). The percentages of SA-β-gal positive cells were calculated from 5 fields of view (c). d HDFs were pretreated with different concentrations of DMF, exposed to 200 µM H2O2 for 2 h, and then cultured in fresh medium at different timepoints (6–72 h). The cell proliferation was assessed by MTT assay. All the data are presented as the means ± S. D. of 3 three independent experiments. *P < 0.05 versus control. #P < 0.05 versus H2O2-treated cells
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
HDFs were washed twice with PBS and the total RNA was extracted by easy-Blue™ kit, according to the manufacturer’s protocol. RNA concentration was read using a GeneQuant pro RNA/DNA calculator (Amersham Biosciences, Uppsala, Sweden). According to the manufacturer’s protocol, cDNA was synthesized with total RNA using a High-capacity RNA-to-cDNA kit (Applied Biosystems, Foster city, CA). The cDNA was mixed with SIRT1 primer (4331182) (Applied Biosystems; Thermo Fisher Scientific, Inc) and then performed using CFX96™ Real-Time System (Bio-Rad). Cycling conditions were performed as follows: preparation at 50 °C for 2 min, denaturation at 95 °C for 10 min, and followed by 40 cycles at 95 °C for 10 s and at 60 °C for 30 s. The data were analyzed using StepOne™ software.
SIRT1 activity assay
SIRT1 activity was performed using the SIRT1 fluorometric drug discovery kit (Enzo Life Sciences, New York, NY, USA), according to the manufacturer’s instruction. Briefly, HDFs were harvested and lysed with RIPA buffer. Lysates (50 μg/well) were incubated in SIRT1 assay buffer (100 μM Fluor de Lys-SIRT1 substrate, 5 μM TSA and 200 μM NAD+) for 45 min at 37 °C, the reaction was stopped by 2 × SIRT1 Developer (contains 2 M Nicotinamide) for 15 min at room temperature. Fluorescence of the samples were read using a SpectraMax M3 instrument with an excitation set to 355 nm and emission set to 460 nm.
Transfection with siRNA
Instructions for using SIRT1 siRNA and scrambled siRNA were provided by Santa Cruz Biotechnology (CA, USA). Cells were transfected with Sirt1 siRNA or scrambled siRNA (80 nM) for 18 h in Opti-MEM®I Reduced Serum Medium (GIBCO BRL) using Lipofectamine 2000 reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Following transfection, the medium was changed, and the cells were used in other experiment.
Statistical analysis
All experiments were operated three times. The results were expressed as mean ± standard deviation (S. D.). One-way ANOVA analysis (R software) was used for data comparisons within multiple groups, with P < 0.05 considered as statistically significant.
Results
DMF pretreatment inhibits H2O2-induced cellular senescence
In this study, we evaluated the effect of DMF on viability of HDFs to determine the cytotoxic potential of it. Up to a concentration of 80 μM, no cytotoxic effects could be detected using MTT assay (Fig. 1b). To establish a senescent model, HDFs were exposed to 200 µM H2O2 for 2 h and subsequently cultured for another 72 h (Fig. S1). We found that HDFs presented senescent cellular morphology, meanwhile, ac-p53 and p21 Cip1/WAF1, senescence markers, significantly increased and reached maximal. Pretreatment with DMF (20–80 µM, 12 h) resulted in decreased ac-p53, p21Cip1/WAF1, and p16Ink4α expression (Fig. 2a). In contrast, pRb and cyclin D1 levels increased (Fig. 2a). Next, we examined the activity of SA-β-gal, a specific cytoplasmic senescent cells marker [23]. We found that DMF pretreatment of cells reduced cytosolic positive stain for senescence (blue) (Fig. 2b) and SA-β-gal positive cells percentage, concentration-dependently (Fig. 2c). Cell proliferation result showed that DMF pretreatment increased HDF growth rate, compared with H2O2 treatment only (Fig. 2d).
DMF treatment induces SIRT1 expression in HDFs
Since SIRT1, a longevity gene, plays a vital role in cellular senescence [16], and can be activated by many flavonoids [27], we examined DMF effect on SIRT1 expression and activity. HDFs were treated with different DMF concentrations for 12 h. DMF up-regulated SIRT1 expression and activity, concentration-dependently (Fig. 3a–c). Optimum SIRT1 induction was achieved at 80 µM DMF. Treating cells with DMF (80 µM) increased SIRT1 expression and activity, time-dependently (Fig. 3d–f). SIRT1 induction by DMF occurred within 3 h, reached maximum at 12 h, and then decreased at 24 h post treatment (Fig. 3d–f).
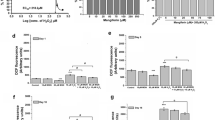
SIRT1 expression was up-regulated by DMF treatment in HDFs. a-c SIRT1 expression was measured 12 h after treatment with indicated concentrations of DMF. d-f Cells were treated with 80 µM DMF and harvested at indicated time points. The expression of SIRT1 and β-actin were measured by Western blotting and quantified by densitometry based on immunoblot images (a, d). SIRT1 mRNA was determined using RT-qRCR (b, e). The SIRT1 activity was performed using a SIRT1 fluorometric kit (c, f). All the data are presented as the means ± S. D. of 3 three independent experiments. *P < 0.05 versus control
SIRT1 inhibition ceases the protective effect of DMF against H2O2-induced premature senescence
From the above-mentioned results, DMF reduced H2O2-induced HDF senescence and up-regulated SIRT1 expression. To investigate the role of SIRT1 in DMF protection against premature senescence, we suppressed SIRT1 in HDFs by transfecting SIRT1 siRNA and used scrambled siRNA as control. Efficient silencing of SIRT1 in HDFs was confirmed by Western blotting, and SIRT1 activity (Fig. 4a, b). We found that SIRT1 inhibition prevented DMF-mediated ac-p53, p21Cip1/WAF1, and p16Ink4α reduction and abolished its protective role on SA-β-gal positive cells percentage, while pRb and cyclin D1 expression levels decreased (Fig. 4a, c, d). These results demonstrated that DMF-induced SIRT1 expression played an important role in preventing H2O2-induced premature senescence.
SIRT1 inhibition abrogated DMF effect against H2O2-induced premature senescence. HDFs were pre-transfected with two siRNAs targeting SIRT1 or a scrambled siRNA (80 nM, 18 h) in the presence or absence of DMF (80 µM, 12 h) before H2O2 (200 μM, 2 h) exposure and then incubated in fresh medium for 72 h. a The expression of SIRT1, ac-p53, p21Cip1/WAF1, p16Ink4α, pRb, cyclin D1, and β-actin were measured by Western blotting and quantified by densitometry based on immunoblot images. b The SIRT1 activity was performed using a SIRT1 fluorometric kit. c SA-β-gal staining was performed. d The percentages of SA-β-gal positive cells were calculated from 5 fields of view. All the data are presented as the means ± S. D. of 3 three independent experiments. *P < 0.05 vs. control. #P < 0.05 versus H2O2-treated cells
DMF treatment attenuates H2O2-induced premature senescence via its inhibitory effect on the activation of Akt signaling
Previous studies have shown that H2O2 activates various kinases, which are associated with cellular senescence [28, 29]. Next, we examined the role of H2O2 exposure on Akt, ERK, p38, and JNK activation by checking their phosphorylation levels in HDFs. Cells exposed to 200 µM H2O2 time-dependently increased Akt phosphorylation, which peaked around 3–6 h and slowly declined thereafter (Fig. 5a). ERK activation by H2O2 immediately increased by 0.25–0.5 h, and reduction thereafter (Fig. 5a). Conversely, p38 and JNK activation were not detected after H2O2 exposure (Fig. 5a). We speculated that activation of either Akt or ERK, or both Akt and ERK, might lead to H2O2-induced premature senescence in HDFs. Pretreatment of the cells with LY294002 and PD98059 inhibited Akt and ERK phosphorylation, respectively, we found that selective PI3 K inhibition by LY294002 dramatically reduced SA-β-gal positive cells percentage after H2O2 exposure, whereas no reduction was detected in PD98059-pretreated cells (Fig. 5b, c). Moreover, 80 µM DMF (15%) showed more effective protection than 20 µM LY294002 (53%) (Fig. 5c). To further investigate DMF inhibition effect on Akt activation, HDFs were pretreated with LY294002 (as a positive control) and DMF prior to 3 h of incubation with H2O2 (Fig. 5d). We found that DMF pretreatment of cells significantly inhibited Akt phosphorylation (Fig. 5d). These results suggested that DMF could attenuate H2O2-induced premature senescence by blocking the Akt pathway.
DMF reduced H2O2-induced premature senescence via its inhibitory effect on the activation of PI3 K/Akt pathway. a HDFs were exposed in 200 µM H2O2 all the time until harvested at indicated time points. The phosphorylation of Akt, ERK, p38, and JNK kinases were measured by Western blotting and quantified by densitometry based on immunoblot images. b, c HDFs were pretreated with PD98059 (10–20 µM, 30 min), LY294002 (10–20 µM, 30 min), and DMF (80 µM, 12 h) before H2O2 (200 µM, 3 h) exposure and then incubated in fresh medium for 72 h. SA-β-gal staining was performed (b). The percentages of SA-β-gal positive cells were calculated from 5 fields of view (c). d HDFs were pretreated with LY294002, and DMF before H2O2 (200 µM, 3 h) exposure. The p-Akt and β-actin were measured by Western blotting and quantified by densitometry based on immunoblot images. All the data are presented as the means ± S. D. of 3 three independent experiments. *P < 0.05 vs. control. #P < 0.05 versus H2O2-treated cells
Discussion
Natural compounds with anti-aging activities can protect against the aging process and ameliorate age-related developmental diseases, through modulation of various mechanisms, including energy homeostasis, cellular metabolism, and stress resistance [30]. The potential of DMF, a class of flavonoid isolated from D. odorifera, as a natural chemical with anti-oxidant properties, has been demonstrated in various cells types. D. odorifera, an important traditional Chinese medicine, has been widely used to treat blood disorders, ischemia, swelling, necrosis, and rheumatic pain [31]. Extracts of this plant exhibited a wide range of bioactivities such as anti-allergic, anti-inflammatory, and anti-oxidant activities [22, 31]. In recent years, the study of DMF’s effect on cell signal transduction has attracted more attention. Im et al. have reported that DMF has anti-osteoporosis effect; inhibits nuclear factor kappa-B ligand-induced osteoclast differentiation [32]. It has also been reported to protect against glutamate-induced oxidative injury in HT22 cells [22]. In this study, we demonstrated that DMF protects HDFs from H2O2-induced premature senescent phenotype and senescence-associated molecular induction via up-regulation SIRT1 expression. DMF also inhibits Akt activation, characteristic of reducing HDF senescence (Fig. 6).
A schematic illustration summarizing the protective effect of DMF against oxidative stress (H2O2)-induced premature senescence in HDFs. Up-regulation of SIRT1 by DMF inhibited cellular senescence through p53/p21Cip1/WAF1 pathway and p16Ink4α/Rb pathway. Meanwhile, inhibition of PI3 K/Akt signaling pathway by DMF also prevented H2O2-induced premature senescence
Because senescent cells are characterized by altered cell cycle arrest, giving rise to typical senescent phenotypes and altered gene expression [1], the effect of DMF was confirmed by cell proliferation, SA-β-gal staining, and expression of related molecules such as ac-p53, p21Cip1/WAF1, and p16 Ink4α, pRb and cyclinD1. In line with our results, Cilostazol reportedly protects endothelial cells from H2O2-induced cellular senescence via reduction of p53 acetylation and SA-β-gal positive cells [33]. Salidroside considerably reversed senescence-like phenotypes in oxidative stress and protected human fibroblast cells from premature senescence, by regulating the expression of senescence-related molecules expression such as p53, p21, and p16 [34]. DMF, Cilostazol, and Salidroside all protect cells from H2O2-induced cellular senescence via reducing SA-β-gal positive cells and abrogating H2O2-induced cell enlargement and flattened morphology. DMF and Cilostazol reduced H2O2-induced acetylation of p53.
In order to explore the mechanism by which DMF prevents premature senescence in HDFs, we investigated the possible involvement of SIRT1, the closest homolog of Sir2, shown to regulate numerous physiological process, including DNA repair, stress resistance, cellular survival, and senescence [35]. Recently, researchers have identified a significant array of phytochemicals from plants and these phytochemicals act as SIRT1 activator to improve various stress-induced senescence. For example, resveratrol, ginsenoside Rb1 protect the human endothelium from H2O2-induced oxidative injury and senescence, via Sirt1 activation [36, 37]. Our study showed that DMF treatment up-regulated SIRT1 expression and activity, concentration- and time-dependently, at a higher than basal level. Interestingly, SIRT1 expression reversed H2O2-induced premature senescence; however, SIRT1 inhibition induced premature senescence-like phenotypes with up-regulated p53 acetylation [16, 36]. This agrees with our results. We found that the SIRT1 siRNA transfection abolished the DMF anti-senescent effect. These results suggested that SIRT1 induction by DMF was essential to preventing H2O2-induced premature senescence.
The Akt and MAPK pathways are well-known mediators of cellular stress in a diverse array of cell types. These kinases play vital roles in senescence process. Akt pathway activation can induce senescence in human cells via mTORC1 and p53; however, its inhibition prevents H2O2-senescent phenotype and cell cycle arrest in normal human diploid fibroblasts [6]. MAPKs have three main pathways: ERK, p38 MAPK, and JNK pathways [17]. ERK-, activated by stress stimuli and oxidative stress, induced senescence correlates with β-galactosidase activity and induction of classical senescence-related genes, p16Ink4α, p53, p21, p14-p19ARF, and senescence-associated heterochromatin and DNA damage foci [38]. Our results showed that the Akt and ERK pathways were selectively activated by H2O2 in HDFs. HDFs treated with Akt kinase inhibitor LY294002, but not with a selective ERK inhibitor, had reduced SA-β-gal positive cell percentages after H2O2 exposure, which is consistent with the previous reports using other cells [6]. Recent studies have discovered that flavonoids can also prevent cellular senescence via Akt activation pathway inhibition. Consistent with our findings, Choi et al. reported that 7,8-dihydroxyflavone attenuates TNF-α-induced skin aging in Hs68 HDFs via Akt signaling pathway down-regulation [39].
In all, our work provides reliable evidence supporting the protective effect of DMF against cellular oxidative stress-induced senescence, through SIRT1 activation and Akt pathway suppression. This finding demonstrates that DMF has the potential to ameliorate the aging process and attenuate age-related diseases in humans.
References
Muller M (2009) Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal 11(1):59–98. https://doi.org/10.1089/ars.2008.2104
Chen QM (2000) Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann N Y Acad Sci 908:111–125. https://doi.org/10.1111/j.1749-6632.2000.tb06640.x
Freund A, Orjalo AV, Desprez PY, Campisi J (2010) Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16(5):238–246. https://doi.org/10.1016/j.molmed.2010.03.003
Toussaint O, Medrano EE, Von Zglinicki T (2000) Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol 35(8):927–945. https://doi.org/10.1016/S0531-5565(00)00180-7
Frippiat C, Chen QM, Remacle J, Toussaint O (2000) Cell cycle regulation in H2O2-induced premature senescence of human diploid fibroblasts and regulatory control exerted by the papilloma virus E6 and E7 proteins. Exp gerontol 35(6–7):733–745. https://doi.org/10.1016/S0531-5565(00)00167-4
Wang Y, Meng A, Zhou D (2004) Inhibition of phosphatidylinostol 3-kinase uncouples H2O2-induced senescent phenotype and cell cycle arrest in normal human diploid fibroblasts. Exp Cell Res 298(1):188–196. https://doi.org/10.1016/j.yexcr.2004.04.012
Seo HR, Choi MJ, Choi JM, Ko JC, Ko JY, Cho EJ (2016) Malvidin protects WI-38 human fibroblast cells against stress-induced premature senescence. J Cancer Prev 21(1):32–40. https://doi.org/10.15430/JCP.2016.21.1.32
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge S (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75(4):805–816. https://doi.org/10.1016/0092-8674(93)90499-G
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75(4):817–825. https://doi.org/10.1016/0092-8674(93)90500-p
Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP (2001) Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21WAF1/Cip1-dependent mechanism. Mol Biol Cell 12(8):2229–2244. https://doi.org/10.1091/mbc.12.8.2229
Geng Y, Yu Q, Sicinska E, Das M, Bronson RT, Sicinski P (2001) Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc Natl Acad Sci USA 98(1):194–199. https://doi.org/10.1073/pnas.011522998
Knudsen ES, Buckmaster C, Chen TT, Feramisco JR, Wang JYJ (1998) Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev 12(15):2278–2292
Stein GH, Lf D, Soulard A, Dulic V (1999) Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 19(3):2109–2117
Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T (2002) Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 21(10):2383–2396. https://doi.org/10.1093/emboj/21.10.2383
Zhou N, Lin X, Dong W, Huang W, Jiang W, Lin LB, Qiu Q, Zhang XJ, Shen JL, Song ZJ, Liang X, Hao J, Wang DW, Hu ZM (2016) SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep 6:22628. https://doi.org/10.1038/srep22628
Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y (2007) Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43(5):571–579. https://doi.org/10.1016/j.yjmcc.2007.08.008
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192(1):1–15. https://doi.org/10.1002/jcp.10119
Astle MV, Hannan KM, Ng PY, Lee RS, George AJ, Hsu AK, Haupt YG, Hannan RD, Pearson RB (2012) AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene 31(15):1949–1962. https://doi.org/10.1038/onc.2011.394
Yun HM, Park KR, Quang TH, Oh H, Hong JT, Kim YC, Kim EC (2015) 2, 4, 5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/β-catenin pathway. Cell Death Dis 6(7):e1819. https://doi.org/10.1038/cddis.2015.185
Yu X, Wang W, Yang M (2007) Antioxidant activities of compounds isolated from Dalbergia odorifera T. Chen and their inhibition effects on the decrease of glutathione level of rat lens induced by UV irradiation. Food Chem 104(2):715–720. https://doi.org/10.1016/j.foodchem.2006.10.081
Lee DS, Jeong GS (2014) Arylbenzofuran isolated from Dalbergia odorifera suppresses lipopolysaccharide-induced mouse BV2 microglial cell activation, which protects mouse hippocampal HT22 cells death from neuroinflammation-mediated toxicity. Eur J Pharmacol 728:1–8. https://doi.org/10.1016/j.ejphar.2013.12.041
An RB, Jeong GS, Kim YC (2008) Flavonoids from the heartwood of Dalbergia odorifera and their protective effect on glutamate-induced oxidative injury in HT22 cells. Chem Pharm Bull 56(12):1722–1724. https://doi.org/10.1248/cpb.56.1722
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92(20):9363–9367. https://doi.org/10.1073/pnas.92.20.9363
Cheng XS, Li MS, Du J, Jiang QY, Wang L, Yan SY, Yu DM, Deng JB (2010) Neuronal apoptosis in the developing cerebellum. Anat Histol Embryol 4:21–27. https://doi.org/10.1111/j.1439-0264.2010.01033.x
Lee DS, Li B, Keo S, Kim KS, Jeong GS, Oh H, Kim YC (2013) Inhibitory effect of 9-hydroxy-6,7-dimethoxydalbergiquinol from Dalbergia odorifera on the NF-kappaB-related neuroinflammatory response in lipopolysaccharide-stimulated mouse BV2 microglial cells is mediated by heme oxygenase-1. Int Immunopharmacol 17:828–835. https://doi.org/10.1016/j.intimp.2013.08.024
Yu HB, Li DY, Zhang HF, Xue HZ, Pan CE, Zhao SH, Wang L (2010) Resveratrol inhibits invasion and metastasis of hepatocellular carcinoma cells. Zhong Xi Yi Jie He Xue Bao 9:3117–3124
Porcu M, Chiarugi A (2005) The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci 26(2):94–103. https://doi.org/10.1016/j.tips.2004.12.009
Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M (2000) Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20(10):2175–2183. https://doi.org/10.1161/01.ATV.20.10.2175
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EWT, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Mo L, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773(8):1263–1284. https://doi.org/10.1016/j.bbamcr.2006.10.001
Ferrari CKB and Torres EAFS (2003) Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed Pharmacother 57(5–6):251–260. https://doi.org/10.1016/s0753-3322(03)00032-5
Chan SC, Chang YS, Wang JP, Chen SC, Kuo SC, Kuo SC (1998) Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med 64(2):153–158. https://doi.org/10.1055/s-2006-957394
Im NK, Choi JY, Oh H, Kim YC, Jeong GS (2013) 6, 4’-Dihydroxy-7-methoxyflavanone inhibits osteoclast differentiation and function. Biol Pharm Bull 36(5):796–801. https://doi.org/10.1248/bpb.b12-00964
Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y (2008) Cilostazol inhibits oxidative stress–induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol 28(9):1634–1639. https://doi.org/10.1161/ATVBAHA.108.164368
Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG, Li RG, Song DQ, Y-yY Li, Li DD, Wang Z (2010) Salidroside protects human fibroblast cells from premature senescence induced by H2O2 partly through modulating oxidative status. Mech Ageing Dev 131(11–12):723–731. https://doi.org/10.1016/j.mad.2010.10.003
Hubbard BP, Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35(3):146–154. https://doi.org/10.1016/j.tips.2013.12.004
Song Z, Liu Y, Hao BS, Yu SJ, Zhang H, Liu DH, Zhou B, Wu L, Wang M, Xiong ZJ, Wu CD, Zhu JM, Qian XX (2014) Ginsenoside Rb1 prevents H2O2-induced HUVEC senescence by stimulating sirtuin-1 pathway. PLoS ONE 9(11):e112699. https://doi.org/10.1371/journal.pone.0112699
Kao CL, Chen LK, Chang YL, Yung MC, Hsu CC, Chen YC, Lo WL, Chen SJ, Ku HH, Hwang SJ (2010) Resveratrol protects human endothelium from H2O2-induced oxidative stress and senescence via Sirt1 activation. J Atheroscler Thromb 17(9):970–979. https://doi.org/10.5551/jat.4333
Cagnol S, Chambard JC (2010) ERK and cell death: mechanisms of ERK-induced cell death-apoptosis, autophagy and senescence. FEBS J 277(1):2–21. https://doi.org/10.1111/j.1742-4658.2009.07366.x
Choi JW, Lee J, Park YI (2017) 7, 8-Dihydroxyflavone attenuates TNF-α-induced skin aging in Hs68 human dermal fibroblast cells via down-regulation of the MAPKs/Akt signaling pathways. Biomed Pharmacother 95:1580–1587. https://doi.org/10.1016/j.biopha.2017.09.098
Acknowledgements
This study was supported by Wonkwang University in 2020. The authors would like to thank Nam-Woo Choi for his encouragement until the publishing of this paper.
Author information
Authors and Affiliations
Contributions
BSL, RZZ, and BMC: Participated in research design, BSL: Conducted experiments, BSL: Performed data analysis, BSL and BMC: Wrote or contributed to the writing of the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, B.S., Zhu, R.Z. & Choi, BM. 6,4′-dihydroxy-7-methoxyflavanone protects against H2O2-induced cellular senescence by inducing SIRT1 and inhibiting phosphatidylinositol 3-kinase/Akt pathway activation. Mol Cell Biochem 476, 863–872 (2021). https://doi.org/10.1007/s11010-020-03951-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03951-z