Abstract
Baicalein (5,6,7-trioxyflavone-7-O-beta-D-glucuronide) derived from the Chinese herb Scutellaria baicalensis is well known as a lipoxygenase inhibitor. We investigated baicalein-mediated inhibitory effects on vascular smooth-muscle cell (VSMC) proliferation and intimal hyperplasia by balloon angioplasty in the rat. In vascular injury studies, baicalein significantly suppressed intimal hyperplasia by balloon angioplasty. Baicalein significantly inhibited cell proliferation via a lipoxygenase-independent pathway using [3H]thymidine incorporation, 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT), and flow cytometry assays. At the concentrations used, no cytotoxic effect on cell culture was found. Baicalein blocks cell-cycle progression in S/G2/M phase, consistent with the cell-cycle effects, baicalein significant inhibited cyclin D1, p42/44 mitogen-activated protein kinase (MAPK), and Akt phosphorylation without change in the other cell-cycle regulatory proteins. Furthermore, baicalein attenuated serum-induced deoxyribonucleic acid (DNA) binding activity of nuclear factor kappa B (NF-κB). These results show that baicalein blocks cell proliferation via blocking cell-cycle progression and proliferating events, including p42/44 MAPK and Akt activations as well as NF-κB activation. It also inhibits intimal hyperplasia after balloon vascular injury in the rat, indicating the therapeutic potential for treating restenosis after arterial injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The structure of the arterial wall and its vasoactive properties are mainly derived from vascular smooth-muscle cells (VSMCs), which represent a prominent cell type in this tissue. VSMCs are involved in several pathological conditions, such as neointima formation, restenosis after acute vascular injury, and chronic pathological processes such as atherosclerosis (Ross 1993). Efforts to inhibit VSMCs proliferation in vascular injury models, either by modulating cellular mediators of the proliferative response or by interfering with the cell-cycle machinery, have provided insights into neointima formation. Cell-cycle progression is a tightly controlled event regulated by cyclins, cyclin-dependent kinases (CDKs), their substrate proteins, CDK inhibitors, and tumour-suppressor gene products p53 and retinoblastoma proteins (Rb) (Golias et al. 2004).
Cell proliferation is initiated by the transduction of mitogenic signals from the cell-surface receptor to nucleus via an activation cascade, receptor protein tyrosine kinase, p42/44 mitogen-activated protein kinase (MAPK) (Katz et al. 2007), and PI3-K/Akt signaling pathway (Mehdi et al. 2007). p42/44 MAPK is a serine/threonine kinase activated by a dual phosphorylation on threonine and tyrosine residues (Zhang and Liu 2002). Phosphorylated and activated p42/44 MAPK translocates into the nucleus, where it phosphorylates several nuclear transcription factors, including nuclear factor kappa B (NF-κB), ultimately leading to initiation of the gene transcription, transition of cells from a quiescent to a proliferative state, deoxyribonucleic acid (DNA) synthesis, and cell division (Chen et al. 2001a, b; Hoshi et al. 2000). Phosphorylation of the serine/threonine kinase Akt is an important signaling pathway that mediates cell proliferation and survival signals in response to growth factors and cytokines (Manning and Cantley 2007). Akt also signals activation of NF-κB through phosphorylation and activation of inhibitor-kappa B kinase-alpha (IKK-α) (Adhikari et al. 2006).
NF-κB has been reported to play a pivotal role in regulating gene expression controlling inflammation, cell differentiation, apoptosis, and proliferation (Courtois and Gilmore 2006). Immunohistochemical studies indicate that human atherosclerotic tissues express NF-κB proteins (Brand et al. 1996). The expression of NF-κB is enhanced in vascular tissue during VSMC proliferation after lumen injury (Cercek et al. 1997). These studies indicate that NF-κB is involved in VSMC proliferation in vitro and in vivo. Under normal conditions, NF-κB is present in the cytoplasm as an inactive heterotrimer consisting of p50, p65, and inhibitor κB (IκB) subunits. On activation, degradation of IκB exposes nuclear localization signals on the p50/p65 heterodimer, leading to nuclear translocation and binding to a specific sequence in DNA, which in turn results in gene transcription. IκB degrades immediately after injury in vascular walls. These findings suggest that IκB plays a key role in regulating NF-κB activation in VSMC proliferation of vascular walls (Landry et al. 1997).
Baicalein (5,6,7-trioxyflavone-7-O-beta-D-glucuronide) is a flavonoid from Scutellaria baicalensis. Previous studies have shown that baicalein exhibits a multitude of pharmacological activities, including anti-inflammatory (Hong et al. 2002), antiallergic (Kimata et al. 2000), anticancer (Pidgeon et al. 2002; Lee et al. 2005; Chao et al. 2007), and antioxidative (Shao et al. 2002) activities. Among them, antithrombotic, antiproliferative, and antioxidative effects lead to cardiovascular protective properties. These findings have highlighted the fact that baicalein has the therapeutic potential to treat atherosclerosis and restenosis. To our knowledge, the study reported here is the first to show direct evidence that baicalein inhibits rat VSMC proliferation in vivo after arterial injury. We also examined the effect of baicalein on VSMC proliferation induced by serum and focused on transcription factor NF-κB, cell-cycle progression regulators, p42/44 MAPK, and Akt signaling pathways.
Materials and methods
Restenosis model and histological examination
Wistar rats were maintained in accordance with the Institutional Animal Care and Use Committee procedures and guidelines. Vascular injury was induced by balloon-catheter inflation of the rat common carotid artery (Peng et al. 2004). The rats were anesthetized with an intraperitoneal administration of sodium chlorohydrate (37 mg/kg), and a 2-Fr Fogarty arterial embolectomy balloon catheter was inserted into the left carotid artery. The catheter was passed through the entire length of the artery three times to cause the vascular injury. After removal of the catheter from the extracarotid artery, the wound was ligated and closed. Vehicle [0.5% carboxymethylcellulose (CMC)] or baicalein (10 mg/kg per day) were orally administered into the rat 3 days before to 14 days after the vascular injury. The right carotid artery remained untreated and served as the intraindividual control. However, 14 days after injury, rats were euthanatized with intraperitoneal administration of pentobarbital (60 mg/kg), and left and right carotid arteries were removed and fixed with 4% formaldehyde and embedded in paraffin. The embedded tissues were sectioned at 5-μm thick, stained with hematoxylin–eosin, and analyzed using microscopy.
Cell culture
VSMCs were isolated from 200-g male Wistar rat. Aortic strips were cut into small pieces and placed in six-well culture plates containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Gibco). Strips were incubated at 37°C in a humidified, 5% CO2 atmosphere for 10 days to allow VSMC migration from the vessel onto the dish. The medium was changed every 3 days, and cells were subcultured by 0.05% trypsin/0.02% ethylenediaminetetraacetate (EDTA) within six passages for the following experiments.
[3H]Thymidine incorporation assay
Confluent VSMCs were trypsinized, suspended in DMEM supplemented with 10% FBS, and seeded at 1.0 × 104 cells per well into 96-well plates. After 24 h, the cells were washed twice with phosphate buffered saline (PBS) and starved with serum-free DMEM for 24 h. Baicalein was dissolved in DMSO, and DMSO was used as vehicle control (final concentration 0.1%) in all in vitro experiments. The cells were incubated with or without indicated reagents and 10% FBS for 48 h and then harvested for DNA synthesis detection. Before harvest, cells were incubated with [3H]thymidine (1 μCi/ml, Amersham Pharmacia) for 16 h, processed and harvested with Filter-Mate (Packard), and incorporated radioactivity was determined.
MTT assay
The alteration of cell number was determined by a colorimetric 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay. In brief, after treatment of cells with or without the indicated agent and/or serum for 48 h, cells were washed twice with PBS and incubated with 0.5 mg/ml MTT (Sigma) for 4 h. The reagent was reduced by living cells to form an insoluble blue formazan product. After the incubation period, cells were washed with PBS, solubilized with DMSO, and quantified using an enzyme-linked immunosorbent assay (ELISA) reader at the absorbance of 550 nm.
Lactate dehydrogenase (LDH) assay
Lactate dehydrogenase (LDH) released into cell culture medium is an index of cytotoxicity and evaluates the permeability of the cell membrane. After an incubation of 24 h with various drug concentrations in serum, the culture supernatants were collected. The LDH assay was performed using the CytoTox96 Nonradioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The percentage of LDH released was expressed as a proportion of the LDH released into the medium compared with the total amount of LDH present in cells treated with 2% Triton X-100.
FACScan flow cytometric assay
After cells were treated with vehicle (0.1% DMSO) or baicalein for the indicated times, they were harvested by trypsinization, fixed with 70% (v/v) alcohol at 4°C for 30 min, and washed with PBS. They were then centrifuged and resuspended with 0.5 ml propidium iodide solution containing Triton X-100 (0.1%, v/v), RNase (100 μg/ml), and propidium iodide (80 μg/ml). DNA content was analyzed with the FACScan and CellQuest software (Becton Dickinson, Mountain View, CA USA).
Protein extraction and Western blot analysis
VSMCs were rendered quiescent by serum starvation for 2 days. After cell treatment with the indicated agent for different time periods [p42/44 MAPK and phosphorylated p42/44 MAPK (Santa Cruz), 10 min; phosphorylated Akt (Upstate Biotechnology), 15 min; phosphorylated IκB-α, 30 min; IκB-α, 1 h; cyclins, CDKs, p21, and p27 (Santa Cruz), 24 h], they were washed twice with PBS pH 7.4 and harvested on ice-cold buffer A containing 20 mM Hepes, pH 7.4, 2 mM EDTA, 1 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM Na3VO4, 1% Triton, 10% glycerol, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, and 400 μM phenylmethylsulfonyl fluoride. The cell lysate was centrifuged and the supernatant used for Western blot analysis as described previously (Lu et al. 2006). Signal detection was performed with an enhanced chemiluminescence detection kit (Amersham).
Detection of NF-κB activity
DNA binding activities of NF-κB were determined using electrophoretic mobility shift assay (EMSA). VSMCs were activated with stimulants for 1 h (NF-κB) and collected with a cell scraper. Nuclear extracts were prepared and applied to gel shift assay as described previously (Pan et al. 2003). Briefly, 2 μg of nuclear extracts were incubated with a 35-base-pair double-stranded 32P-labeled probe encoding the κB consensus sequence (5′-AGT TGA GGG GAT CCC CCC AGG C-3′) in the binding buffer containing 10 mM Tris-HCl, 40 mM NaCl, 10% glycerol, 1 mM EDTA, 1 mM dithiothreitol, 1% Nonidet P-40, 1% deoxycholate, and 3 μg/ml polydeoxyinosinic-deoxycytidylic acid at room temperature for 30 min. Then, samples were applied to native 5% polyacrylamide gels and analyzed on autoradiography. For competition assay, 20-fold molar excess unlabeled consensus oligonucleotide was added 30 min prior to addition of the labeled probe. Components of NF-κB proteins were identified by supershift assay using antibodies against p65 antibodies.
Statistical analysis
Data are presented as the mean ± standard error (SE) for the indicated number of separate experiments. Statistical significance was assured by one-way analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons. A P value <0.05 was considered significant statistically.
Results
Baicalein inhibits intimal hyperplasia by balloon angioplasty
Eighteen rats were assigned to orally receive vehicle (0.5% CMC) or baicalein (10 mg/kg per day) beginning 3 days before balloon angioplasty. At day 14 after angioplasty, animals were euthanatized to detect vascular injury and intimal thickening. As demonstrated in Fig. 1, the carotid artery without balloon angioplasty exhibited normal vessel thickness in the media layer (Fig. 1a), and the injured vessel showed a profound neointimal thickening (Fig. 1b). However, there was an obvious reduction in neointimal thickness in the section from the baicalein-treated animals (Fig. 1c), and data are also quantified by the neointima/media ratio of common carotid arteries after balloon injury from each group of animal studies (Fig. 1d).
Responses of rat carotid arteries to balloon injury. Representative observations are sections from uninjured vessel (a), balloon-injured vessel (b), and baicalein-treated balloon-injured vessel (c). All pictures were taken at ×200 magnification. Data are also quantified by the neointima/media ratio of common carotid arteries after balloon injury from each group of animals (d). Data are expressed as means ± standard error of six animals in each group. # P < 0.01 and **P < 0.01 compared with basal and control, respectively. (N neointima layer, M media layer)
Baicalein inhibits VSMC proliferation
VSMCs proliferate after vascular injury in response to several growth factors and cytokines such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and angiotensin II (Ross 1993; Griendling et al. 1997). In our study, we use 10% FBS instead of a single growth factor to induce cell proliferation. Because FBS contains a range of growth factors, including PDGF, FGF, transforming growth factor, serotonin, and thrombin (Wang et al. 2000), we used FBS to mimic the multiple factors environment in vivo. To examine the effect of baicalein on the regulation of cell growth, [3H]thymidine incorporation and MTT assay were used to determine DNA synthesis and cell number, respectively. As demonstrated in Fig. 2a,b, baicalein inhibited the serum-induced increase of DNA synthesis \(\left( {{\text{IC}}_{50} = 1.97 \times 10^{ - 5} {\text{M}}} \right)\) and cell number \(\left( {{\text{IC}}_{50} = 2.32 \times 10^{ - 5} {\text{M}}} \right)\) in a concentration-dependent manner, suggesting the antiproliferative action of baicalein. Furthermore, baicalein showed little influence on the release reaction of lactate dehydrogenase (Fig. 2c), revealing that the antiproliferative action of baicalein was not due to the cytotoxic effect.
Effect of baicalein on proliferation and cell number of rat vascular smooth-muscle cells. Cells were rendered quiescent for 24 h, treated with or without baicalein. Then vehicle or fetal bovine serum (FBS) (10%, v/v) was added for 48 h. Sixteen h before termination of the incubation period, [3H]thymidine was added to the cells (a). After the final treatment, cells were harvested to detect [3H]thymidine incorporation. (b), the cell number was detected using MTT assay after the incubation period, as described in Materials and methods. Cells were incubated in the absence or presence of baicalein for 24 h, and then the culture medium was obtained for to detect lactate dehydrogenase (LDH) release reaction, as described in Materials and methods (c). Data are expressed as mean ± standard error of six determinations. # P < 0.01 compared with basal group; *P < 0.05 and **P < 0.01 compared with control group
Lipoxygenase metabolites do not reverse the action to baicalein
Baicalein is well known as a lipoxygenase inhibitor (Butenko et al. 1993; Sekiya and Okuda 1982). We determined whether the antiproliferative effect of baicalein resulted from lipoxygenase inhibition. Data showed that lipoxygenase metabolites 12- and 15-hydroxyeicosatetraenoic acid (HETE), either alone or combined, could not induce the proliferative effect (Fig. 3a). Furthermore, the combination of 12- and 15-HETE also could not reverse the antiproliferative action to baicalein (Fig. 3a,b), suggesting that the antiproliferative effect of baicalein was not via the inhibition of cellular lipoxygenase.
Effect of lipoxygenase metabolites and baicalein on the cell viability of rat vascular smooth-muscle cells. Cells were rendered quiescent for 48 h, and then treated with or without 12-hydroxyeicosatetraenoic acid (HETE) (100 nM), 15-HETE (100 nM), or baicalein (50 μM) for 48 h (a). Cells were rendered quiescent for 48 h, and then treated with or without 12-HETE (100 nM), 15-HETE (100 nM), or baicalein (50 μM), and then vehicle or fetal bovine serum(FBS) (10% v/v) was added for 48 h (b). After the incubation period, the cell number was detected using thr 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay method, as described in the Materials and methods. Data are expressed as mean ± standard error of six determinations. # P < 0.01 compared with basal group; **P < 0.01 compared with control group
Baicalein inhibits cell-cycle progression and related protein expression
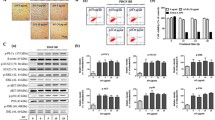
To determine whether baicalein influences the cell-cycle machinery, we examined cell-cycle progression using flow cytometric assessment. We found that about 96% of cells were arrested in the G0/G1 phase of the cell cycle when the cells were rendered quiescent by the deprivation of serum. After supplementation with 10% FBS for 24 h, a significant increase of S and G2/M phases of the cell cycle was observed (2.9-fold compared with the basal of 1-fold); however, this effect was completely abolished by 50 μM baicalein (Fig. 4a). We next examined the cell-cycle regulatory proteins, which represent the entering of the cell cycle from G0/G1 to S phase. Baicalein significantly inhibited cyclin D1 without change in cyclin E expression, (CDK)-2 and 4, and retinoblastoma protein (Rb) proteins. Data showed a profound increase of cyclin D1 expression after stimulation of cells with serum; baicalein completely abolished this serum action (Fig. 4b). These results suggest that baicalein could effectively inhibit serum-induced cell-cycle progression and retain the cells in the quiescent state.
Regulation of cell-cycle progression and regulatory proteins by baicalein. Cells were rendered quiescent for 48 h, and then fetal bovine serum (FBS) was added for 24 h in the absence or presence of baicalein (30 μM and 50 μM). Cells were then harvested to detect cell-cycle progression using flow cytometric methods (a) and cell-cycle regulatory proteins expression using the Western blot method (b). Data are expressed as mean ± standard error of four determinations. # P < 0.01 compared with basal group; **P < 0.01 compared with control group
Baicalein inhibits phosphorylation of p42/44 MAPKs and Akt/PKB
We measured the effect of baicalein on serum-induced activation of p42/44 MAPKs, which act downstream of several types of mitogenic stimulation and upstream of several transcriptional events. The phosphorylated p42/44 MAPKs were examined as the determination of MAPK activation. Data showed that serum induced MAPK phosphorylation and therefore kinase activity. Both baicalein and PD98059, a selective MAPK inhibitor, significantly inhibited the effects of serum addition (Fig. 5a), revealing that baicalein behaves as an upstream regulator of p42/44 MAPK activation. In addition, PI 3-kinase is a ubiquitously expressed enzyme family that plays a crucial role in the regulation of several cellular functions, including cell proliferation. To determine the effect of baicalein and PD98059 on the PI 3-kinase-signaling pathway, we measured their action on Akt/protein kinase B (PKB) phosphorylation, which is downstream of PI 3-kinase occurring in response to growth stimuli and cell-survival signaling pathway. As demonstrated in Fig. 5b, serum induced a marked increase of Akt/PKB phosphorylation that was completely inhibited by baicalein and LY294002 (10 μM), a selective inhibitor of PI 3-kinase activation. However, PD98059 significantly increased rather than decreased the amount of phosphorylated Akt/PKB (Fig. 5b). Thus, baicalein and LY294002 exhibited the inhibitory and PD98059 the stimulatory effect on serum-induced PI 3-kinase activation.
Effect of baicalein and PD98059 on p42/44 mitogen-activated protein kinase (MAPK) and Akt/protein kinase B (PKB) phosphorylation in rat vascular smooth-muscle cells. Cells were rendered quiescent for 24 h then incubated in the absence or presence of baicalein (50 μM), or PD98059 (10 μM), or LY294002 (10 μM) for 1 h, and vehicle or fetal bovine serum was added to the cells for 10 min. Cells were harvested for to detect phosphorylated-p42/44 MAPK and p42/44 MAPK (a) and phosphorylated-Akt/PKB and Akt (b) using Western blot detection, as described in Materials and methods. Data are expressed as mean ± standard error of four determinations. # P < 0.01 compared with basal group; *P < 0.05 and **P < 0.01 compared with control group
Baicalein blocks serum-mediated IκB-α degradation and NF-κB activation
It is well established that the NF-κB activity is regulated by IκB proteins and that phosphorylation and degradation of IκB-α results in activation of NF-κB. As shown in Fig. 6a, exposure of quiescent cells to serum for 10 min stimulated a profound increase of IκB-α phosphorylation; both baicalein and PD98059 (30 μM) significantly inhibited the serum-induced effect. Additionally, the treatment of quiescent cells with serum for 60 min evoked a marked degradation of IκB-α. This serum action also completely reversed in the presence of baicalein or PD98059 (Fig. 6b). Furthermore, we determined the DNA binding activity of NF-κB by EMSA using an oligonucleotide containing NF-κB consensus sequence. Data showed that both baicalein and PD98059 effectively inhibited serum-induced DNA binding activity of NF-κB (Fig. 6c).
Effect of baicalein and PD98059 on phosphorylation (a) and degradation (b) of inhibitor kappa B alpha (IκB-α) and nuclear factor kappa B (NF-κB) deoxyribonucleic acid (DNA) binding activity (c). Cells were rendered quiescent for 24 h, treated with or without the indicated agent for 1 h, and then vehicle or fetal bovine serum (FBS) was added for 10 min (a) or 1 h (b, c). After treatment, total cellular proteins were extracted to detect IκB-α phosphorylation and degradation using immunoblot by specific antibodies (a, b). The nuclei were extracted, and electrophoretic mobility shift assay was performed to detect the DNA binding activity of NF-κB (1 basal, 2 FBS control, 3 baicalein, 4 PD98059, 5 p65 antibody), as described in Materials and methods. Data are expressed as mean ± standard error of three determinations. # P < 0.01 and **P < 0.01 compared with basal and control, respectively
Discussion
Herbal medicines used by ancient people for thousands of years are now being manufactured in many countries as drugs with standardized quality and quantities of ingredients. Baicalein, one of the major flavonoids contained in the dried roots of S. baicalensis, which is widely used in traditional Chinese medicine as a treatment for inflammation, fever, and allergic diseases (Lai et al. 2003). In our laboratory, we have examined many of purified compounds and crude extracts from herbal medicines of Chinese medicinal prescriptions to find out their active component against VSMCs proliferation (Pan et al. 2003; Guh et al. 1996), as it is a causative factor in several vascular diseases, such as atherosclerosis and restenosis after acute vascular injury.
The in vivo animal model of balloon injury has been the most frequently used in restenosis study. Rats are regularly used because of the reproducible major formation of intimal hyperplasia within 2 weeks after balloon injury (Clowes and Schwartz 1985). Our data showed for the first time that baicalein has an in vivo antiproliferation activity to block intimal thickening in the rat model. When baicalein was administrated orally, the conjugated metabolite appeared in the bloodstream within 10 min, indicating very rapid absorption and simultaneous glucuronidation/sulfation. When compared with intravenous bolus administration with dose correction, the absolute absorption was 40% (Lai et al. 2003).
As baicalein effectively inhibited neointima formation after balloon injury, the in vivo effect would be carried out to examine its therapeutic mechanism. Although baicalein has been reported to have in vitro antiproliferative activity in VSMCs (Huang et al. 1994; Dethlefsen et al. 1994; Natarajan et al. 1994; Nishio and Watanabe 1997; Preston et al. 2006), the exact molecular mechanisms are not known. In our study, baicalein exhibited a marked antiproliferative effect in VSMCs using [3H]thymidine incorporation, MTT assay, and flow cytometric assessment. We tested the hypothesis that baicalein could inhibit VSMC proliferation as it did in endothelial cells or tumor cells. There are two reasons indicating that the antiproliferative effects of baicalein is not contributed to the inhibition of lipoxygenase. One is that the IC50 value of the antiproliferative effect (about 10 μmol level) is 30–100 times that of lipoxygenase inhibition (about 0.3 μM). The other is that lipoxygenase metabolites 12- and 15-HETE, either alone or in combination, could not induce the proliferative effect. They also could not reverse the antiproliferative action of baicalein, suggesting that the antiproliferative effect of baicalein was not via cellular lipoxygenase inhibition.
It has been suggested that several second messengers could inhibit VSMC proliferation, such as cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), and some prostaglandins (Hayashi et al. 2000; Kronemann et al. 1999). In our study, baicalein could not effectively induce the formation of cAMP and cGMP, and the exposure of 10 μM indomethacin (a potent cyclooxygenase inhibitor) did not influence the antiproliferative action of baicalein (data not shown). This indicates that the antiproliferative effect of baicalein is not simply through generation of the above second messengers. Furthermore, reactive oxygen species (ROS) are suggested to regulate the cell proliferative effect (Kamata and Hirata 1999), and baicalein was found to exhibit the free radical scavenging activity in a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (about 90% scavenging activity). However, in our unpublished data, serum induced little generation of ROS. This rules out the possibility that the free radical scavenging activity of baicalein mediates its antiproliferative action.
Cell-cycle control is a highly regulated process that involves a complex cascade of events. Modulation of the expression and function of cell-cycle regulatory proteins provides an important mechanism for inhibiting cell growth (Nigg 1995). Several reports have shown that baicalein caused an apparent accumulation of prostate cancer cells in the G1 phase (Chen et al. 2001a, b), increased S phase in lung cancer cells (Lee et al. 2005), and elevated G2/M phases in bladder and hepatoma cancer cell lines (Chao et al. 2007; Chang et al. 2002). Our results indicated that baicalein suppressed serum-induced VSMC proliferation and blocked serum-induced progression of the VSMC cell cycle from the G1 to S phase. Data from primary VSMCs indicate that cyclin D1 plays a regulatory role in progression of the cell cycle at the G1/S transition. In this study, we found that the levels of cyclin D1 protein but not other cell-cycle regulatory proteins, such as cyclin E, CDK-2, CDK-4, and Rb, in serum-induce VSMCs were decreased by baicalein. We suggest that the inhibitory effect of baicalein on VSMC proliferation is related to the arrest of cell-cycle progression.
It has been suggested that MAPK-mediated signaling regulates cell-cycle progression induced by growth factors. Nofer and the colleagues reported that in response to stimulation, increased protein expressions of cyclin D1 and cyclin E were also induced in VSMC (Nofer et al. 2001). In our study, both baicalein and PD98059 significantly inhibited MAPK activation. However, only baicalein profoundly reduced cyclin D1 expression, indicating that there existed an undefined modulator, which was regulated differently by baicalein and PD98059.
Additionally, MAPK activation induced by growth factors also causes the translocation of NF-κB to the nucleus (Hoshi et al 2000). This early event, within the first 2 h, regulates the cell proliferative effect in VSMCs. In our study, we determined the effect of baicalein and PD98059 on serum-induced NF-κB activation. Initially, we examined their effects on IκB-α phosphorylation and its following degradation, as these two events are essential for nuclear translocation and activation of NF-κB. Both baicalein and PD98059 significantly inhibited serum-induced IκB-α phosphorylation and degradation and therefore blocked NF-κB nuclear translocation. There are several lines of evidence suggesting that p42/44 MAPK plays a role in NF-κB activity regulation in VSMCs (Hoshi et al. 2000). The blockade of NF-κB-related cascade might be explained by the inhibition of MAPK-mediated signaling in our work.
Recently, it was suggested that the PI 3-kinase-mediated pathway plays a key role in cyclin D1 expression and entrance of the S phase in a cell cycle (Gille and Downward 1999). Furthermore, PI 3-kinase-activated Akt/PKB phosphorylation is sufficient to induce E2F transcriptional activity, and inhibition of PI 3-kinase inhibits the cyclin/CDK-mediated pathway (Brennan et al. 1997). This reveals the significance of PI 3-kinase/PKB-involved signaling on the regulation of the cell-cycle machinery. Our data showed that baicalein other than PD98059 significantly inhibited serum-induced activation of PI 3-kinase, explaining its distinct regulation on cyclin D1 expression. Moreover, PD98059 exhibited a complete blockade of the MAPK pathway and then directed serum stimulation to the PI 3-kinase-mediated pathway, resulting in the increase of Akt/PKB phosphorylation (Fig. 5).
In conclusion, we suggest that baicalein exhibits an antiproliferative effect to serum action via inhibition of p42/44 MAPK, NF-κB transcription factor, PI 3-kinase, and cell-cycle machinery. Baicalein also inhibits intimal hyperplasia after balloon vascular injury in the rat, indicating the therapeutic potential for treating restenosis after arterial injury.
References
Adhikari N, Charles N, Lehmann U, Hall JL (2006) Transcription factor and kinase-mediated signaling in atherosclerosis and vascular injury. Curr Atheroscler Rep 8:252–260
Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D (1996) Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest 97:1715–1722
Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA (1997) Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity 7:679–689
Butenko IG, Gladtchenko SV, Galushko SV (1993) Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesis in vitro by flavonoid baicalein from Scutellaria baicalensis georgy roots. Agents Actions 39:C49–C51
Cercek B, Yamashita M, Dimayuga P, Zhu J, Fishbein MC, Kaul S, Shah PK, Nilsson J, Regnstrom J (1997) Nuclear factor-kappaB activity and arterial response to balloon injury. Atherosclerosis 131:59–66
Chang WH, Chen CH, Lu FJ (2002) Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med 68:128–132
Chao JI, Su WC, Liu HF (2007) Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and surviving associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther 6:3039–3048
Chen F, Castranova V, Shi X (2001a) New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 159:387–397
Chen S, Ruan Q, Bedner E, Deptala A, Wang X, Hsieh TC, Traganos F, Darzynkiewicz Z (2001b) Effects of the flavonoid baicalein and its metabolite baicalein on androgen receptor expression, cell cycle progression and apoptosis of prostate cancer cell lines. Cell Prolif 34:293–304
Clowes AW, Schwartz SM (1985) Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res 56:139–145
Courtois G, Gilmore TD (2006) Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25:6831–6843
Dethlefsen SM, Shepro D, D’Amore PA (1994) Arachidonic acid metabolites in bFGF-,PDGF-, and serum-stimulated vascular cell growth. Exp Cell Res 212:262–273
Gille H, Downward J (1999) Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem 274:22033–22040
Golias CH, Charalabopoulos A, Charalabopoulos K (2004) Cell proliferation and cell cycle control: a mini review. Int J Clin Pract 58:1134–1141
Griendling KK, Ushio-Fukai M, Lassegue B, Alexander RW (1997) Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension 29:366–370
Guh JH, Yu SM, Ko FN, Wu TS, Teng CM (1996) Antiproliferative effect in rat vascular smooth muscle cells by osthole, isolated from Angelica pubescens. Eur J Pharmacol 298:191–197
Hayashi S, Morishita R, Matsushita H, Nakagami H, Taniyama Y, Nakamura T, Aoki M, Yamamoto K, Higaki J, Ogihara T (2000) Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension 35:237–243
Hong T, Jin GB, Cho S, Cyong JC (2002) Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med 68:268–271
Hoshi S, Goto M, Koyama N, Nomoto K, Tanaka H (2000) Regulation of vascular smooth muscle cell proliferation by nuclear factor-kappaB and its inhibitor, I-kappaB. J Biol Chem 275:883–889
Huang HC, Wang HR, Hsieh LM (1994) Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol 251:91–93
Kamata H, Hirata H (1999) Redox regulation of cellular signalling. Cell Signal 11:1–14
Katz M, Amit I, Yarden Y (2007) Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta 1773:1161–1176
Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H (2000) Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy 30:501–508
Kronemann N, Nockher WA, Busse R, Schini-Kerth VB (1999) Growth-inhibitory effect of cyclic GMP- and cyclic AMP-dependent vasodilators on rat vascular smooth muscle cells: effect on cell cycle and cyclin expression. Br J Pharmacol 126:349–357
Landry DB, Couper LL, Bryant SR, Lindner V (1997) Activation of the NF-kappa B and I kappa B system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am J Pathol 151:1085–1095
Lee HZ, Leung HW, Lai MY, Wu CH (2005) Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res 25:959–964
Lai MY, Hsiu SL, Tsai SY, Hou YC, Lee Chao PD (2003) Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J Pharm Pharmacol 55:205–209
Lu PH, Kung FL, Kuo SC, Chueh SC, Guh JH (2006) Investigation of anti-tumor mechanisms of K2154: characterization of tubulin isotypes, mitotic arrest and apoptotic machinery. Naunyn Schmiedebergs Arch Pharmacol 374:223–233
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Mehdi MZ, Azar ZM, Srivastava AK (2007) Role of receptor and nonreceptor protein tyrosine kinases in H2O2-induced PKB and ERK1/2 signaling. Cell Biochem Biophys 47:1–10
Natarajan R, Gonzales N, Lanting L, Nadler J (1994) Role of the lipoxygenase pathway in angiotensin II-induced vascular smooth muscle cell hypertrophy. Hypertension 23:I142–147
Nigg EA (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays 17:471–480
Nishio E, Watanabe Y (1997) Role of the lipoxygenase pathway in phenylephrine-induced vascular smooth muscle cell proliferation and migration. Eur J Pharmacol 336:267–273
Nofer JR, Junker R, Pulawski E, Fobker M, Levkau B, von Eckardstein A, Seedorf U, Assmann G, Walter M (2001) High density lipoproteins induce cell cycle entry in vascular smooth muscle cells via mitogen activated protein kinase-dependent pathway. Thromb Haemost 85:730–735
Pan SL, Huang YW, Guh JH, Chang YL, Peng CY, Teng CM (2003) Esculetin inhibits Ras-mediated cell proliferation and attenuates vascular restenosis following angioplasty in rats. Biochem Pharmacol 65:1897–1905
Peng CY, Pan SL, Guh JH, Liu YN, Chang YL, Kuo SC, Lee FY, Teng CM (2004) The indazole derivative YD-3 inhibits thrombin-induced vascular smooth muscle cell proliferation and attenuates intimal thickening after balloon injury. Thromb Haemost 92:1232–1239
Pidgeon GP, Kandouz M, Meram A, Honn KV (2002) Mechanism controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res 62:2721–2727
Preston IR, Hill NS, Warburton RR, Fanburg BL (2006) Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 290:L367–L374
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Sekiya K, Okuda H (1982) Selective inhibition of platelet lipoxygenase by baicalein. Biochem Biophys Res Commun 105:1090–1095
Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, Yuan CS (2002) Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol 282:H999–H1006
Wang HL, Kilfeather SA, Martin GR, Page CP (2000) Effects of tetrandrine on growth factor-induced DNA synthesis and proliferative response of rat pulmonary artery smooth muscle cells. Pulm Pharmacol Ther 13:53–60
Zhang W, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18
Acknowledgments
This work was supported by research grants of the National Science Council of the Republic of China (NSC-96–2628-B002–109-MY3 and NSC96–2321-B-002–031-MY2).
Author information
Authors and Affiliations
Corresponding authors
Additional information
C.-Y. Peng and Y.-W. Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Peng, CY., Pan, SL., Huang, YW. et al. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-κB activity in vascular smooth-muscle cells. Naunyn-Schmied Arch Pharmacol 378, 579–588 (2008). https://doi.org/10.1007/s00210-008-0328-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-008-0328-1