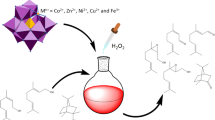
Abstract
Keggin heteropolyacids (i.e. H3PW12O40, H3PMo12O40 and H4SiW12O40) were converted to potassium lacunary salts and afterwards their vacancy were filled by metal cations (i.e. Cu2+, Co2+, Fe3+, Ni2+ or Al3+). These solid catalysts were assessed on oxidation of benzaldehyde to benzoic acid by hydrogen peroxide. Remarkably, among heteropoly salts investigated, the K6SiW11CoO39 was the most active catalyst. High conversion (ca. 90%) and benzoic acid selectivity (ca. 90%) were achieved. The activity of solid catalyst remained unaltered after successive cycles of reuse. Effect of catalyst nature, temperature, reactants concentration on conversion and selectivity were assessed.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Benzoic acid has been also widely used as raw material to industrial production of cosmetics, fibers, plasticizers, dyestuffs [1]. At an industrial scale, benzoic acid has been produced through toluene oxidation in liquid or vapor phase, in a one pot-process that involves expensive stoichiometric oxidant and high reaction temperature; however, only low yields are achieved [2].
Aldehyde oxidation to carboxylic acids is one key step for synthesis of manifold fine chemicals useful for pharmaceutical and fragrances industries, material science or bioorganic chemistry, and has been traditionally accomplished by hazardous strong stoichiometric metal oxidants that are toxic and unfriendly environmentally [3,4,5,6].
To overcome the drawbacks of stoichiometric oxidation processes, the use of green oxidants together with recyclable solid catalysts may effectively make the production of benzoic acid from benzaldehyde more economic and benign to the environment. Indeed, in last decades the homogeneous or heterogeneous catalysts besides clean oxidants have gradually replaced stoichiometric oxidation reagents.
Different than molecular oxygen, hydrogen peroxide is liquid, nonflammable, and atom efficient [7]. In addition, it is also inexpensive and a green acceptable oxidant, because produces only water as a by-product. In particular, hydrogen peroxide has been successfully used to oxidizing aldehydes, but it always requires the presence of metallic salts or oxides in order to allow that the oxygen-transfer reactions to proceed [8,9,10,11]. Nevertheless, some of based peroxide methods require additives to a rigorous pH control, the use of phase transfer catalysts or even toxic solvents [12, 13].
Solid supported catalysts with a high surface area are able to activate hydrogen peroxide in solution, and are an attractive option to oxidizing of alcohols and aldehydes [14,15,16]. Among the different supported catalysts used to activate hydrogen peroxide, Keggin-type polyoxometalates have received significant attention due to their versatility and diversity structural, and have been anchored or impregnated in a variety of supports [17,18,19]. Although the immobilization of homogeneous catalyst on solid support remains an area extensively explored, there are disadvantages that motivate the search for alternatives.
Recently, Farina et al. pointed some drawbacks of supported catalysts, which deserves be highlighted herein; the first one is that several dopants commonly supported on solids are unstable under the reaction conditions, and suffer leaching or deactivation during the reactions [20]. Secondly, in some cases, occurs a decreasing on the rate and selectivity of solid-catalyzed reactions, if it is compared the reactions with the soluble catalyst. The last and more important issue is that the immobilization process generates an extra-cost that is added to the process, in addition to time spent to synthesize both support and solid-supported catalyst.
An alternative to the supported catalysts is use of insoluble lacunary heteropoly salts as solid catalysts [21,22,23]. In special, when transition metal are included in the structure of these lacunary salts, the activity of Keggin-type polyoxometalates can be significantly improved, resulting in active and selective solid catalysts, which can be effectively recovered and reused [24,25,26].
In present study, we have used metal substituted Keggin-type heteropoly salts as catalysts to oxidizing benzaldehyde to benzoic acid with hydrogen peroxide. The main feature of this manuscript is correlating the activity of potassium salts of metal substituted Keggin HPAs substituted with their structural properties and chemical composition. To do it, we have assessed the effects of main reaction parameters on conversion and reaction selectivity. In addition, the issues of leaching and reusability of catalyst were also assessed.
2 Experimental Section
2.1 Chemicals
All the reagents and chemicals were acquired from commercial sources (Sigma Aldrich) and used without any further purification.
2.2 Synthesis of Catalysts
K8−xSiW11Mx+O39 (Mx+ = Cu2+, Fe3+, Co2+, Ni2+ and Al3+) HPAs salts were prepared in according with the modified method described in the literature [21, 27]. A scheme of catalysts synthesis starting from precursor metal nitrate and silicotungstic heteropolyacid is depicted in Scheme 1.
To an aqueous solution containing H4SiW12O40·n⋅H2O (ca. 6.29 mmol; 100 mL), it was added KCl (ca. 13.4 mmol) with vigorous stirring. The slow addition of an aqueous solution of KHCO3 (ca. 1 molL−1) adjusted the pH to 5.5. The resulting solution was filtered to take out insoluble material (when required) and then it was concentrated on a rotary evaporator at 313 K to give a white precipitate. The white solid (K8SiW11O39·nH2O) was separated by filtration and then it was dried under ambient conditions [28].
The metal cation was directly incorporate into the framework of lacunar heteropolyanion (i.e., SiW11O398−). To do it, 5 g of K8SiW11O39 salt was dissolved in 30 ml of deionized water (ca. 353 K). Afterward, it was slowly added an appropriate amount of aqueous solution containing metal precursor and vigorously stirred during 1 h. The resulting transparent solution was cooled to room temperature. Upon adding the transparent solution to the 100 ml of methanol–ethanol mixture (1:1 volume ratio), a precipitate was immediately formed. The solid product was filtered, washed with methanol and dried in vacuum oven overnight at 353 K, providing the K8−xSiW11Mx+O39 solid catalyst (Mx+ = Cu2+, Fe3+, Co2+, Ni2+ and Al3+).
All the catalysts were heated to 473 K for 3 h in air prior the characterization steps and catalytic tests. The same procedure was used to synthesizing others metal substituted catalysts (i.e., K7−xPW11Mx+O39 or K7−xPMo11Mx+O39).
2.3 Catalysts Characterization
X-rays diffraction pattern of the powdered catalysts was recorded with a X-ray Diffraction System model D8-Discover Bruker using Ni filtered Cu-kα radiation (λ = 1.5418 Å), operating at 40 kV and 40 mA. The measurements were performed in step of 0.05° with a counting time of 1.0 s in the 2θ range of 5°–80°.
FT-IR/ATR spectra of the heteropolyacid salt catalysts were recorded on an FT-IR Varian 660 spectrometer with reflectance accessory utilizing KBr plates under ambient conditions.
Catalysts acidity was measured by potentiometric titration in a potentiometer Bel, model W3B, in accordance with the procedure reported by Pizzio et al. [29]. Typically, an adequate amount of HPA was suspended in CH3CN and stirred by 24 h. The amount of acid sites was determined by titration with n-butylamine solution in toluene (ca. 0.025 molL−1).
The textural properties of salts were studied by H2 desorption/adsorption using NOVA 1200e High Speed, Automated Surface Area and Pore Size Analyzer Quantachrome Instruments. The samples were previously degassed by 1 h. The specific surface area was calculated by Brunauer–Emmett–Teller equation applied to the desorption/adsorption isotherms.
Scanning electron microscopy (SEM) images were obtained using a JEOL JSM-6010/LA microscope. The SEM equipment was equipped with an energy dispersive spectrometry system (EDS) for analysis of the sample chemical composition.
2.4 Catalytic Tests
Typically, a 25 mL three-necked glass flask, equipped with a sampling system and a reflux condenser was charged with CH3CN (ca. 10 mL), benzaldehyde (ca. 6.0 mmol), and an adequate amount of HPA salt catalyst. The reaction was carried out using magnetic stirring and heating to 333 K temperature for over 8 h.
The effects of main reaction parameters (i.e. reactants stoichiometry, temperature, type and catalyst load) were investigated. Blank-reactions were performed for each molar proportion. The activity of the most active metal exchanged lacunar HPA salt was compared to the precursors.
The reaction progress was followed by GC analysis of samples periodically collected (GC Shimadzu, capillary column, FID). To recycling the catalyst, after the end of the reaction, the suspension was centrifuged and solid removed by filtration, three times washed with CH3OH, dried in an oven at 373 K, and reused in another catalytic run.
3 Results and Discussion
3.1 Catalysts Characterization
3.1.1 FT-IR Spectroscopy Analyses
The FT-IR study was performed to assess whether ok Keggin anion structure is retained or not during the course of the removal of tungsten atom and the introduction of metal cation into heteropolyacid. So, the FT-IR spectra of silicotungstic Keggin heteropolyacid, intermediate salts and cobalt(II) substituted lacunar heteropoly salt are shown in the Fig. 1.
The fingerprint region of FT-IR spectrum of the H4SiW12O40 heteropolyacid shows four main bands at 1010, 980 and 920 and 790 cm−1. In general, literature describes five typical bands for silicotungstic anion [30]. For instance, Pizzio and Blanco have found that FT-IR spectrum of H4SiW12O40 displayed the absorption bands at 1020, 982, 926, 884 and 778 cm−1 [31]. Holclajtner-Antunonovi et al. found the following vibration frequencies for these bands characteristic for the Keggin anion of silicotungstic acid: 1017, 981, 928, 880 and 785 cm−1. Those authors attributed these bands to the vibrations υas(W=Od), υas(Si–Oa), υas(W–Ob–W) and υas (W–Oc–W), respectively [32]. The absorption peak at 982 cm−1 (vas W=O) shifting to 976 cm−1 was probably attributed to the effect of stannous ion at a larger radius [33].
The first intermediate (i.e., K4SiW12O40) presents these bands at the same wavenumber. However, as can be seen in Fig. 1a, there was a shift to the region of higher energy. When the pH was adjusted to 5.5, one unit of WO was removed from Keggin anion, and the lacunary salt became majority in the equilibrium.
When lacunary salts are synthesized from phosphotungstic or phosphomolybdic heteropolyacids, a splitting occur for absorption band corresponding to the vibration of the P–O bond, generating absorption bands at wavenumber 1081 and 1037 cm−1 [34]. However, the same it was not observed when lacunar silicotungstic salts were synthesized.
After the insertion of Co(II) cation into Keggin anion structure, we realize a shifting of stretching frequencies toward lower wavenumber in relation to the precursor lacunary potassium salt. Patel et al. have attributed this shifting to the introduction of transition metal into octahedral lacuna [24]. The presence of nitrate anions may be also confirmed by characteristic absorption bands of this anion around 1350 cm−1 [35].
To comparison, the FT-IR spectra of cobalt substituted HPAs (K5PW11CoO39, K6SiW11CoO39), as well as their precursor heteropolyacid are displayed in Fig. 2. Spectral data of K6PMo11CoO39 were similar to the K6PW11CoO39 and were omitted by simplification.
The spectrum of the bulk H3PW12O40 shows the following characteristic absorption bands: νas(P–Oa) at 1080 cm−1, νas(W=Od) at 960 cm−1 an νas(W–Ob–W inter octahedral) at 886 cm−1. Herein, the vasW–O–W intra-octahedral that generally is observed close to 810 cm−1 was not observed. In a Keggin type unit, Oa refers to the oxygen atom common to PO4 tetrahedron and one group W3O13; conversely, Ob links two W3O13 groups; Oc binds two octahedral WO6 inside a W3O13 group and Od is the terminal oxygen of Keggin anion [36].
The splitting of absorption bands corresponding to the stretching of P–O bond was observed at frequencies of 900 and 1050 cm−1, endorsing thus the formation of lacunary potassium salts [37, 38]. This splitting is attributed to the decreasing on symmetry of group PO4, resulting from removal of unit WO [39]. The presence of bands at frequency below the 500 cm−1 in both cobalt substituted potassium salts as well as the band at wavenumber around 1350 cm−1 indicates the presence of Co2+ and nitrate ions, respectively.
The Fig. 3 shows that all the FT-IR spectra of metal substituted potassium salts presented the absorption bands characteristic for NO3− anions. In addition, similarly to the observed in FT-IR of cobalt salts, the presence of bands at frequency below the 500 cm−1 suggest that metal cation was included into Keggin anion.
3.1.2 Analyses of X-ray Diffraction Patterns
First of all, it noteworthy that water molecules number per unitary cell strongly affects the XRD patterns of heteropolyacids. For instance, Derrick et al. recorded data from silicotungstic acid and showed the existence of stable phases of composition H4SiW12O40⋅n H2O where n = 24, 14, 6 and 0 [40]. We prepared H4SiW12O40·6H2O heteropolyacid to 150 °C and through direct comparison of DRX spectrum conclude that their data fits well with those recorded by us. It means that when we heated the H4SiW12O40 to 200 °C no significant changes happened into their structure.
The powder X-ray diffraction patterns of K4SiW12O40, K8SiW11O39, and K6SiW11CoO39 indicates that the synthesized salts were crystalline in nature (Fig. 4). Heteropolyacid H4SiW12O40 shows main XRD peaks in the region of 5° < 2θ < 30° corresponds to the Keggin anion.
Comparing diffraction patterns of synthesized salt K8SiW11O39 with literature data (JCPD file no. 34-0205) we have found that they perfectly correspond to the cubic phase [41].
From Fig. 4, it is clear that, all the three synthesized materials show similar peaks pattern as their parent H4SiW12O40. It is suggestive that the Keggin anion is present in their framework. Nonetheless, the introduction of potassium cations into anions structure, made appear new diffraction lines along all spectra in the range of angles between 5° and 60°. The potassium derivative lacunar salt as well as the cobalt substituted salt displayed a number of diffraction peaks greater than both precursor acid and saturated potassium salt (Fig. 4).
The shifting in 2θ value for Co(II) substituted lacunar salt compare to the other salts and the appearance of strong diffraction peak at 2θ equal to 28° may be due to the introduction of cobalt cation into lacuna [24]. This shifting to high angle (i.e. 2 θ) is an evidence that distance between crystal planes was decrease; it means that unitary cell was also decreased.
The Fig. 5a, b present the powder DRX patterns of silicotungstic acid lacunary potassium salts after before and after the introduction of M3+ cations (a) or M2+ (b), respectively.
It can be observed that the introduction of M3+ cations (i.e., Fe3+ and Al3+) in the Keggin anion of the silicotungstic potassium salts reduced both the number and the intensity of diffraction lines if compared to the precursor (i.e. lacunary potassium salt).
On the other hand, regardless M2+ cation introduced in the Keggin anion structure, all the lacunar salts synthesized were highly crystalline and presented the characteristics peaks of silicotungstic Keggin anion. The number of diffraction peaks was higher than that observed for acid or non-lacunar potassium salt. Depending on the type of metal cation introduced, new diffraction signals were observed. This effect can attributed to change of arrange of structure of unitary cell, for instance of cubic to hexagonal and so on.
3.1.3 Potentiometric Curves
The potentiometric curves of metal substituted silicotungstic Keggin heteropolyacid salts are displayed in the Fig. 2. It can be seen a strong decrease on initial electrode potential value, immediately after the addition of base minimum volume, indicating that the protons were virtually removed.
Moreover, after this initial period the potential remained practically constant. This behavior is completely distinct than precursor heteropolyacid ones (Fig. 6).
This fact is useful to understand because the FT-IR spectra of potassium heteropoly salts displayed an absorption band at 1620 cm−1, which is attributed to the cation di-hydronium (H5O2+). It means that even after the tentative of replacing the protons, a minimum portion of acidity persist, yet. Nonetheless, this remaining acidity is so small. For this reason, a minimum volume of n-butylamine was enough to completely neutralize Brønsted acid sites.
3.1.4 N2 Adsorption Studies
BET surface areas, size and pores volume are shown in Table 1. In general, the surface area of heteropolyacids increased after exchanges of protons by potassium cations. The data of surface areas and porosity of the phosphotungstic and phosphomolybdic catalysts have presented the same trend that silicotungstic catalysts, and were omitted by simplification.
Although Pizzio et al. have reported that the surface area of potassium salts are very lower than cesium salts, for instance, ca. 5 m2g−1 (K3.8H0.2SiW12O40) whereas 169 m2g−1 (Cs3.8H0.2SiW12O40), the surface area of potassium salts synthesized herein was two times higher, nearly 12.5 m2g−1 (Table 1) [31].
Table 1 shows the results of the texture parameters of Keggin silicotungstic potassium salts substituted or not by metal cations. The introduction of metal cations slightly reduced the surface area of potassium salts (from 12 to 10 m2g−1), excepted in the case of iron salt. The pore volume (< 0.02 cm3 g−1) as well as the pore sizes showing values in the region between 1.10 and 1.50 nm confirm that all the catalysts are microporous (i.e., microporous solids < 2 nm, mesoporous solids between 2 and 50 nm, macroporous solids > 50 nm). These porosity and isothermal adsorption characteristics for heteropolysalts are supported by the literature data [41].
The Fig. 7 displays the adsorption/desorption isotherms pore volume of the porous of silicotungstic catalysts. All have the same profile, indicating that the porosity type is the same for all synthesized catalysts. The isotherms reflect Type II characteristics, which are typical of not porous solids, suggesting that there was physic adsorption in multilayers [41].
The quick initial increase corresponds to the formation of the first layer; so, an increase in pressure forms the second layer of adsorbed molecules, followed by another layer. It was observed the total reversibility of the adsorption–desorption isotherm (absence of hysteresis cycle) for all catalysts, a condition that is present in these systems.
Thermogravimetric analysis of metal-substituted lacunary potassium heteropolyacid salts shows two region of loss weight; the first one before 200 °C assigned to loss of all water molecules (Fig. 8). The second one, attributed to decomposition of Si–O–W framework followed by the noticeable peak in DSC curves around 620–635 °C. The final products are oxides mixture.
The introduction of metal into Keggin anion resulted in a decreasing of temperature related to the lacunary salt framework decomposition, probably consequence of loss symmetry resulting from exchange of tungsten atom by a lower radium metal cation.
The data obtained via EDS analysis confirmed the samples chemical composition of all the catalysts synthesized. In general, only a slight difference between theoretical and experimental values was observed, regardless the metal substituted lacunary potassium silicotungstic salt. So, we omitted these data for simplification.
3.2 Catalytic Tests
In order to select the most active catalyst an initial screening of metal substituted lacunary potassium salt was done and the main results are displayed in Table 2.
Although oxidant excess, blank-reactions had a poor conversion (ca. 10%). Conversely, in the presence of silicotungstic catalysts the conversion and selectivity of reactions were dependent of catalyst nature. When catalysed by the precursor heteropolyacid, the reaction was highly selective to benzoic acid (ca. 96%), however, only a conversion of ca. 24% was reached. On the other hand, the introduction of metal in the Keggin anion structure improved the conversions, mainly when Ni2+ and more remarkably Co2+ were the cations introduced. Notwithstanding, different than H4SiW12O40, all the metal substituted lacunary catalysts were insoluble in the reaction solution.
Among the HPAs salts assessed, the K6SiW11CoO40 was the most active and selective (Entry 7, Table 2). Literature has described the activity of cobalt complexes in the oxidation of alcohol [40, 41].
Recently, Patel et al. have assessed the activity of (Co, Mn or Ni)-substituted-H3PMo12O40 lacunary salts on benzyl alcohol oxidation with hydrogen peroxide [25]. Those authors found that cobalt salt was the most active catalyst. Nonetheless, to achieve conversions around ca. 74%, a long reaction time was required (ca. 24 h), in reactions carried out at temperature of ca. 383 K and 1:3 alcohol: hydrogen peroxide proportion [25].
Due to higher efficiency of cobalt lacunary salt, we have decided investigate the effect of heteropolyanion on activity of cobalt catalysts. Thus, we carried out reactions in the presence of different cobalt lacunary salts and their respective precursor heteropolyacids (Fig. 9).
Heteropolyacids had a different behaviour in terms of conversion and selectivity on reactions of oxidation assessed. Although the H3PMo12O40 or H3PW12O40-catalyzed reactions have achieved conversions higher than that in the presence of H4SiW12O40, this late was the most selective catalyst for benzoic acid (Table 3). On the other hand, among the cobalt lacunar salts that containing the silicotungstic Keggin anion was the most active and selective.
An important observation is that if we compared the reactions catalysed by the cobalt lacunar salt and their precursors (i.e., Co(NO3)2 and H4SiW12O40) we can conclude that there was a synergism between Keggin anion and the Co2+ cation, which resulted in a very active catalyst.
To better understand the effects of modification on the heteropolyacid catalysts structure we carried out reactions with the all the precursor and synthesis intermediate of cobalt lacunar salt (Fig. 10).
The Fig. 8 show that the conversion of silicotungstic heteropolyacid into potassium salt (i.e. K4SiW12O40) followed by the conversion of this late into lacunar salt (i.e. K8SiW11O39) improved the conversion of oxidation reactions of benzaldehyde to benzoic acid. Tungsten catalysts have been active catalysts in oxidation reactions with hydrogen peroxide, where “peroxotungstate” intermediates are responsible by removal of proton or incorporation of the oxygen atom into substrates [40]. Thus, seems reasonable suppose that opening a vacancy into heteropolyanion structure enhance the activity of lacunar catalyst, favouring the oxidation of benzaldehyde.
The Co(NO3)2-catalyzed reaction achieved the lowest conversion (ca. 15%), although has been highly selective (entry 1, Table 3). However, the Fig. 10 shows clearly that the introduction of Co(II) cation into structure of lacunary Keggin anion improved notably its activity, without compromise the selectivity (Entry 7, K6SiW11CoO39, Table 3). This fact once more reinforce that possibly exists a synergic effect between Co2+ cation and Keggin silicotungstic anion.
Because the K6SiW11CoO39 catalyst was the most active and selective, it was selected to investigate the effect of main reaction parameters. Figure 11 show kinetic curves of reactions with different load of catalyst.
An increasing on catalyst load from 0.33 to 2 mol% resulted in an increase on both initial rate and final conversion of reactions. Nonetheless, above this load no beneficial effect was observed because the reactions carried out with 2.0 or 2.6 mol% had equal conversion rates and selectivity (Fig. 12).
The rate initial of reaction was increased by the increase on temperature, while the reaction selectivity toward benzoic acid varied of 85–100% (Fig. 13).
While the use of higher load oxidant did not affect the benzoic acid selectivity, the opposite happened with the conversion; a benzaldehyde: H2O2 proportion higher than 1:8 triggered a strong decreasing on conversion. Possibly, a leaching of active specie due to higher water amount generated increasing on aqueous hydrogen peroxide concentration in the reaction solution may be provoked this lowering.
The reuse and recycle of K6SiW11CoO39 catalyst was assessed (Table 4). The catalyst was successfully recovered and reused for three times without loser activity or selectivity.
Although high catalyst recovery rate, one other experiment was carried out in order to assure the heterogeneity of the reaction. The reaction was carried during 30 min; the catalyst was filtered and washed with heated CH3CN. After removal the catalyst, we monitored the reaction progress until complete 4 h (Fig. 14).
After the removal of catalyst, no significant conversion was observed, an indicative that it is a genuinely heterogeneous reaction.
4 Conclusions
In this paper, the oxidation of benzaldehyde by hydrogen peroxide was performed in the presence of a class of solid catalysts even little explored: cobalt(II) substituted silicotungstic heteropolyacid lacunary potassium salt. This solid Lewis acid-catalyzed reaction, benzaldehyde was selectively converted to benzoic acid (ca. 98%) with high conversion (ca. 90%), in reactions faster than those promoted by solid supported catalysts. This selective process is an attractive alternative to the solid supported-catalyzed process because avoid the laborious synthesis of both support and metal catalyst doped-support. Among metal exchanged heteropolyacid lacunar salts evaluated, K6SiW11CoO39 was the most active and selective. The lacunary catalysts containing metal cations were easily synthesized through stoichiometric reaction between precursor heteropolyacid and metal nitrate. These catalysts have addition advantages of being a solid and reusable without lost activity.
References
Chumbhale VR, Paradhy SA, Anilkumar M, Kadam ST, Bokade VV (2005) Chem Eng Res Des 83:75
Ilyas M, Sadiq M (2009) Catal Lett 128:337
Yuan ZH, Chen BZ, Zhao JS (2011) Chem Eng Sci 66:5137
Mukhopadhyay C, Datta A (2008) Catal Commun 9:2588
Sedelmeier J, Ley SV, Baxendale IR, Baumann M (2010) Org Lett 12:3618
Dash S, Patel S, Mishra BK (2009) Tetrahedron 65:707
Muzart J (2003) Tetrahedron 59:5789
Sato K, Hyodo M, Takagi J, Aoki M, Noyori R (2000) Tetrahedron Lett 41:1439
Yan H, Liu C, Luo G (2005) Pet Sci Technol 23:1511
Tandon PK, Baboo R, Singh AK, Purwar GM (2005) Appl Organomet Chem 19:1079
Bernini R, Coratti A, Provenzano G, Fabrizi G, Tofani D (2005) Tetrahedron 61:1821
Cong Z-Q, Wang CI, Chen T, Yin BZ (2006) Synth Comm 36:679
Jeong SY, Kim N, Lee JC (2014) Bull Korean Chem Soc 35:345
Zhou L, Dong B, Tang S, Ma H, Chen C, Yang X, Xu J (2013) J Energ Chem 22:659
Farsani MR, Yadollahi B (2014) J Mol Catal A 393:8
Dong BB, Zhang BB, Wu HY, Li SD, Zhang K, Zheng XC (2013) Micropor Mesopor Mat 176:186
Sousa JLC, Santos ICMS., Simões MMQ, Cavaleiro JAS, Nogueira HIS, Cavaleiro AMV (2011) Catal Commun 12:459
Li B, Ma W, Han C, Liu J, Pang X, Gao X (2012) Micropor Mesopor Mat 156:73
Chen Y, Cao Y, Zheng GP, Dong BB, Zheng XC (2014) Adv Powder Technol 25:1351
Hübner S, de Vries JG, Farina V (2016) Adv Synth Catal 358:3
Khenkin A, Neumann R (2002) Adv Synth Catal 344:1017
Ma B, Zhang Y, Ding Y, Zhao W (2010) Catal Commun 11:853
Balula SS, Santos ICMS., Cunha-Silva L, Carvalho AP, Pires J, Freire C, Cavaleiro JAS, de Castro B, Cavaleiro AMV (2013) Catal Today 203:95
Pathan S, Patel A (2013) Appl Catal A 459:59
Pathan S, Patel A (2011) Dalton Trans 40:348
Hill CL, Prosser-McCartha CM (1995) Coordin Chem Rev 143:407
Jonnevijlle F, Tourné CM, Tourné GF (1982) Inorg Chem 21:2751
Miura YK, Kamiya Y (2012) Chem Lett 41:331
Pizzio LR, Vásquez PG, Cáceres CV, Blanco MN (2003) Appl Catal A 256:125
Moffat JB (2001) Metal-oxygen clusters: the surface and catalytic properties of heteropoly oxometalates. Plenum, New York
Pizzio LR, Blanco MR (2007) Microporous Mesoporous Mat 103:40
Bajuk-Bogdanovi D, Holclajtner-Antunonovi I, Todorovi M, Mio UB, Zakakrzewska J (2008) J Serb Chem Soc 73:197
Rocchiccioli-Deltcheff C, Fournier M, Franck R, Thouvenot R (1983) Inorg Chem 22:207
Choi JH, Kim JK, Park DR, Kang TH, Song JH, Song IK (2013) J Mol Catal A 371:111
Da Silva MJ, Liberto NA, Leles LCA, Pereira UA (2016) J Mol Catal A 422:69
Kholdeeva OA, Vanina MP, Timofeeva MN, Maksimovskaya RI, Trubitsina TA, Melgunov MS, Burgina EB, Mrowiec-Bialon J, Jarzebski AB, Hill CL (2004) J Catal 226:363
Das S, Punniyamurthy T (2003) Tetrahedron Lett 44:6033
Hida T, Nogusa H (2009) Tetrahedron 65:270
Berry FJ, Derrick GR, Mortimer GR M (2014) Polyhedron 68:17
Dong X, Yua C, Wang D, Zhang Y, Wu P, Hu H, Xue G (2017) Matter Res Bull 85:152
Wu N, Li B, Ma W, Chunying H (2014) Micropor Mesopor Mat 186:155
Acknowledgements
The authors are grateful for the financial support from CAPES, CNPq and FAPEMIG (Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, M.J., de Andrade Leles, L.C., Natalino, R. et al. An Efficient Benzaldehyde Oxidation by Hydrogen Peroxide over Metal Substituted Lacunary Potassium Heteropolyacid Salts. Catal Lett 148, 1202–1214 (2018). https://doi.org/10.1007/s10562-018-2326-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2326-3