Abstract
Toluene oxidation over platinum supported on zirconia under solvent free conditions in a batch reactor was studied in the temperature range 60–90 °C. Molecular oxygen was used as oxidant. The catalyst was highly active and selective for benzoic acid formation. Some conversion to benzyl benzoate, trans-stilbene and methyl biphenyl carboxylic acid was also observed when the reaction was studied for >3 h. The reaction is taking place at considerably lower temperature than the reported solvent free oxidation reactions of toluene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Selective catalytic oxidation of toluene to corresponding alcohol, aldehyde and carboxylic acid by molecular oxygen is of great economical and industrial importance. Industrially, the oxidation of toluene to benzoic acid (BzOOH) with molecular oxygen is a key step for phenol synthesis in the Dow Phenol process and for ε-caprolactum formation in Snia-Viscosia process [1–7]. Toluene is also a representative of aromatic hydrocarbons categorized as hazardous material [8]. Thus development of methods for the oxidation of aromatics such as toluene is also important for environmental reasons. The commercial production of benzoic acid via the catalytic oxidation of toluene is achieved by heating a solution of the substrate, cobalt acetate and bromide promoter in acetic acid to 250 °C, with molecular oxygen, at high pressure. Although complete conversion is achieved, however, the use of acidic solvents and bromide promoter results in difficult separation of products and catalyst, large volume of toxic waste and equipment corrosion. The system requires very expensive, specialized equipments, fitted with extensive safety features. Operating under such extreme conditions consumes large amount of energy. Therefore, attempts are being made to make this oxidation more environmentally benign by performing the reaction in the vapor phase using a variety of solid catalysts [9, 10]. However, liquid-phase oxidation is easy to operate and achieve high selectivity under relatively mild reaction conditions. Many efforts have been made to improve the efficiency of toluene oxidation in the liquid phase, however, most investigation still focus on homogeneous systems using volatile organic solvents. Employing heterogeneous catalysts in liquid-phase oxidation of toluene without solvent would make the process more environmentally friendly. Bastock et al. have reported [11] the oxidation of toluene to benzoic acid in solvent free conditions using a commercial heterogeneous catalyst Envirocat EPAC in the presence of catalytic amount of carboxylic acid as promoter at atmospheric pressure. The reaction was performed at 110–150 °C, with oxygen flow rate of 400 mL/min. The isolated yield of benzoic acid was 85% in 22 h. Subrahmanyan et al. [12] have performed toluene oxidation in solvent free conditions using vanadium substituted aluminophosphate or aluminosilicates as catalyst. Benzaldehyde (BzH) and benzoic acid were the main products when tert-butyl hydro peroxide was used as the oxidizing agent while cresols were formed when H2O2 was used as oxidizing agent. Raja et al. [13, 14] have also reported the solvent free oxidation of toluene using zeolite encapsulated metal complexes as catalysts. Air was used as oxidant (3.5 MPa). The highest conversion (45.1%) was achieved with manganese substituted aluminum phosphate with high benzoic acid selectivity (83.4%) at 150 °C in 16 h. Li et al. [15, 16] have also reported manganese oxide and copper manganese oxide to be active catalysts for toluene oxidation to benzoic acid in solvent free conditions with molecular oxygen (1.0 MPa) at 190–195 °C. Recently it was observed in this laboratory [17] that when toluene was used as a solvent for benzyl alcohol (BzOH) oxidation by molecular oxygen at 90 °C in the presence of Pt/ZrO2 as catalyst, benzoic acid was obtained with 100% selectivity. The mass balance of the reaction showed that some of the benzoic acid was obtained from toluene oxidation. This observation is the basis of the present study for investigation of the solvent free oxidation of toluene using Pt/ZrO2 as catalyst.
2 Experimental
2.1 Material
ZrOCl2 · 8H2O (Merck, 8917), NH4OH (BDH, 27140), AgNO3 (Merck, 1512), PtCl4 (Acros, 19540), benzyl alcohol (Merck, 9626), benzaldehyde (Scharlu, BE0160), toluene (BDH, 10284), phenol (Acros, 41717), benzoic acid (Merck, 100136) and trans-stilbene (Aldrich, 13,993-9) were used as received.
H2 (99.999%) was prepared using hydrogen generator (GCD-300, BAIF). Nitrogen and Oxygen were supplied by BOC Pakistan Ltd. and were further purified by passing through traps (C.R.S.Inc.202268) to remove traces of water and oil. Traces of oxygen from nitrogen gas were removed by using specific oxygen traps (C.R.S.Inc.202223).
2.2 Catalyst Preparation and Characterization
Monoclinic zirconia was used as a support for platinum. It has been shown earlier that monoclinic zirconia was a more effective catalyst than tetragonal zirconia for alcohol oxidation in solvent free conditions [18, 19].
Zirconia [17–20] was prepared using an aqueous solution of zirconyl chloride with the drop wise addition of NH4OH for 4 h (pH 10) with continuous stirring. The precipitate was washed with triply distilled water using a Soxhlet’s apparatus for 24 h. The Cl−1 test with AgNO3 was found to be negative. Precipitate was dried at 110 °C for 24 h. After drying it was calcined with programmable heating at a rate of 0.5 °C/min to reach 950 °C and was kept at the same temperature for 4 h. Nabertherm C-19 programmed control furnace was used for calcinations. Supported Catalysts were prepared by incipient wetness technique. For this purpose calculated amount (wt%) of the precursor compound (PtCl4) was taken in a crucible and triply distilled water was added to make a paste. Then the required amount of the support (ZrO2) was mixed with it to make a paste. The paste was thoroughly mixed and dried in an oven at 110 °C for 24 h and then grounded. The catalyst was sieved and 80–100 mesh portions were used for further treatment. The grounded catalyst was calcined again at the rate of 0.5 °C/min to reach 750 °C or 950 °C and was kept at the same temperature for 4 h, after which it was reduced in H2 flow at 280 °C for 4 h.
BET surface area of the catalyst was determined using a Quanta chrome (Nova 2200e) surface area and pore size analyzer. Samples were degassed at 110 °C for 2 h prior to determination. XRD analyses were performed on a JEOL (JDX-3532) X-ray diffractometer using CuKα radiation with a tube voltage of 40 kV and 20 mA current. SEM was recorded on a JEOL-l 50 H Super Probe 733.
2.3 Reaction Procedure
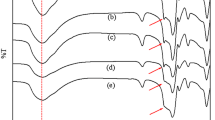
All the experiments were carried out in a magnetically stirred Pyrex glass three-necked batch reactor (Height 19 cm, ID 3 cm) provided with reflux condenser and a mercury thermometer, for measuring the reaction temperature. Reaction temperature was maintained by using heating tapes. A predetermined quantity of Toluene (10 mL) was taken in the reactor, Pt/ZrO2 (0.2 g) was added and O2 (at atmospheric pressure) was passed through the reaction mixture (usually at 40 mL min−1), at a fixed temperature, (60–90 °C). Samples were withdrawn from the reaction mixture at predetermined time intervals. The reaction mixture was filtered to remove the catalyst and analyzed. The changes in the concentration of toluene and its products benzyl alcohol, benzaldehyde, benzoic acid, benzyl benzoate, trans-stilbene and methyl biphenyl carboxylic acid were monitored by UV–VIS spectroscopy, gas chromatography and thin layer chromatography [15, 16, 21, 22]. Thin layer chromatographic analysis was carried out using standard chromatographic plates (Merck) with silica gel 60 F254 support (Merck, TLC: 105,554 and PLC: 113,793). Ethyl acetate (10%) in cyclohexane was used as eluent. Identification of products was made by comparison of the UV–Visible and FTIR spectra of the products with that of the standards. Shimadzu UV-160A was used for UV–Visible Spectra. Diffuse reflectance spectra of solids (trans-Stilbene) were recorded on Shimadzu, IRPrestigue-21, FTIR-8400S, using diffuse reflectance accessory [DRS-8000A]. Solid samples were diluted with KBr before measurement. The spectra were recorded with resolution of 4 cm−1 with 50 accumulations.
3 Results and Discussion
3.1 Catalyst Characterization
BET surface area was 65 and 183 m2 g−1 for ZrO2 and Pt/ZrO2, respectively. Figure 1 shows SEM images which reveal that the Pt/ZrO2 has smaller particle size than that of ZrO2 which may be due to further temperature treatment or reduction process. The high surface area of Pt/ZrO2 in comparison to ZrO2 could be due to its smaller particle size.
Figure 2a, b shows the diffraction pattern for uncalcined ZrO2 and ZrO2 calcined at 950 °C. Diffraction pattern for ZrO2 calcined at 950 °C was dominated by monoclinic phase (major peaks appears at 2θ = 28.18° and 31.38°) [23–25]. Figure 2c, d shows XRD patterns for a Pt/ZrO2 calcined at 750 °C both before and after reduction in H2. The figure revealed that Pt/ZrO2 calcined at 750 °C exhibited both the tetragonal phase (major peak appears at 2θ = 30.94°) and monoclinic phase (major peaks appears 2θ = 28.18° and 31.38°). The reflection was observed for Pt at 2θ = 39.79° which was not fully resolved due to small content of Pt (~1 wt%) as also concluded by Perez-Hernandez et al. [26].The reduction processing of Pt/ZrO2 affects crystallization and phase transition, resulting in certain fraction of tetragonal ZrO2 transferred to monoclinic ZrO2 as also reported else where [27]. However, the XRD pattern of Pt/ZrO2 calcined at 950 °C (Fig 2e, f) did not show any change before and after reduction in H2 and were fully dominated by monoclinic phase. However, a fraction of tetragonal zirconia was present as reported by Liu et al. [20].
3.2 Catalytic Activity
In this work we first studied toluene oxidation at various temperatures (60–90 °C), with oxygen or air passing through the reaction mixture (10 mL of toluene and 200 mg of 1(wt)% Pt/ZrO2) with continuous stirring (900 rpm). The flow rate of oxygen and air was kept constant at 40 mL/min. Table 1 present these results. The known products of the reaction were benzyl alcohol, benzaldehyde and benzoic acid. The mass balance of the reaction showed some loss of toluene (~1%). Conversion rises with temperature from 9.6% to 37.2%. The selectivity for benzyl alcohol is higher than benzoic acid at 60 °C. At 70 °C and above the reaction is more selective for benzoic acid formation70 °C and above. The reaction is highly selective for benzoic acid formation (>70%) at 90 °C. Reaction can also be performed in air where 18.8% conversion is achieved at 90 °C, with 25% selectivity for benzyl alcohol, 16.5% for benzaldehyde and 51.6% for benzoic acid. Comparison of these results with other solvent free systems shows that Pt/ZrO2 is very effective catalyst for toluene oxidation. Higher conversions are achieved at considerably lower temperatures and pressure than other solvent free systems [11–16]. The catalyst is used with out any additive or promoter. The commercial catalyst (Envirocat EPAC) requires trimethylacetic acid as promoter with a 1:1 ratio of catalyst and promoter [11]. The turnover frequency (TOF) was calculated as the molar ratio of toluene converted to the platinum content of the catalyst per unit time (h−1). TOF values are very high even at the lowest temperature of 60 °C.
3.2.1 Time Profile Study
The time profile of the reaction is shown in Fig. 3, where a linear increase in conversion is observed with the passage of time. An induction period of 30 min is required for the products to appear. At the lowest conversion (<2%) the reaction is 100% selective for benzyl alcohol (Fig. 4). Benzyl alcohol is the main product until the conversion reaches ~14%. Increase in conversion is accompanied by increase in the selectivity for benzoic acid. Selectivity for benzaldehyde (~20%) is almost unaffected by increase in conversion. This reaction was studied only for 3 h. The reaction mixture becomes saturated with benzoic acid, which sublimes and sticks to the walls of the reactor.
3.2.2 Effect of Oxygen Flow Rate
Effect of the flow rate of oxygen on toluene conversion was also studied. Figure 5, shows this effect. It can be seen that with increase in the flow rate both toluene conversion and selectivity for benzoic acid increases. Selectivity for benzyl alcohol and benzaldehyde decreases with increase in the flow rate. At the oxygen flow rate of 70 mL/min the selectivity for benzyl alcohol becomes ~0 and for benzyldehyde ~4%. This shows that the rate of reaction and selectivity depends upon the rate of supply of oxygen to the reaction system.
3.2.3 Appearance of Trans-Stilbene and Methyl biphenyl carboxylic acid
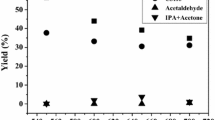
Toluene oxidation was also studied for the longer time of 7 h. In this case 20 mL of toluene and 400 mg of catalyst (1% Pt/ZrO2) was taken and the reaction was conducted at 90 °C as described earlier. After 7 h the reaction mixture was converted to a solid apparently having no liquid and therefore, the reaction was stopped. The reaction mixture was cooled to room temperature and more toluene was added to dissolve the solid and then filtered to recover the catalyst. Excess toluene was recovered by distillation at lower temperature and pressure until a concentrated suspension was obtained. This was cooled down to room temperature, filtered and washed with a little toluene and sucked dry to recover the solid. The solid thus obtained was 11.2 g. Preparative TLC analysis showed that the solid mixture was composed of five substances. These were identified as benzaldehyde (yield, mol%, 2.2%), benzoic acid (29.6%), benzyl benzoate (3.4%), trans-stilbene (5.3%) and 4-methyl-2-biphenylcarboxylic acid (10.8%).The rest (~4%) could be identified as tar due to its black color. Figure 6 shows the conversion of toluene and the yield (mol%) of these products. Trans-stilbene and methyl biphenyl carboxylic acid were identified by their melting point and UV–Visible and IR spectra. The Diffuse Reflectance FTIR spectra (DRIFT) of trans-stilbene (both of the standard and experimental product) is given in Fig. 7. The oxidative coupling of toluene to produce trans-stilbene has been reported widely [28–32]. Kai et al. [32] have reported the formation of stilbene and bibenzyl from the oxidative coupling of toluene catalyzed by PbO. However, the reaction was conducted at a higher temperature (525–570 °C) in the vapor phase. Daito et al. [33] have patented a process for the recovery of benzyl benzoate by distilling the residue remaining after removal of un-reacted toluene and benzoic acid from a reaction mixture produced by the oxidation of toluene by molecular oxygen in the presence of a metal catalyst. Beside the main product, benzoic acid, they have also given a list of >20 by products. Most of these byproducts are due to the oxidative coupling/oxidative dehydrocoupling of toluene. Methyl biphenyl carboxylic acid (mp 144–146 °C) is one of these by products identified in the present study. Besides these by products they have also recovered the intermediate products in toluene oxidation benzaldehyde and benzyl alcohol and esters formed by esterification of benzyl alcohol with a variety of carboxylic acids inside the reactor. The absence of benzyl alcohol (Figs. 3, 6) could be due to its esterification with benzoic acid to form benzyl benzoate.
Conversion of toluene after 7 h of reaction. TL: toluene, BzH: benzaldehyde, BzOOH: benzoic acid, BzB: benzyl benzoate, t-ST: trans-stilbene, MBPA: methyl biphenyl carboxylic acid reaction. Conditions: toluene (20 mL), catalyst (400 mg), pO2 (101 kPa), flow rate (40 mL/min), agitation (900 rpm), temperature (90 °C)
4 Conclusions
Pt/ZrO2 is an active catalyst for toluene partial oxidation to benzoic acid at 60–90 °C in solvent free conditions. The rate of reaction is limited by the supply of oxygen to the catalyst surface. Selectivity of the products depends upon the reaction time on stream. With a reaction time ≤3 h benzyl alcohol, benzaldehyde and benzoic acid are the only products identified. After 3 hours of reaction time benzyl benzoate, trans-stilbene and methyl biphenyl carboxylic acid appear along with benzoic acid and benzaldehyde. In both the cases benzoic acid is the main product (selectivity ≥60%).
References
Collinn DE, Richery FA (1987) In: Kent JA (eds) Reigle handbook of industrial chemistry, Ch. 22. C. B. S., New Delhi, p. 800
Kaeding WW, Lindblom RO, Temple RG (1955) US Patent, 2 727 926
Toland WG Jr (1956) US Patent, 2 762 838
Bujis W (1999) J Mol Catal A 146:237
Fraga-Dubreuil J, Garcia-Serna J, Garcia-Verdugo E, Dudda LM, Aird GR, Thomas WB, Poliakoff M (2006) J Supercritical Fluids 39:220
Bujjs W, Frijns LHB, Offermanns MRJ (1993) US Patent, 5 210 331
Pennington J (1984) In: Heaton CA (eds) An introduction to industrial chemistry, Ch. 9. Leonard Hill, London, p 323
U.S. Environmental Protection Agency (1999) Integrated risk information system (IRIS) on toluene, National Center for Environmental Assistance, Office of Research and Development, Washington DC
Bulushev DA, Rainone F, Kiwi-Minsker L (2004) Catal Today 96:195
Worayingyong A, Nitharach A, Poo-arporn Y (2004) Sci Asia 30:341
Bastock TE, Clark JH, Martin K, Trentbirth BW (2002) Green Chem 4:615
Subrahmanyama Ch, Louisb B, Viswanathana B, Renkenb A, Varadarajan TK (2005) Appl Catal A Gen 282:67
Raja R, Thomas JM, Dreyerd V (2006) Catal Lett 110:179
Thomas JM, Raja R (2006) Catal Today 117:22
Li X, Xu J, Wang F, Gao J, Zhou L, Yang G (2006) Catal Lett 108:137
Li X, Xu J, Zhou L, Wang F, Gao J, Chen C, Ning J, Ma H (2006) Catal Lett 110:255
Ilyas M, Sadiq M (2007) Chem Eng Technol 30:1391
Ilyas M, Sadiq M (2007) Chin J Catal 28:413
Ilyas M, Sadiq M (2008) Chin J Chem 26:941
Liu H, Feng l, Zhang X, Xue Q (1995) J Phys Chem 99:332
Zhao Y, Wang G, Li W, Zhu Z (2006) Chemom Intell Lab Sys 82:193
Christoskova ST, Stoyanova M (2002) Water Res 36:2297
Souza LD, Suchopar A, Zhu K, Balyozova D, Devadas M, Richards RM (2006) Microporous Mesoporous Mater 88:22
Ferino I, Casula MF, Corrias A, Cutrufello M, Monaci GR, Paschina G (2000) Phys Chem Chem Phys 2:1847
Ding J, Zhao N, Shi C, Du X, Li J (2006) J Alloys Compd 425:390
Perez-Hernandwz R, Aguilar F, Gomez-Cortes A, Diaz G (2005) Catal Today 107–108:175–180
Zhan Y, Cai G, Xiao Y, Wei K, Cen T, Zhang H, Zheng Q (2004) Guang Pu Xue Yu Guang Pu Fen Xi 24:914–917
Montgomery PD, Moore RN, Knox WK (1976) US Patent, 3965206
Lee TP (1978) US Patent, 4091044
Williamson AN, Tremont SJ, Solodar AJ (1981) US Patent, 4255604, 4268704, 4278824
Hupp SS, Swift HE (1979) Ind. Eng. Chem., Prod. Res. Dev. 18:117
Kai T, Nomoto R, Takahashi T (2002) Catal Lett 84:75
Daito N, Ueda S, Akamine R, Horibe K, Sakura K (2002) US Patent, 6491795
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilyas, M., Sadiq, M. Oxidation of Toluene to Benzoic Acid Catalyzed by Platinum Supported on Zirconia in the Liquid Phase-Solvent Free Conditions. Catal Lett 128, 337–342 (2009). https://doi.org/10.1007/s10562-008-9750-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9750-8