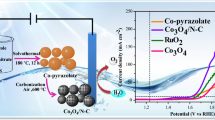
Abstract
The advanced oxygen evolution catalysts in alkaline solution play a growing role in alternative energy devices due to the need for clean and sustainable energy. In this paper, we report the cobalt phosphate nanoparticles embedded in N-doped carbon (Co3(PO4)2@N-C) using N,N′-piperazinebis (methylene-phosphonic acid) as both phosphate and carbon sources by two-step, hydrothermal method. The prepared Co3(PO4)2@N-C annealed at 600 °C exhibits advanced OER performance, with a current density of 10 mA cm−2 at a lower overpotential of 290 mV, a Tafel slope of 82 mV dec−1 and superior durability in 1.0 M KOH solution. This kind of material with MOF as precursor has wide application prospect in electro-chemistry field, especially for OER.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) are important for the water splitting [1]. The HER is a relatively simple reaction that is easily prone to many materials in a low potential [2]. Nevertheless, the OER is a more complicated reaction with higher overpotential, because there are four successive electron transfer procedures and low kinetics [3,4,5]. Generally, in the OER, the thermodynamic potential value is 1.23 V at about 25 °C (vs RHE) [6]. However, for the presence of the extra potential (also called overpotential), we must adopt to a higher potential to promote the electrocatalytic OER reaction. Thus, significant efforts have been made to explore highly efficient electrocatalysts for the OER.
Metal–organic frameworks (MOFs), as the superior electrocatalysts in aqueous alkaline solutions, have received notable attention in recent years due to the structural and chemical multiformity [7,8,9]. MOFs have become popular as pyrolytic precursors for synthesis of porous electrocatalysts [10,11,12,13]. MOFs are easily prepared with metal@N-C components that are well scattered within the frameworks and can be transformed into active metal@N-C structures for OER/ORR by an annealing procedure [14,15,16,17]. For instance, Zhang et al. reported a porous Co3O4/C nanowire prepared through thermally annealing a Co-based MOF, which can be used as catalysts for OER [18]. Lin et al. synthesized Co9S8@CoS@CoO@C nanoparticles using MOF as the precursor, which possessed excellent catalytic activity for the OER [19].
To date, transition metal phosphides have aroused widespread concern, owing to their plentiful reserves, environmental-friendly property [20,21,22,23]. In recent years, in order to enhance the electrocatalytic activity of transition metal phosphides electrocatalysts, various cobalt-containing phosphides have been prepared. Meanwhile, a number of cobalt phosphides or cobalt phosphates have been investigated as the OER electrocatalysts [21, 24, 25]. For example, Li et al. reported the Co-Pi electrocatalyst modified TiO2 nanowire with co-catalytic effect, which had excellent catalytic properties [26].
Herein, we prepared the cobalt phosphate nanoparticles embedded in N-doped carbon (Co3(PO4)2@N-C) through hydrothermal method. During the synthesis process, Co(NO3)2·6H2O reacted with N,N′′-piperazinebis(methylene-phosphonic acid) (PMP) in water by hydrothermal method, then the obtained precipitate was annealed at 600, 700, 800, 900 °C for 3 h in air to get Co3(PO4)2@N-C catalysts. The PMP not only acted as the phosphate source, but also was thermally decomposed into N doped carbon (N-C) coating on cobalt phosphate nanoparticles during the pyrolysis process, which enhanced the electrocatalytic performance. The prepared Co3(PO4)2@N-C annealed at 600 °C exhibits a current density of 10 mA cm−2 at a lower overpotential of 290 mV in 1.0 M KOH solution. Besides, the catalysts have good catalytic stability over continuous 1000 cycles with negligible drops of the current density, and little decay (5.7%) in OER activity up to 8 h of continuous operation at 1.52 V versus RHE.
2 Experimental
2.1 Chemicals
All reagents were used without further purification. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O, Shanghai Titanchem Co. Ltd., ≥ 99.8%), PMP was prepared by the method reported by Alhendawi et al. [27]. Potassium hydroxide (KOH, Shanghai Titanchem Co. Ltd., ≥ 85.0%). Distilled water was utilized in all experimental procedures.
2.2 Synthesis of Cobalt Phosphate Catalysts
Generally, 2.46 mmol (0.716 g) of Co(NO3)2·6H2O was dissolved in 20 mL of distilled water under magnetic stirring, then 1.23 mmol (0.337 g) of PMP was added. 1.0 M KOH solution was added dropwise to the mixture to adjust a final reaction pH of 7. The mixture was stirred for another 10 min, and transferred to Teflon-lined stainless steel autoclave and maintained at 200 °C for 72 h. After being cooled to room temperature, the purple powder was filtered under vacuum and washed thoroughly with distilled water. Dried at 60 °C overnight. As-prepared purple powder was named Co-PMP. To obtain the final product, the Co-PMP powder was then annealed at 600, 700, 800, 900 °C in air at a heating rate of 5 °C min−1. After kept at different temperature for 3 h, the powder was cooled down to room temperature at a cooling rate of 5 °C min−1. Finally, purple Co3(PO4)2@N-C powder at different temperature was obtained.
2.3 Characterization
X-ray diffraction (XRD) patterns were performed on a Bruker-Axs D8 Advance X-ray diffractometer in a wide angle range(2θ = 5–35°) with Cu Kα radiation, operating at 40 kV and 40 mA. The morphology of the samples was operated on SU70 field-emission scanning electron microscopy (FE-SEM) instrument at 10 kV, and elemental mappings were obtained at 20 kV. Samples for SEM were gold sputtered before the analyses. The high-resolution transmission electron microscopy (HRTEM) characterization was carried out on a Tecnai F30 microscope at an accelerating voltage of 300 kV. N2 adsorption–desorption isotherms were employed on a Quantachrome NOVA 2000e sorption analyzer (Fig. S2). The X-ray photoelectron spectroscopy (XPS) data was acquired on an ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo Scientific) using Al Kα radiation. TG were performed in Netzsch STA449 F3 Jupiter (Fig. S3).
2.4 Evaluation of the Electrocatalytic Activity Toward OER
Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) measurements were carried out on an Autolab electrochemical workstation (NOVA 2.1). The catalytic activity for OER was evaluated at room temperature in a conventional three-electrode system with electrochemical workstation in 1.0 M KOH solution. The electrode of glassy carbon (5 mm diameter, 0.196 cm2) was used as the working electrode. The Pt foil and an Ag/AgCl-saturated electrode were used as the counter electrode and reference electrode. In order to prepare the working electrode, 5 mg of catalysts were dispersed in a mixture of 950 μL ethanol and 50 μL 5 wt% Nafion solution with sonication for 60 min. After this process, the catalysts (20 μL) were dropped onto a glassy carbon electrode and then fully dried at room temperature for 12 h before measurements (loading ~ 0.510 mg cm−2). Linear sweep voltammetry was carried out at a scan rate of 10 mV S−1 for the polarization curves from 1.0 to 1.7 V. All the measured potentials were referred to RHE with the following equation: E (RHE) = EAg/AgCl + 0.197 + 0.059pH.
3 Results and Discussion
To obtain the final products, the Co-PMP powder was then annealed at different temperature. Because the electrochemical reaction of the amorphous products calcined at below 600 °C was very complex, in which both anode and cathode reaction were included, we chose samples of other temperatures for the OER test. Figure 1 presented the PXRD pattern of Co3(PO4)2@N-C powder annealed at different temperature (600, 700, 800 and 900 °C). The diffraction pattern exhibits peak at 20.52°, 21.91°, 25.65°, 27.68° and 36.78°, corresponding to (011), (101), (210), (021) and (031) planes of Co3(PO4)2 (JCPDS No. 77-0225), respectively. No peaks from carbon and nitrogen are observed, because of the low concentration; In addition, peaks appearing in 29.62°, 30.14° can be indexed to Co2P2O7 (JCPDS No. 34-1378), indicating that a small amount of Co2P2O7 was doped in the Co3(PO4)2@N-C. This phenomenon increases the number of active coordinated sites and can be beneficial to electrocatalytic application for OER. Obviously, with the increase of pyrolysis temperature, the crystallinity was getting better. With the increase of the thermal treatment temperature, the internal defects of the material gradually decreased, and the carbon element gradually disappeared (Table S2 see Supporting Information).
The electrocatalytic activity of Co3(PO4)2@N-C catalysts at different temperature (600, 700, 800 and 900 °C) for OER was also evaluated (Fig. 2). The Tafel slope can be fitted to an equation: η = blog(J) + a, where η presents the overpotential and current density is indicated by J, b is the Tafel slope. As shown in Fig. 2a, b, the overpotential at a current density of 10 mA cm−2 were 290, 300, 320, 340 mV, respectively; Besides, the corresponding Tafel slope were 82, 97, 126, 101 mV dec−1, respectively. The overpotential at a current density of 10 mA cm−2 and Tafel slope are important metrics, a good OER electrocatalyst should possess a low overpotential and Tafel slope, therefore, the Co3(PO4)2@N-C products annealed at 600 °C were much superior to others. To summarize, according to the results of PXRD (Fig. 1) and N2 adsorption–desorption isotherms (Fig. S2 see Supporting Information), we surmised that there are three reasons for the good OER performance of the Co3(PO4)2@N-C catalysts annealed at 600 °C. Firstly, the Co3(PO4)2@N-C catalysts annealed at 600 °C belong to poor crystallinity material, which also were doped with Co2P2O7. Compared with the better crystalline materials, there will be more active sites because of the presence of the small clusters caused by the internal defects [28, 29]; Then, the Co3(PO4)2@N-C catalysts annealed at 600 °C possess the larger specific surface areas, which could increase the density of the surface reactive sites and the contact areas [30]. Finally, the N-doped carbon layers act as a bridge linking nanoparticles, which can enhance the electrochemical performance [31]. The stability of Co3(PO4)2@N-C catalysts for OER was measured by the i–t tests at a constant potential of 1.52 V versus RHE, it can be clearly seen that Co3(PO4)2@N-C catalysts annealed at 600 °C exhibit superior durability, with little decay (5.7%) in OER activity up to 8 h of continuous operation (Fig. 2c). Further stability test showed that the Co3(PO4)2@N-C catalysts annealed at 600 °C almost consistent with the OER polarization curves as initial catalyst after 1000 cycles, only with negligible increases of the overpotential (Fig. 2d).
a OER polarization curves of Co3(PO4)2@N-C annealed at 600, 700, 800 and 900 °C, sweep rate: 10 mV S−1 in 1 M KOH. b Corresponding Tafel slope plots. c The i–t curve of Co3(PO4)2@N-C annealed at 600 °C at 1.52 V versus RHE. d OER polarization curves of Co3(PO4)2@N-C annealed at 600 °C before and after 1000 cycles
To get an in-depth understanding of the morphology and element composition of the top OER performance catalysts, which are Co3(PO4)2@N-C nanoparticles annealed at 600 °C, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were obtained (in Fig. 3). As shown in Fig. 3a–c, it can be observed that the sample contains a great number of nanoparticles with diameter about 200 nm. In addition, SEM images and the corresponding elemental mappings of the Co3(PO4)2@N-C catalysts annealed at 600 °C are shown in Fig. 3d–i, which present Co3(PO4)2@N-C composites were mainly comprised of cobalt, phosphorus and oxygen with trace amounts of carbon and nitrogen elements, inferring that pyrolysis process from Co-PMP can obtain nitrogen-doped carbon scaffold encapsulated in situ with Co3(PO4)2 nanoparticles. The N-doped carbon layers act as a network connection structure, which may enhance the electrochemical kinetics and further improve the OER performance.
The XPS was used to characterize the elements state of the Co3(PO4)2@N-C catalysts annealed at 600 °C (Fig. S4 see Supporting Information). The full XPS spectra provided evidence for presence of Co, P, O, and N as well as C (Fig. S4a). As shown in Fig. S4b, for the Co 2p XPS spectrum, two major peaks of 2p3/2 and 2p1/2 (resulting from the spin–orbit splitting), located at 781.7 and 797.8 eV, respectively, which can be assigned to Co2+ [32, 33]. The P 2p spectrum (Fig. S4c) clearly demonstrates the existence of phosphorus atoms in two chemical environment. The P 2p XPS displays two peaks, the 2p3/2 and 2p1/2 peaks were observed at 133.4 and 134.4 eV binding energies [34, 35]. The O 1s spectrum can be discovered by two sub-peaks (Fig. S4d), centred at 531.5 and 533 eV, which are characteristics of O2− ions in oxygen-deficient regions within the matrix of Co3(PO4)2 nanoparticles. Two peaks at 400.1 and 403.7 eV in the N 1s spectrum can be attributed to the presence of pyrrolic-type nitrogen atoms and the oxidized nitrogen, respectively (Fig. S4e) [36]. There are three resolved peaks in the C 1s spectrum. The first peak present at 284.7 eV, which is characteristic of C–C peak; The other two peaks centered at 285.8 and 287.8 eV were assigned to carbon binding with surface nitrogen and oxygen groups (C–N, C=O) respectively [37,38,39,40].
4 Conclusion
In summary, we prepared cobalt phosphate nanoparticles embedded in N-doped carbon (Co3(PO4)2@N-C annealed at 600 °C), which are prepared via a simple hydrothermal process using PMP as both the phosphate source and carbon source. The obtained materials display superior electrocatalytic activity for OER. Firstly, the N-doped carbon layers act as a bridge linking nanoparticles, which can enhance the electrochemical performance. Then, small amounts of doping of Co2P2O7, the poor crystallinity and larger specific surface areas contribute to more active sites and contact areas, improving the catalytic activity and stability efficiently.
References
Zou XX et al (2013) Efficient oxygen evolution reaction catalyzed by low-density Ni-doped Co3O4 nanomaterials derived from metal-embedded graphitic C3N4. Chem Commun 49(68):7522–7524
Landon J et al (2012) Spectroscopic characterization of mixed Fe–Ni oxide electrocatalysts for the oxygen evolution reaction in alkaline electrolytes. ACS Catal 2(8):1793–1801
Najafpour MM et al (2012) Nano-sized manganese oxide-bovine serum albumin was synthesized and characterized. It is promising and biomimetic catalyst for water oxidation. RSC Adv 2(30):11253–11257
Chen S et al (2013) Three-dimensional N-doped graphene hydrogel/NiCo double hydroxide electrocatalysts for highly efficient oxygen evolution. Angew Chem Int Ed 52(51):13567–13570
Kanan MW, Nocera DG (2008) In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321(5892):1072–1075
Han L, Dong SJ, Wang EK (2016) Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv Mater 28(42):9266–9291
Chaikittisilp W et al (2014) Synthesis of nanoporous carbon–cobalt–oxide hybrid electrocatalysts by thermal conversion of metal–organic frameworks. Chemistry 20(15):4217–4221
Gascon J et al (2014) Metal organic framework catalysis: Quo vadis? ACS Catal 4(2):361–378
Xia W et al (2015) Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ Sci 8(7):1837–1866
Xu X et al (2012) Spindle-like mesoporous alpha-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett 12(9):4988–4991
Zhao SL et al (2014) Carbonized nanoscale metal–organic frameworks as high performance electrocatalyst for oxygen reduction reaction. ACS Nano 8(12):12660–12668
Wang H et al (2014) Preparation, characterization and bifunctional catalytic properties of MOF(Fe/Co) catalyst for oxygen reduction/evolution reactions in alkaline electrolyte. Int J Hydrog Energy 39(28):16179–16186
Shang NZ et al (2016) Ag/Pd nanoparticles supported on amine-functionalized metal–organic framework for catalytic hydrolysis of ammonia borane. Int J Hydrog Energy 41(2):944–950
Dou S et al (2016) Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ Sci 9(4):1320–1326
Yu HY et al (2016) Cu, N-codoped hierarchical porous carbons as electrocatalysts for oxygen reduction reaction. ACS Appl Mater Interfaces 8(33):21431–21439
Ma TY et al (2016) Interacting carbon nitride and titanium carbide nanosheets for high-performance oxygen evolution. Angew Chem Int Ed 55(3):1138–1142
Liu YY et al (2016) Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J Mater Chem A 4(5):1694–1701
Ma TY et al (2014) Metal–organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J Am Chem Soc 136(39):13925–13931
Long JY, Gong Y, Lin JH (2017) Metal–organic framework-derived Co9S8@CoS@CoO@C nanoparticles as efficient electro- and photo-catalysts for the oxygen evolution reaction. J Mater Chem A 5(21):10495–10509
Bendi R et al (2016) Metal organic framework-derived metal phosphates as electrode materials for supercapacitors. Adv Energy Mater 6(3)
Li XY et al (2016) ZIF-67-derived Co-NC@CoP-NC nanopolyhedra as an efficient bifunctional oxygen electrocatalyst. J Mater Chem A 4(41):15836–15840
Liu YR et al (2016) Novel CoP hollow prisms as bifunctional electrocatalysts for hydrogen evolution reaction in acid media and overall water-splitting in basic media. Electrochim Acta 220:98–106
Pu ZH et al (2017) General strategy for the synthesis of transition-metal phosphide/N-doped carbon frameworks for hydrogen and oxygen evolution. ACS Appl Mater Interfaces 9(19):16187–16193
Chang JF et al (2015) Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal 5(11):6874–6878
Surendranath Y, Kanan MW, Nocera DG (2010) Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J Am Chem Soc 132(46):16501–16509
Ai GJ et al (2015) Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 7(15):6722–6728
Alhendawi H et al (2013) A new layered zirconium biphosphonate framework covalently pillared with N,N′-piperazinebis(methylene) moiety: synthesis and characterization. J Porous Mater 20(5):1189–1194
Zhou W et al (2011) Amorphous iron oxide decorated 3D heterostructured electrode for highly efficient oxygen reduction. Chem Mater 23(18):4193–4198
Bergmann A et al (2015) Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat Commun 6:8625
Zhao J et al (2014) Self-template construction of hollow Co3O4 microspheres from porous ultrathin nanosheets and efficient noble metal-free water oxidation catalysts. Nanoscale 6(13):7255–7262
Chen ZY et al (2017) Ag-enhanced catalytic performance of ordered mesoporous Fe–N-graphitic carbons for oxygen electroreduction. Catal Lett 147(11):2745–2754
Yang J et al (2010) Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J Phys Chem C 114(1):111–119
Xing M et al (2014) Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. J Mater Chem A 2(43):18435–18443
Hu GR et al (2008) Comparison of AlPO4- and Co3(PO4)(2)-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochim Acta 53(5):2567–2573
Yuan CZ et al (2016) Cobalt phosphate nanoparticles decorated with nitrogen-doped carbon layers as highly active and stable electrocatalysts for the oxygen evolution reaction. J Mater Chem A 4(21):8155–8160
Zhang CZ et al (2013) Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2(1):88–97
Nagaiah TC et al (2012) Mesoporous nitrogen-rich carbon materials as catalysts for the oxygen reduction reaction in alkaline solution. ChemSusChem 5(4):637–641
Shruthi TK et al (2014) Functionalization of graphene with nitrogen using ethylenediaminetetraacetic acid and their electrochemical energy storage properties. RSC Adv 4(46):24248–24255
Li XZ et al (2015) MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: extraordinary bi-functional electrocatalysts for OER and ORR. J Mater Chem A 3(33):17392–17402
Zhou WJ et al (2015) N-doped carbon-wrapped cobalt nanoparticles on N-doped graphene nanosheets for high-efficiency hydrogen production. Chem Mater 27(6):2026–2032
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Feng, P., Cheng, X., Li, J. et al. Co3(PO4)2 Nanoparticles Embedded in Nitrogen-Doped Carbon as an Advanced Electrocatalyst for OER in Alkaline Solution. Catal Lett 148, 214–219 (2018). https://doi.org/10.1007/s10562-017-2251-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2251-x