Abstract
A new hybrid organic–inorganic material denoted by ZrPMP·1.7H2O, where PMP = N,N′-piperazinebis(methylenephosphonic acid), is prepared through the decomposition of zirconium fluoro-complex in the presence of PMP. This material is characterized by X-ray diffractometry, solid 31P NMR and FT-IR spectrophotometries and thermal analyses. All of the above mentioned analyses provide strong evidence that this material is structurally related to α-type layered zirconium phosphate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The area of chemistry that explores the porous materials has received a great impulse in the last 40 years. In addition to the classic porous materials (e.g. activated charcoal, silica, alumina, zeolites), synthetic materials have been developed with analogous structures to those observed in Nature. An important landmark in this field has been the preparation of synthetic zeolites which have a significant role as they could be used as catalysts, adsorbents and ion exchangers.
An important effort has been dedicated to the development of other porous materials with analogous three-dimensional structures. To this effect, the chemistry of metallic phosphates [1] with laminar structure has received a great impulse, because they are very versatile materials. Among these metallic phosphates, layered zirconium phosphate (ZrP) can be easily converted into porous 3D materials by the concourse of well-designed organic moieties.
Basically, layered structures formed by zirconium phosphate (ZrP) are produced by octahedra–tetrahedra combinations in two main different formats, named α- and γ-phases, where zirconium coordination varies from one to another (Fig. 1). In both α and γ structures zirconium coordinates octahedrically to six oxygens of six surrounding phosphates. However, in the former all phosphates (HPO4) are equivalent and they all use three oxygens to bond to zirconium, whereas in the γ-phase phosphates are classified into two types: (1) PO4 uses its four oxygens to coordinate to zirconium, (2) H2PO4 uses only two oxygens to do that. Thus the molecular formulas for α- and γ-ZrP are Zr(HPO4)2·H2O and Zr(PO4)(H2PO4)·2H2O, respectively [2, 3].
Moreover, pillared γ-ZrP is formed when the topotactic phosphate/phosphonate exchange reactions occur at the same time on the facing surfaces of two adjacent layers by a molecule with two phosphonic groups (diphosphonic acid) [2, 3]. While pillared α-ZrP is obtained by allowing zirconium fluoro-complexes to decompose in presence of diphosphonic acids [2, 3].
If the pillars have a suitable height and are sufficiently spaced, microporous materials are thus obtained. Pillars could be either rigid or non-rigid, the latter being the most versatile as the porosity of the materials can be controlled by simple chemical means [4–9]. The hallmark features of these materials come from their ability to combine the properties of both the organic pendant groups with those of the inorganic host [10]. Such an approach can result in the design and development of hybrid organic–inorganic materials with tailor-made properties. These materials can function as molecular sieves of controlled pore size [10], shape-selective catalysts [11–13], molecular sorbents [14, 15], and stationary phases for chiral molecular recognition [16, 17].
The goal of this research work is to synthesize and characterize a new layered pillared organic–inorganic 3D framework by decomposing zirconium fluoro-complex in the presence of PMP (Fig. 2).
2 Experimental
All chemicals and reagents used were of analytical grade. All of them were purchased from Aldrich Co., and used as supplied. PMP was synthesized using a literature modified procedure [18].
2.1 Synthesis of N,N′-piperazinebis(methylenephosphonic acid) (PMP)
Piperazine hexahydrate (33.82 g, 0.174 mol), crystalline phosphorous acid (57.12 g, 0.697 mmol) previously dissolved in 70 ml of distilled water and 52.5 ml of concentrated HCl (10 M) were heated to reflux (125 °C) in a two-necked flask fitted with condenser and syringe. In the course of ca. 1.5 h 37 % aqueous formaldehyde solution (103.8 ml, 1.393 mol) was added dropwise. The reaction mixture was kept at the same reflux temperature for 24 additional hours. Upon cooling to room temperature the product was crystallized as a white solid. Then it was filtered, washed portionwise with cold distilled water (3 × 200 ml), and dried for 24 h at 120 °C. The yield was 42.1 g (88.3 %). 1H NMR (D2O at pH = 9) δ (ppm): 2.80 (m, 8H, N–CH2CH2–N); 3.25 (d, 2 J HP = 10.1 Hz, 4H, N–CH2P); Solid-state MAS 13C NMR (CP-MAS) δ (ppm): 49.4 (N–CH2CH2–N); 53.4 (N–CH2P); elemental analysis (%), calcd. for C6H16N2O6P2: C, 26.29; H, 5.88; N, 10.22; found: C, 26.26; H, 5.92; N, 10.20; MS (EI) m/z: 274.0 [M]+.
2.2 Synthesis of ZrPMP·1.7H2O
Two clear solutions, named A and B, are separately prepared. Solution A was obtained by mixing, at room temperature, ZrOCl2·8H2O (0.403 g, 1.25 mmol) previously dissolved in 48 % HF (0.27 ml, 7.45 mmol, Zr/HF molar ratio = 1:6) and 1 l distilled water. Solution B was prepared by mixing, at room temperature, PMP (0.383 g, 1.25 mmol, Zr/PMP molar ratio = 1:1) and 1 l 2.5 × 10−3 M NaOH aqueous solution (PMP/NaOH molar ratio = 1:2). Solution B was then added dropwise to solution A at room temperature to obtain a clear solution. The final solution was heated at 80 °C in a polypropylene bottle with a good stopper for 2 weeks. The solid product was centrifuged, washed with distilled water (3 × 50 ml) and dried in the oven for 48 h at 105 °C. Finally, the solid was conditioned 3 days in a desiccator containing a saturated solution of BaCl2 to obtain 340 mg of the product.
2.3 Characterization
Solution 1H NMR spectra were recorded on Bruker AV-300 spectrometers. Chemical shifts were given in ppm relative to TMS using the corresponding solvent signal as internal reference. Solid-state 31P and 13C NMR spectra were recorded under cross-polarization and magic-angle techniques (CP-MAS) on a Bruker AV-400 WB spectrometer. Chemical shifts were given in ppm relative to TMS using adamantane as external standard. X-ray powder diffraction (XRD) patterns were recorded at room temperature on Siemens D-5000 diffractometers with Cu Kα radiation (λ = 0.154 nm) and Ni filter at 40 kV, 30 mA, a scanning rate of 5° min−1, and a 2θ angle ranging from 3° to 70°. Infrared spectra were recorded on a FTIR-8201PC spectrometer (Shimadzu-Japan) using KBr disk in the range 4,000–400 cm−1. Elemental analyses were performed on a Perkin Elmer II 2400 CHN analyzer. Zr and P content of the material was determined using a literature described procedure [19]. Thermogravimetric analyses was performed on a Mettler-Toledo TGA/STDA 851e apparatus and recorded at 10 °C/min. The TGA–DTA curves have been obtained under nitrogen sweeping. Structure modeling was carried out on PC computers using the Hyperchem release 7 and the molecular mechanics method MM+.
3 Results and discussion
The method used to prepare ZrPMP·1.7H2O is closely related to that used for the preparation of α-zirconium bis(monohydrogen phosphate) (α-ZrP) [20], i.e. by direct precipitation method by which zirconium fluoro-complex is allowed to decompose in presence of phosphonic acids. As an important point, this method was described and highlighted for the first time by Alberti et al. in [21], has been used to prepare a large number of α-type zirconium phosphonates materials. Concerning materials, they have been used in so many applications (such as, protonic conductivity, shape-selective catalysis and molecular recognition) which reflect the significance of increasingly interest of these materials [22].
In practice, ZrPMP·1.7H2O is prepared using the above mentioned method (Scheme 1). It was necessary to treat PMP with NaOH to dissolve it in water (see “Experimental” section) because of its dipolar character (zwitter ion). The strongly basic hydroxide ion is expected to pull hydrogen ion away from the weakly basic amino groups to yield the soluble form of PMP.
Regarding the performed analyses, elemental analysis of ZrPMP·1.7H2O (found: 23.30 % Zr, 15.55 % P, 18.42 % C, 4.20 % H and 7.02 % N; calculated: 23.27 % Zr, 15.80 % P, 18.39 % C, 3.96 % H and 7.15 % N) is in accordance with a molecular formula of Zr[(O3PCH2)2N2C4H8)]·1.7H2O deduced also from thermogravimetric analysis (Fig. 3).
As an important point to be mentioned, the Zr/P mole ratio (1:2) and the total absence of fluorine in the chemical formula of our new material (Zr[(O3PCH2)2N2C4H8)]·1.7H2O) emphasize the complete decomposition of the zirconium fluoro-complex during the course of the reaction.
In the context of thermogravimetric analysis, Fig. 3 shows the TGA and DTA curves of Zr(PMP)·1.7H2O. They reveal two weight loss intervals. The first one (25–150 °C) corresponds to dehydration (8.43 %). The second weight loss interval (150–700 °C) caused by decomposition and volatilization of the organic fragments (27.06 %). The later percentage is in consistent with the calculated amount of the PMP in the formula of Zr(PMP).1.7H2O mentioned above (28.62 %).
Concerning the X-ray power pattern of Zr(PMP)·1.7H2O (Fig. 4), it is typical of poly-phase material where there are several small reflections, but most important, the first intense reflection that occurs at two theta value of 9.4°, which corresponds to d spacing of 0.94 nm.
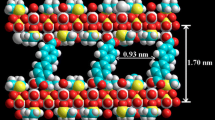
Figure 5 shows the structure of the most stable conformer of PMP (chair conformation) as calculated by MM+ molecular mechanics method. The expected molecular dimensions are also indicated. In an effort to calculate an expected structure for Zr(PMP)·1.7H2O, let us consider it as organic derivative of α-ZrP. Additionally, the thickness of one α-ZrP layer, calculated as the shortest distance between the centers of oxygens of the P–OH groups present on the opposite sides of one layer, is 0.63 nm [3]. Therefore, the measured interlayer distance for Zr(PMP)·1.7H2O (0.94 nm) leaves ca. 0.31 nm for PMP molecules inside the solid matrix. Figure 5 shows a possible arrangement of PMP molecules inside the interlayer region of Zr(PMP)·1.7H2O complying with the observed interlayer distance (0.94 nm).
Also, Fig. 5 shows that the interlayer layer gallery of ZrPMP·1.7H2O is very crowded with N,N′-piperazinebis(methylene) moieties. Therefore, this materials is not expected to exhibit appreciable microporosity.
Regarding Fig. 6, it shows the FT-IR spectra of free PMP and Zr(PMP)·1.7H2O. Free PMP shows the following key stretching peaks: 1,100 and 1,128 cm−1 (C–N), 1,268 cm−1 (P=O), 2570 cm−1 (O=PO–H), 2,850–3,000 cm−1 (C–H) and 3,427 cm−1 (N–H).
With respect to ZrPMP·1.7H2O, the FT-IR spectrum (Fig. 6) is almost composite of those of ZrP and free PMP. The peak at 550 cm−1 is assigned to δ (PO4). While the band at 1,634 cm−1 is ascribed to the bending vibrations of water molecules. The broad band in the region 3,500–3,000 cm−1 belongs to symmetric and asymmetric stretching vibrations of water molecules.
Regarding the solid-state 31P NMR spectrum of ZrPMP·1.7H2O (Fig. 7), the presence of only one resonance at −2.50 ppm indicates that the two phosphonate phosphorus atoms of PMP are chemically equivalent and both of them are connected to three metal atoms. This assignment is made on the basis of similar resonances in compounds that are structurally well established [23–25]. Therefore, it is appropriate to say that ZrPMP·1.7H2O has a layered structure similar to that of α-zirconium phosphate (Fig. 2); whereas this fact is consistent with both the calculated chemical formula and structure of ZrPMP·1.7H2O mentioned above.
4 Related work
Taddei et al. [26, 27] highlighted that two different materials were synthesized based on Zr and N,N′-piperazinebis(methylenephosphonate) system: Zr2H4[(O3PCH2)2N2C4H8]3·9H2O 1 and ZrF2(O3PCH2)2(NH)2C4H8 2. It was found that 1 made of channels running along the c axis, while 2 has a layered structure. These inherent structural differences between 1 and 2 were mainly attributed the pH value of the starting precipitation solution, that can influence the protonation level of N atoms of the piperazine moiety.
The mentioned justification strengthen the conclusion of this research study, where the starting pH values for 1, 2 and our new material (Zr[(O3PCH2)2N2C4H8)].1.7H2O) were 7, 2, 3, respectively (Table 1). Therefore, these materials have different structural features and belong to different phase-types of zirconium aminophosphonates.
It is worth mentioning, the reaction conditions used to prepare our new material (Zr[(O3PCH2)2N2C4H8)]·1.7H2O) are more similar to those used in case of compound 2 (Table 1) as well as both of them have pillared-layered structures.
For the purposes of clarity regarding the main difference, there is an essentiality to express the variations between the two compounds in terms of structure. In this study, zirconium is coordinated to six oxygen atoms of six surrounding aminophosphonates as in the usual α-layered structure (fluorine is absent). While in the related work, zirconium is coordinated to four oxygen and two fluorine atoms, and therefore, the two materials have different chemical compositions and phase-types.
Table 1 shows the comparison between the conditions under which compounds 1, 2 and Zr[(O3PCH2)2N2C4H8)]·1.7H2O are prepared.
To summarize the significant points, FT-IR, chemical formula of ZrPMP·1.7H2O calculated from thermal analyses, the absence of fluorine in this formula, Zr/P mole ratio (1:2), the presence of only one peak at −2.50 ppm in its 31P NMR spectra, X-ray diffraction pattern and molecular modeling provide strong evidence that zirconium is octahedrally coordinated to six oxygen atoms. Therefore, Zr[(O3PCH2)2N2C4H8)]·1.7H2O is organic–inorganic material structurally related to α-ZrP.
5 Conclusion
It is clearly seen that the present research study demonstrates that N,N′-piperazinebis(methylenephosphonic acid) (PMP) can be covalently liked to the inorganic framework of zirconium phosphate (ZrP). Accordingly, a new hybrid organic–inorganic material (ZrPMP·1.7H2O) is obtained. The data obtained provide strong evidence that the crystalline structure of this material is similar to that of α-ZrP.
References
A. Clearfield in Metal phosphate chemistry ed. by K.D. Karlin. Progress in Inorganic Chemistry, vol 47, (Wiley, New York, 1998), p. 373–510
G. Alberti, Sci. Technol. 80, 607 (1998)
G. Alberti, M. Casciola, U. Costantino, R. Vivani, Adv. Mater. 8(4), 291 (1996)
G. Alberti, S. Murcia-Mascarós, R. Vivani, Mater. Chem. Phys. 35, 187 (1993)
G. Alberti, F. Marmottini, S. Murcia-Mascarós, R. Vivan, Angew. Chem. Int. Ed. Engl. 33, 1594 (1994)
E. Brunet, H.M.H. Alhendawi, C. Cerro, M.J. de la Mata, O. Juanes, J.C. Rodríguez-Ubis, Microporous Mesoporous Mater. 138, 75 (2011)
E. Brunet, H.M.H. Alhendawi, M. Alonso, C. Cerro, L. Jiménez, O. Juanes, M.J. de la Mata, A. Salvador, M. Victoria, E. Rodríguez-Payán, J.C. Rodríguez-Ubis, J. Phys. Conf. Ser. 232, 012017 (2010)
E. Brunet, H.M.H. Alhendawi, C. Cerro, M.J. de la Mata, O. Juanes, J.C. Rodríguez-Ubis, Chem. Eng. J. 158, 333 (2010)
G. Alberti, E. Brunet, D. Chiara, M.J. de la Mata, O. Juanes, J.C. Rodríguez-Ubis, R. Vivan, Angrew Chem Int Ed 22(38), 3351 (1999)
G. Alberti, in Recent Development in Ion Exchange, ed. by P.A. Williams, M.J. Hundson (Elsevier Applied Sciences, London, 1987), pp. 233–248
B.Z. Wan, R.G. Anthony, G.Z. Peng, A. Clearfield, J. Catal. 101, 19 (1986)
G. Alberti, U. Costantino, J. Mol. Catal. 27, 235 (1984)
M. Dines, P.M. Digiacomo, K.P. Callahan, P.C. Griffith, R.H. Lane, R.E. Cooksey (1982) Proc. Am. Chem. Soc. 223
G. Cao, M.E. Garcia, M. Alcalá, L.F. Burgess, T.E. Mallouk, J. Am. Chem. Soc. 114, 7574 (1992)
T.E. Mallouk, J.A. Gavin, Acc. Chem. Res. 31, 209 (1998)
E. Brunet, H.M.H. Alhendawi, O. Juanes, J.C. Rodríguez-Ubis, J. Mex. Chem. Soc. 53(3), 155 (2009)
E. Brunet, Chirality 14, 135 (2002)
K. Moedritzer, R.R. Irani, J. Org. Chem. 31, 1603 (1966)
D.N. Bernhart, A.R. Wreath, Anal. Chem. 27, 440 (1955)
G. Alberti, E. Torracca, J. Inorg. Nucl. Chem. 30, 317 (1968)
G. Alberti, U. Costantino, S. Allulli, N. Tomassini, J. Inorg. Nucl. Chem. 40, 1113 (1978)
G. Alberti in Comprehensive supramolecular chemistry, vol. 7, Chap 5, ed. by G. Alberti, T. Bein (Pergamon, New York, 1996), p. 151–187
U. Costantino, M. Nocchetti, R. Vivani, J. Am. Chem. Soc. 124, 8428 (2002)
R. Zeng, X. Fu, C. Gong, J. Mater. Sci. 41, 4771 (2006)
A. Clearfield, D. Poojary, B. Zhang, B. Zhoa, A. Derecskei-Kovas, Chem. Mater. 12, 2745 (2000)
M. Taddei, F. Costantino, R. Vivani, Inorg. Chem. 49, 9664 (2010)
M. Taddei, F. Costantino, V. Manuali, R. Vivani, Inorg. Chem. 50, 10835 (2011)
Acknowledgments
The authors wish to thank the Association of Arab Universities for financial support and Mr. Kamal Alhendawi for English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alhendawi, H., Brunet, E., Payán, E.R. et al. A new layered zirconium biphosphonate framework covalently pillared with N,N′-piperazinebis(methylene) moiety: synthesis and characterization. J Porous Mater 20, 1189–1194 (2013). https://doi.org/10.1007/s10934-013-9702-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-013-9702-6