Abstract
Grafts made from human amniotic membrane are used to prevent recurrence of pterygium after excision. The success of the procedure can be affected by the quality of preparation and preservation of the grafts. We prospectively evaluated the safety and efficacy of cryopreserved amniotic membrane prepared at the research tissue bank of the Biotechnology Research Center in Tripoli, Libya, and used as adjunct therapy in primary pterygium excision. Twenty-six patients (15 males and 11 females) aged 21–78 years and indicated for primary pterygium excision were transplanted at the Tripoli Eye Hospital with the amniotic membrane grafts. Sixteen patients (62 %) were available for all three follow-up visits scheduled at 1, 3 and 6 months post-surgery. By the third visit, two patients (12.5 %) developed granuloma and three (18.8 %) had pterygium recurrence. The grafts were used after cryopreservation for ≤180 days or >180 days, but statistical analysis showed that the complications were not associated with the length of storage. Moreover, the high rate of complications in this study was not caused by use of cryopreserved AM. In conclusion, locally produced cryopreserved AM is safe as an adjunct therapy for treatment of primary pterygium excision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pterygium is a common benign growth of the conjunctiva on the sclera, which impairs vision. Its pathogenesis is linked to environmental factors such as frequent exposure to ultraviolet light in hot, dry, windy and dusty areas and smoky environments (Koranyi et al. 2004). The tumor suppressor gene p53 has been implicated in the pathogenesis of pterygium, as well as several other genes involved in DNA repair, cell proliferation and migration, and angiogenesis, indicating the interaction of genetic and environmental factors (Liu et al. 2013).
Pterygium is commonly treated by surgical excision, and recurrence is the most common complication following surgery. There are different surgical options: bare sclera, conjunctival and limbal autografts, mitomycin C, lamellar keratoplasty and amniotic membrane (AM) transplantation (Hirst 2003). AM is the innermost layer lining the foetal sac in the uterus. It is widely used as a wound healing material in many disciplines, including ophthalmology. AM is processed using different protocols: fresh, lyophilized, and cryopreserved at −80 °C (Alireza et al. 2007). The preservation method may affect its biological and functional properties when transplanted on the eye surface. Cryopreservation of AM significantly impairs viability and proliferative capacity of its cells; hence, AM does not trigger immune reactions in the transplanted eye (Kruse et al. 2000). AM serves as a biological scaffold containing epidermal growth factor (EGF), transforming growth factor-β (TGF-β) and other biomolecules that promote host cell proliferation and wound healing (Koizumi et al. 2000; Alió et al. 2005). The content AM comprising of collagen IV, collagen VII, laminin, laminin 5, and fibronectin, and soluble proteins TIMP-1 and IL-1ra has been reported to be not significantly changed after preservation for 24 months at −80 °C (Thomasen et al. 2011). On the other hand, other studies reported that levels of EGF (Gicque et al. 2009) and TGF-β (Hopkinson et al. 2006), which are important in wound healing, are reduced by cryopreservation. The latter report gives credence to the assumption that cryopreserved AM functions as a structural scaffold that enhances corneal epithelia proliferation rather than as a biochemical wound healing material (Kruse et al. 2000).
Pterygium excision is frequently associated with recurrence. In bare sclera excision, the recurrence rate is as high as 38.09 % (Alpay et al. 2009) and may reach 89 % (Khan et al. 2010). However, recurrence can be reduced considerably by using limbal or conjunctival autografts (Li et al. 2012; Kocamis and Bilgec 2014). Lamellar keratoplasty and AM with or without topical application of anti-metabolities such as mitomycin C have been used. Mitomycin C has also been used alone (Salman and Dina 2011), but its use on bare sclera is controversial due to its rare but potentially serious side effects (Norliza et al. 2006; Kheirkhah et al. 2012). Some studies indicated that conjunctival and limbal autografts are associated with similar (Kangkeng et al. 2012) or lower rates of pterygium recurrence than AM (Kaufman et al. 2013), and lower than that of bare sclera (Besharati et al. 2008). Others reported that AM grafts were associated with less frequent recurrence than conjunctival autografts and advocated the use of AM (Ma et al. 2000). We therefore investigated the rate of pterygium recurrence and post-surgery complications and its relation to cryopreservation duration of AM.
Materials and methods
Placenta procurement
Procurement and processing of placenta and AM tissue were ethically approved by the Libyan National Committee for Biosafety and Bioethics, and informed consent was obtained from each donor. Placentas were collected between August 2012 and October 2013 from six healthy mothers during elective cesarean deliveries at the Gynaecology Department of the Tripoli Medical Centre, Tripoli. The donors were screened for HBV, HCV and HIV by using chemiluminescent microparticle immunoassay (CMIA) (Abbot ARCHITECT® 1000sr, USA). Donors were assessed for compatibility with tissue safety criteria; they were questioned in detail about their medical and behavioral history as listed in the donation form before signing the consent form.
Amniotic membrane preparation
Placentas were transported to the laboratory in a sterile, 2-l, wide-mouth glass bottle containing 1 l sterile saline solution supplemented with antibiotics (50 mg/ml penicillin, 50 mg/ml streptomycin, 100 mg/ml neomycin) (PSN Sigma, cat# P3664, USA), and amphotericin B (Fungizone™ 2.5 mg/ml, Sigma, cat# A2942, USA). The bottle was transported in a polystyrene foam box filled with ice bags pre-frozen at −80 °C and stored at 2 °C overnight. Following the method described by Kim and Tseng (1995), the placentas were washed twice in 500 ml sterile saline under a Class II safety cabinet (HERASAFE KS9; Thermo Scientific, Germany). The AM was separated from the chorionic membrane by blunt dissection, and the rest of the placenta was placed in a sterile stainless steel pan containing 500 ml Eagle’s Balanced Salt Solution (Sigma, USA; cat #E 3024 EBSS without phenol red) supplemented with PSN antibiotic mixture and amphotericin B antifungal. While submerged, the AM was scraped manually under aseptic conditions to remove all the debris until it became clear and transparent. Sterile circular nitrocellulose filters (pore size 0.2 µm, diameter 47 mm; Whatman®, Cat# 7187, Whatman Paper Limited, England) were used as a whole or cut into strips of different sizes along with pre-cut sterile nitrocellulose strips (pore size 0.2 µm; Protran® Whatman, cat# BA-83, Whatman Paper Limited, England) and used to hold the AM in the preservation medium during cryo-storage. The nitrocellulose filters/strips were placed over the stromal side of the AM, and the AM was cut around the nitrocellulose strips with a few millimeters of AM folded on the other side of the nitrocellulose strip. The AM with the adherent nitrocellulose filters/strips were transferred into 30-ml sterile polystyrene double-bagged containers (Sterilin, code# 128 DB/IRR, UK) containing 1:1 (v:v) mixture of Dulbecco’s Modified Eagle Medium (DMEM Sigma, cat#D 6171, USA) and autoclave-sterilized glycerol (Code 32509033, BioChemika, Sigma, UK) supplemented with PSN antibiotic mixture and amphotericin B antifungal.
For sterility test, the contents of a random bottle from each of the processing batches and 5–10 ml of the prepared mixture of culture medium and glycerol were inoculated in 300 ml Tryptic Soy Broth (3.0 g/l peptic digest of soya bean) (Code# 610053, Liofilchem Biotechnology Products, Italy) and thioglycollate medium (0.5 g/l sodium thioglycollate) (Code# 610050, Liofilchem Biotechnology Products, Italy). The bottles were incubated for 14 days at 32 ± 2 °C to detect any microbial growth as according to ISO 11737 (2006). The other bottles containing the AM strips were vacuum heat sealed (ALLPAX PT-MJ-4 DS; Allpax GmbH and Co. KG, Germany) and stored at −80 °C until released for surgical use. Upon receiving an allograft request from surgeons, the graft bottle was transported to the hospital in a polystyrene foam box filled with ice.
Patients indicated for surgery were informed before the day of the surgery about the study and the voluntary nature of participation. On the day of the surgery, the patient or his/her next of kin signed an informed consent form and the basic demographic data were recorded.
Patients
Twenty-six patients indicated for primary pterygium surgery were transplanted with AM grafts as an adjunctive treatment between October 2012 and December 2013. All of them had been evaluated pre-operatively for visual acuity and by slit-lamp examination of the anterior segment. No inclusion or exclusion criteria were imposed other than clinical indication as judged by the attending surgeons. The indication for AM graft was patients’ complaints of a feeling of discomfort, foreign body sensation, redness and astigmatism effects.
Surgical technique
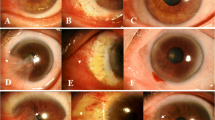
Three surgeons at the Tripoli Eye Hospital participated in this study. The eye was anesthetized with 0.4 % oxybuprocaine hydrochloride (Cebesin, Bausch and Lomb, France). It was adequately exposed using an eyelid speculum and injected subconjunctivally with 1 ml of 2 % lidocaine solution (B-Braun, Germany). The head of the pterygium was excised from the cornea by avulsion, the pterygial fibrovascular growth was removed, and an avascular bed was created. The AM was placed on the eye surface with the epithelial side in contact with the eye and sutured to the episclera using 10-0 vicryl suture. Post-operation management of participants included patching the eye and giving oral antibiotics, analgesics and topical steroids. Patients were examined the following day and after 1 week. They were also scheduled for the follow-ups at 1, 3 and 6 months after surgery. Pictures of the operated eyes were taken pre and 1-day post-surgery with an Optomed Smartscope® M5 digital medical camera (Finland).
Results
Total of 183 AM grafts were prepared from six placentas. Sterility test of a sample from each of the six processing batches at the time of cryopreservation showed that all six batches were sterile.
AM grafts were used as adjunct therapy in pterygium surgery on 26 eyes of 26 patients (15 males, 57.7 %; 11 females, 42.3 %). Almost all of the patients were living in Tripoli or in nearby towns and working outdoors (e.g., drivers, field technicians, and housewives working on farms). Their ages ranged between 21 and 78 years with a mean of 48.2 years. Half of the patients were grafted with membranes cryopreserved for ≤180 days (mean 118 days) and another half with membranes cryopreserved for >180 days (mean 338 days).
Table 1 shows the presence of complications at the follow-ups. By the second visit, two patients developed recurrence of pterygium (7.7 %) and two developed granuloma (7.7 %) resulting in (15.4 %) overall rate of complications. Only 16 out of 26 patients presented themselves for the third visit, and a new case of recurrence was observed. Overall, for these 16 patients there were two cases of granuloma (12.5 %) and three of recurrence (18.8 %), giving an overall rate of complications of 31.3 % by the third follow-up.
By the second follow-up visit, of the 13 patients grafted with AM preserved for the shorter duration, three had recurrence and one developed granuloma. Of the other 13 patients, grafted with longer preserved membranes, only one had recurrence. Fisher’s exact test showed that there was no significant difference between the two groups in the development of granuloma or recurrence (P value = 0.3217), analysis performed using GraphPad Prism®, version 5. No infection or other complication was observed at any of the follow-up visits.
Discussion
Confirmation of sterility is extremely important in the preparation of AM grafts in order to avoid or minimise the potential risks to patients (Qureshi et al. 2010). Sterility test demonstrated the sterility of all the six processing batches, and no complication of infection or inflammation due to infected AM grafts was observed, which is in line with the previous studies (Liu et al. 2010).
Besides sterility, it is important that the preparation procedure and cryopreservation do not induce detrimental changes to the histological and biological properties of amniotic membranes (Thomasen et al. 2011). Previous studies indicated that morphological changes after AM cryopreservation were insignificant (Kruse et al. 2000). Meller et al. (2011) found that AM membrane can be preserved at −80 °C for 1–2 years before use. Essex et al. (2004) used AM preserved at −80 °C for 1–21 weeks and reported that the low success rate of AM grafts was not related to the deep freezing storage time. The AMs we used in surgery had been cryopreserved for up to 417 days or 1.1 year (average 223 days or 0.6 year), and the statistical analysis showed that the development of granuloma or pterygium recurrence was not statistically significant between the shorter (≤180 days) and the longer (>180 days) durations of AM cryopreservation (P value = 0.3217).
The literature shows that the success rate of using AM for preventing recurrence after primary pterygium is widely variable, but the causes of variability is unclear. The pterygium recurrence rate of 18.8 % following excision plus AM graft as obtained in this study is higher than those reported in other studies: 10.7 % (Tekin et al. 2001); 10.9 % (Prabhasawat et al. 1997); 12.9 % (Arain et al. 2012) and 13.8 % (Ebru and Eraslana 2016). On the other hand, our rate is lower than the 27.3 % reported by Kurna et al. (2012). Very high recurrence rates of 40.9 % (Tananuvat and Martin 2004) and 64 % (Essex et al. 2004) have also been reported for cryopreserved AM grafts. In the latter case, the authors suggested that the high rate could have been due to either defects in processing and storage or to the surgical technique.
We did not observe post-surgical complications such as conjunctival inflammation around the surgical site (Fig. 1). Kheirkhah et al. (2011) reported that conjunctival inflammation occurred in 84.2 % of cases after AM grafts were used for primary pterygium, while the recurrence rate of pterygium was 10.5 % and post-surgical pyogenic granuloma was 15.8 %, the latter of which is comparable to our granuloma rate of 12.5 %. We also observed no symptoms of AM graft rejection. This could have been due to the cryopreservation method, which greatly reduces the viability of AM epithelial cells (Kruse et al. 2000; Alireza et al. 2007).
The rate of pterygium recurrence after using AM grafts is influenced by different factors such as inter-donor and intra-donor variations (Hopkinson et al. 2006), differences in the AM content of healing biomolecules such as growth factors and collagens, the health status of the host tissue around the AM graft (Liu et al. 2010), and post-surgery exposure to sun, especially in males (Torres-Gimeno et al. 2012). Other factors such as pterygium size and patient age or sex were found to have no association with pterygium recurrence (Essex et al. 2004).
One limitation of our study is the small sample size, which was further reduced by loss of a large proportion of patients in the follow-ups. Comparing to the second follow up, the third follow up complications were greatly influenced by an addition of only one recurrence case; leading to a noticeable increase in recurrence rate to 18.8 % and the overall complications rate was doubled to 31.3 %. Another limitation was the lack of an −80 °C freezer at the hospital causing logistic difficulties in delivering the grafts on the day of surgery.
Conclusion
The processing procedure and prolonged cryopreservation used in this study to prepare and preserve AM grafts was not associated with infection or inflammation, but the rate of pterygium recurrence was unacceptably high, though this rate was not associated with storage duration. The high rate of complications in this study was caused by the low sample size patients attended the proposed follow ups rather than due to the low AM efficacy. The locally produced cryopreserved AM is safe as an adjunct therapy for treatment of primary pterygium excision. The authors suggest an extended study using appropriate number of patients and independent controls in future trials. To overcome cold chain storage limitations; dried AM with the advantage of room temperature storage would be an interesting option for future applications.
References
Alió L, Abad M, Scorsetti DH (2005) Preparation, indications and results of human amniotic membrane transplantation for ocular surface disorders. Expert Rev Med Dev 2:153–160
Alireza B-R, Hamid-Reza A, Babak A, Mohammad-Ali J (2007) Amniotic membrane transplantation. Iran J Ophthalmic Res 2:56–75
Alpay A, Uğurbaş SH, Erdoğan B (2009) Comparing techniques for pterygium surgery. Clin Ophthalmol 3:69–74
Arain M, Yaqub M, Ameen S, Iqbal Z, Naqvi A, Niazi M (2012) Amniotic membrane transplantation in primary pterygium compared with bare sclera technique. J Coll Phys Surg Pak 22:440–443
Besharati M, Miratashi S, Ahamadi A (2008) Pterygium surgery: amniotic membrane or conjunctival autograft transplantation. Clin Res 1:362–366
Ebru T, Eraslana M (2016) Recurrence after primary pterygium excision: amniotic membrane transplantation with fibrin glue versus conjunctival autograft with fibrin glue. Curr Eye Res 41:1–8
Essex R, Snibson G, Daniell M, Tole D (2004) Amniotic membrane grafting in the surgical management of primary pterygium. Clin Exp Ophthalmol 32:501–504
Gicque J-J, Dua HS, Brodie A, Mohammed I, Suleman H, Lazutina E, James D, Hopkinson A (2009) Epidermal growth factor (EGF) variations in amniotic membrane used for ex vivo tissue constructs. Tissue Eng Pt A 15:1919–1927
Hirst L (2003) The treatment of pterygium. Surv Ophthalmol 48:145–180
Hopkinson A, McIntosh R, Tighe P, James D, Dua HS (2006) Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-β content. Invest Ophthalmol Vis Sci 47:4316–4322
ISO 11737-2 (2006). Sterilization of medical devices—Microbiological methods—part 2: tests of sterility performed in the validation of a sterilization process
Kangkeng Z, Jianhao C, Vishal J, Haoyu C (2012) Comparison of pterygium recurrence rates after limbal conjunctival autograft transplantation and other techniques: meta-analysis. Cornea 31:1422–1427
Kaufman S, Jacobs D, Lee B, Deng S, Rosenblatt M, Shtein R (2013) Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology 120:201–208
Khan N, Ahmad M, Baseer A, Kundi N (2010) To compare the recurrence rate of pterygium excision with bare-sclera, free conjunctival auto graft and amniotic membrane grafts. Pak J Ophthalmol 26:138–142
Kheirkhah A, Nazari R, Nikdel M, Ghassemi H, Hashemi H, Behrouz MJ (2011) Postoperative conjunctival inflammation after pterygium surgery with amniotic membrane transplantation versus conjunctival autograft. Am J Ophthalmol 152:733–738
Kheirkhah A, Hashemi H, Adelpour M, Nikdel M, Rajabi M, Behrouz M (2012) Randomized trial of pterygium surgery with mitomycin C application using conjunctival autograft versus conjunctival–limbal autograft. Ophthalmology 119:227–232
Kim JC, Tseng S (1995) Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit cornea. Cornea 14:473–484
Kocamis O, Bilgec M (2014) Evaluation of the recurrence rate for pterygium treated with conjunctival autograft. Graefes Arch Clin Exp Ophthalmol 252:817–820
Koizumi N, Inatomi T, Quantock A, Fullwood N, Dota A, Kinoshita S (2000) Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 19:65–71
Koranyi G, Seregard S, Kopp ED (2004) Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol 88:911–914
Kruse F, Joussen A, Rohrschneider K, You L, Sinn B, Baumann J, Völcker H (2000) Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefe’s Arch Clin Exp Ophthalmol 238:68–75
Kurna SA, Altun A, Aksu B, Kurna R, Sengor T (2012) Comparing treatment options of pterygium: limbal sliding flap transplantation, primary closing, and amniotic membrane grafting. Eur J Ophthalmol 23(4):480–487
Li M, Zhu M, Yu Y, Gong L, Zhao N, Robitaill MJ (2012) Comparison of conjunctival autograft transplantation and amniotic membrane transplantation for pterygium: a meta-analysis. Graefes Arch Clin Exp Ophthalmol 381:250–375
Liu J, Sheha H, Fu Y, Liang L, Tseng SC (2010) Update on amniotic membrane transplantation. Expert Rev Ophthalmol 5:645–661
Liu T, Liu Y, Xie L, He X, Bai J (2013) Progress in the pathogenesis of pterygium. Curr Eye Res 38:1191–1197
Ma DK, See L, Liau S, Tsai R (2000) Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol 84:973–978
Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP (2011) Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int 108:243–248
Norliza WW, Raihan IS, Azwa JA, Ibrahim M (2006) Scleral melting 16 years after pterygium excision with topical Mitomycin C adjuvant therapy. Cont Lens Anterior Eye 29:165–167
Prabhasawat P, Barton K, Burkett G, Tseng S (1997) Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 104:974–985
Qureshi IZ, Fareeha A, Wajd A (2010) Technique for processing and preservation of human amniotic membrane for ocular surface reconstruction. World Acad Sci Eng Technol 69:763–766
Salman A, Dina E (2011) The recurrence of pterygium after different modalities of surgical treatment. Saudi J Ophthalmol 25:411–415
Tananuvat N, Martin T (2004) The results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograft. Cornea 23:458–463
Tekin N, Kaynak S, Saatci A, Cingil G (2001) Preserved human amniotic membrane transplantation in the treatment of primary pterygium. Ophthalmic Surg Lasers 32:464–469
Thomasen H, Pauklin M, Noellec B, Geerlingd G, Vettere J, Stevenf P, Steuhla K-P, Meller D (2011) The effect of long-term storage on the biological and histological properties of cryopreserved amniotic membrane. Curr Eye 36:247–255
Torres-Gimeno A, Mart´ınez-Costa L, Ayala G (2012) Preoperative factors influencing success in pterygium surgery. BMC Ophthalmol 12:1–7
Acknowledgments
This work was funded by the Libyan Authority for Research Science and Technology, Tripoli Libya. The authors thank the Biotechnology Research Centre for providing the equipment and materials used in this study. Thanks to Dr. Amin Bredan for his valuable editing of the manuscript, and thanks are due to Dr. Abdulmunem Abulayha, Dr. Abdul Munam Fellah and Mohamed Mansur for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Marsit, N., Gafud, N., Kafou, I. et al. Safety and efficacy of human amniotic membrane in primary pterygium surgery. Cell Tissue Bank 17, 407–412 (2016). https://doi.org/10.1007/s10561-016-9554-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-016-9554-9