Abstract
Amniotic membrane, the inner layer of the placenta, has biological properties (e.g. promotes epithelization, reduces fibrosis, secretes antimicrobial products and inhibits immune responses) which make it a useful option for several ophthalmologic procedures, especially those involving the ocular surface. Its use in eye surgery has been reported by other authors. To our knowledge, there is a lack of descriptive studies on surgical indications using amniotic membrane in Mexican population. Here we describe the eight years Amniotic Membrane Bank experience in Mexico, including a detailed protocol of the donors selection, tissue harvesting, preparation, storage and distribution of amniotic membrane since its establishment in 2007. Moreover, we describe the Ophthalmological indications of amniotic membrane transplantation of the total of 1686 amniotic membranes fragments used during eight years. The five most common indications for amniotic membrane transplantation were pterygium (46 %), corneal ulcers (12.6 %), conjunctival surface repair (11.1 %), neoplasms (7.4 %), and persistent epithelial defects (7.3 %). In addition, we compared the indications of amniotic membrane use in two different types of Institutions: general hospitals and ophthalmologic reference hospitals. We found interesting differences between the indications and use rates between these institutions, although pterygium was the most frequent pathology that amniotic membrane fragments were used in both institutions, there was up to a five-fold increase in the use of amniotic membrane for correction of persistent epithelial defects in reference hospitals which could be explained due to the more complex and severe ophthalmological pathologies admitted in reference hospitals. In conclusion, Amniotic Membrane is used in a numerous ocular pathologies and especially on pterygium in our Mexican population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amniotic membrane is the inner layer of the placenta, which is in contact to the amniotic fluid and the foetus, this tissue comprises a single layer of ectodermally derived columnar epithelial cells adhered to a basement membrane that contains large amounts of proteogylcans, which in turn is attached to an underlying layer of connective tissue. Mesenchymal fibroblast-like cells are immersed in the zona spongiosa with abundant content of glycoproteins and proteoglycans, this layer is in contact with the chorion (Mamede et al. 2012). It has been proposed that AM has multiple mechanisms of action: promoting epithelization (Mariappan et al. 2010), reducing fibrosis (Sant’Anna et al. 2011), secreting antimicrobial products (Stock et al. 2007) and inhibiting the immune responses (Dominguez-Lopez et al. 2014; Garfias et al. 2011).
Amniotic membrane tissue is immediately obtained from elective caesareans in consented women; after decontaminating the tissue by using a solution enriched with broad-spectrum antibiotics solution for 24 h at 4 °C, the membrane is cut into 2 × 2 cm pieces, placed on a nitrocellulose support and stored at −80 °C until use (Madhavan et al. 2002). The legislature of most of the countries requires serological testing to identify active infections of the donor at the time of donation, and in some cases a serological re-testing to assure an appropriate “window period” for certain microorganisms; consequently, AM is mostly used preserved rather than fresh (Rahman et al. 2009). For preserving AM, two methods are well-accepted and comprise cryopreservation with glycerol and freeze-drying (Riau et al. 2010). It has been reported that cryopreservation using 50 % glycerol effectively preserves the epithelium and basement membrane, as well as it maintains the AM biological properties (Thomasen et al. 2011). As AM is obtained from a living donor and at least two serological tests have to be performed to deliver the tissue for its use in the clinic, a bank of amniotic membrane is mandatory in vision related institutions.
Although the use of AM in ophthalmology was first reported in 1940, it was not until 1992 that its use formally re-appeared as a significant tool in ocular cell surface therapy (Dua et al. 2004); since then, it has been extensively used. The AM can be employed as a graft or as a patch to cover and repair cell surface defects and surgical incisions performed during surgery (Burman et al. 2004). When using as a graft, AM is intended to be a scaffold for epithelial cells to grow; while used as a patch, AM is considered to be a biological bandage (Rahman et al. 2009). Currently, there are many surgical indications of AM in ophthalmology, such as Stevens-Johnson syndrome, chemical or thermal burns and ocular cicatricial pemphigoid, bullous keratopathy, persistent corneal epithelial defects, perforations, pterygium, tumours and symblepharon amongst others (Rahman et al. 2009).
To our knowledge, there is a lack of descriptive studies of the surgical indications using amniotic membrane in Mexican population. The aim of the present study was to describe the Amniotic Membrane Bank function as well as to describe the AM ophthalmic surgical indications of the Amniotic Membrane Bank from the Institute of Ophthalmology “Conde de Valenciana” from January 2007 to December 2014.
Materials and methods
The Ministry of Health granted the Amniotic Membrane Bank of the Institute of Ophthalmology “Conde de Valenciana” Registration number: 14-TR-09-015-0006. Harvesting, preparation, storage, distribution and follow-up of AM have been coordinated by the Amniotic Membrane Bank of the Institute. A detailed review of the Amniotic Membrane Bank records for data validation was performed. Data including patient age, sex, diagnosis and surgical indications were collected from January 2007 to December 2014. From 1714 records, 1686 (98 %) were complete and included for analysis.
Amniotic Membrane Bank protocol
-
1.
Selection criteria. Women who underwent an elective caesarean were invited to participate as possible donors. A strict clinical history as well as a standardized behavioural risk assessment questionnaire on each possible donor was performed. Pregnant women older than 18 years with 36 to 38 weeks of gestation were included. After a careful explanation of donation, signed of a written informed consent was obtained from all possible donors. Women going into labour were excluded. All women were asked to also donate blood samples to perform serological tests at the time of the surgery.
-
2.
Amnion harvesting. Right after delivery, each placenta was macroscopically examined and roughly cleaned, foetal membranes such as amnion and chorion were dissected from the placenta, and located into a sterile recipient containing balanced salt solution with antibiotics and antimicotics, this procedure was performed at the surgical room. The foetal membranes were transported from the obstetrics hospital to the amniotic membrane bank at 4–8 °C and were maintained at that temperature for additional 24 h in the same solution for decontamination.
-
3.
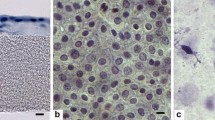
Amnion preparation. Under a biosafety class II cabinet in a clean room, the amnion was carefully and manually separated from the chorion, which was discarded. The amniotic membrane was placed on a nitrocellulose membrane with the epithelium facing up and was cut in 2.5 × 2.5 cm pieces, which were situated in borosilicate glass recipients containing 10 ml of preservation media (RPMI-glycerol) without antibiotics. Immediately after, the membranes were frozen and maintained at −80 °C until use. Representative samples of amniotic membranes were randomly selected to perform histopathological studies. Single layer of columnar epithelial cells adhered on a basement membrane and no leucocytes infiltrate were the main histological characteristics of acceptance of an amnion to be used into the clinic (Fig. 1). During each critical procedure, such as collection, transportation and preparation of amnion, at least three samples were randomly obtained to perform microbial cultures. All cultures must be negative for an amnion acceptance to be used into the clinic.
Fig. 1 Amniotic membrane. Light microscopy photograph of an amniotic membrane. A columnar epithelial cell layer is attached to a basement membrane, which in turn is adhered to a layer of connective tissue. Fibroblast-like cells are immersed in the zona spongiosa which is in contact with the chorion (not shown). Hematoxilin and eosin stained sample. Note the absence of inflammatory infiltrate. Magnification at 63×
-
4.
Amnion storage. Each amnion piece clearly identified was placed on an ultra-freezer at −80 °C. For at least 3 months. At the third month after amnion harvesting, a second blood sample was obtained from the donor to perform serological tests. Table 1 summarizes serological tests and acceptance values criteria.
Table 1 Selection criteria of serological tests results for amniotic membrane transplantation -
5.
Amnion distribution. When amnion was required, a written solicitation was made to the Amniotic Membrane Bank specifying the surgical indications, patient’s demographical data, among many other data. All amnion pieces were sent on dry ice in order to maintain the tissue frozen. For distribution purposes, amniotic membranes were delivered either to reference hospitals (ophthalmological hospitals) or to general hospitals.
-
6.
Quality control assessments: In all the above processes rigorous quality control validations are performed. For the selection criteria, it is needed that a gynaecologist carefully selects the donor paying special attention in the personal antecedents and in the absence of labour. In the hospital there is a donor coordinator, who is in charged for obtaining the consent of each patient; the Ministry of Health authorizes this coordinator for such activity. On amnion harvesting process there are strict microbiology controls. Representative samples from the amniotic liquid and the placenta are obtained in Stuart medium, which is then transferred to TSA (trypticase soy agar). For amnion preparation representative samples for all solutions are cultured on TSA. The gloves for each participant on the preparation are exposed to TSA petri dishes. At the final stage of the preparation, representative samples of the solutions are cultured on TSA. In order to certificate that the clean room as well as the Class II Biosafety hood are adequate to prepare amnion, representative parts of the clean room as well as the hood are randomly selected to be cultured on TSA medium, monthly. All the cultures are performed at 37 °C in a microbiological hood for 48 h in which all have to be negative for acceptance; meanwhile the clean room as well as the hood presenting <10 CFU/5 min are accepted. In the case of fungi, all the samples are treated similarly, except the medium is SDA (Sabouraud Dextrose Agar) and the plates are incubated at 28 °C for 7 days, the results have to be negative for acceptance.
Statistical analysis
Mean and standard deviation (SD) for age of the donor and receptor, time in which AM was obtained and delivered, and the number of AM by year was calculated. Frequency and percentages of diagnoses and indications of the use of AM for the all sample and by type of hospital (reference hospitals or general hospitals) were calculated. Analysis was performed using STATA/MP 13.1 (Stata Corporation, College Station, TX, USA.).
Results
Amniotic membrane procuration
From July 2006 to December 2013, 46 amniotic membranes were procured, processed and stored. Seven of them were discarded because the selection criteria of the serological results were not suitable for transplantation (Table 1); meanwhile, 10 amniotic membranes were designated for research purposes. Therefore, 29 amniotic membranes were used for clinical purposes. In total, 1914 (2.5 × 2.5 cm) amniotic membrane fragments were appropriate for ophthalmological surgeries. The mean number of pieces obtained from each amniotic membrane was 66 (±5). The average age of the donor women was 31 years old (±6.3). (Fig. 2) Cephalopelvic disproportion was the indication of the caesarean surgery in all of the cases. The average time in which amniotic membrane was collected, processed and delivered was three months.
Flowchart of the amniotic membrane selection on the Amniotic Membrane Bank. From the total (46) collected amniotic membranes, seven were discarded after strict selection criteria, since the serological tests results were unsuitable for use as a human transplant; meanwhile, ten of the total were designated for research purposes. Twenty-nine amniotic membranes were accepted for human transplantation. One thousand nine-hundred and fourteen amniotic membrane fragments were obtained, from which, 1686 are analyzed in this report
Ophthalmological indications of amniotic membrane transplantation
A total of 1686 amniotic membranes fragments were used during a period of time of eight years. The number of amniotic membranes used per year ranged from 103 to 459. The mean age of patients was 48.9 years (±19.4) from which 822 (48.7 %) were women and 863 (51.3 %) were men. The majority of the patients 1035 (61.4 %) were from reference hospitals, meanwhile 651 (38.6 %) patients were from general hospitals distributed all over the country.
Considering the total of patients, the five most common indications for amniotic membrane transplantation were pterygium (46 %), corneal ulcers (12.6 %), conjunctival surface repair (11.1 %), neoplasms (7.4 %), and persistent epithelial defects (7.3 %). (Table 2) The distribution of main causes of indications for AM transplantation between reference and general hospitals was different. Although in both types of institutions pterygium was the most indicated pathology in which amniotic membrane was used, around 60 % was distributed to general hospitals, while 37 % of the amniotic membrane fragments were used in patients in the reference hospitals for this common pathology. In contrast to pterygium, amniotic membrane was used in similar frequency for corneal ulcers treatment, around 12 % of amniotic membrane fragments were utilized in both kinds of institutions. The behavior of the amniotic membrane use in conjunctival surface repair was slightly different in both types of institutions, 12 and 9 % in reference hospitals and general hospitals, respectively. Interestingly, in the case of neoplasms, 9 % of the amniotic membrane fragments were used in reference hospitals, while only around 5 % of the pieces were indicated in general hospitals. Similarly, in persistent epithelial defects, amniotic membrane pieces were used four times more frequently in reference hospitals (10.5 %) than in general hospitals (2.2 %) (Table 3).
Discussion
In the present study we have described the function of our Amniotic Membrane Bank. In this context, the selection criteria of donor women as well as the amniotic membrane preparation are similar to those proposed by other authors (Cirman et al. 2014; Paolin et al. 2015). Principal differences are found in the preservation procedure, for example, Paolin et al., perform the cryopreservation process with a programmable cryogenic freezer and maintain the amniotic membrane fragments at −180 °C in vapour phase liquid nitrogen, in contrast and similar to our technique, Cirman et al., preserve the fragments of amnion at −80 °C. Interestingly, Laurent et al. (2014) determined that cryopreservation at −80 °C is an appropriate temperature for preserving at least 40 % of cell viability. Whether differences on temperature affect final clinical results is still a matter for further studies. Another critical issue is the execution of a second serological test from the donor, in order to identify possible pathogens in a “window period”. In our Amniotic Membrane Bank we routinely perform a second serological test for each donor (Table 1); however, the implementation of the use of nucleic acid amplification test (NAT) as it is currently performed in other amniotic membrane banks (Cirman et al. 2014), will significantly narrow the window period (Busch et al. 2005; Weusten et al. 2011). In our protocol of amniotic membrane preparation we have considered mandatory to perform histological description, because it has been clearly determined that leukocytes infiltrating the amnion indicate an intrauterine infection (Holzman et al. 2013), risking the receptor health. Moreover, labour is one of our exclusion criteria; this is supported because it has been demonstrated that uterine labour is an inflammatory process consisting of leukocytes and pro-inflammatory cytokines presence on amnion tissue (Osman et al. 2003). Therefore, when histological analysis is performed, all observations have to be negative for leukocyte infiltrate into the amniotic membrane. For preservation purposes, the AM fragments are commonly stored no more than two years at −80 °C (Cirman et al. 2014), however in our study, three months was the average conservation time, this could be due to a high utilization amniotic membrane rate amongst reference and general hospitals and a low amniotic membrane procuration rate in our Tissue Bank. Although the use of amniotic membrane in ophthalmology has been reported since 1992 (Dua et al. 2004), it was not until 2014, that Mexican Health Legislation considered for the first time the existence of Amniotic Membrane Banks in Mexico to be used for transplantation purposes. In 2003 the first Amnion Bank was described in Mexico in which radiosterilization is included on the preservation technique, however, no further information about its activity has been published (Esther Martinez-Pardo and Lourdes Reyes-Frias 2003). Our Amniotic Membrane Bank was established as a research project with a Federal Grant support from the National Council of Science and Technology (CONACYT-SALUD-C01-076) on 2004, and started activities at the beginning of 2007. Currently, we have two agreements to obtain Amniotic Membrane Tissue, one is with the Mexican City Government and the other is with a Private General Hospital. It is important to mention that function protocol was adapted from the Banque de Tissus Humains at the Saint Louis Hospital in Paris, France.
Our results show that pterygium is the most frequent ocular surface pathology (46 %) in which amniotic membrane is used. These results are in accordance to those recently described by Yang et al., who reported 48 % of pterygium cases treated with amniotic membrane (Yang et al. 2014). It has been reported that pterygium is a frequent disease in population living close to the equator, thus the common factor appears to be the latitude, since pterygia primary occur within the peri-equatorial “pterygium belt” which is located within latitudes 37º north and south of the equator and Mexico is situated in this “pterygium belt” (Nava-Castaneda et al. 2015). This could explain the high frequency indication of amniotic membrane in pterygium in our population. In general hospitals the frequency of use of amniotic membranes for pterygia treatment was 60 %, in comparison to 37 % in reference hospitals, a possible explanation of this difference is because there is an increase in more rare ocular surface pathologies in reference hospitals than in general hospitals; for example, amniotic membrane is used almost five more times on persistent epithelial defects in reference hospitals than in general hospitals, similarly occurs when analysis is made on neoplasms, amniotic membrane is used almost two times more in reference hospitals than in general hospitals. Yang et al., who reported the indications of amniotic membrane grafts in Ontario, present similar results, except that the use of amniotic membrane on neoplasms precedes persistent epithelial defects (Yang et al. 2014); although it is not explicitly referred on the text that the surgeries were performed on reference hospitals, there is evidence that 97 % of all the reported surgeries were performed by ophthalmologic subspecialties surgeons. These results suggest that reference hospitals are in charged to treat more rare and complicated cases of diseases. Interestingly, the use frequency of amniotic membrane on corneal ulcers was similar on both general and reference hospitals (12 %), ranking the second place of our list after pterygium (46 %). In contrast, corneal ulceration is the most frequent condition treated by amniotic membrane transplantation with up to 45 % of the total treated patients in an Italian report (Paolin et al. 2015). This discrepancy on the uses of Amniotic Membranes suggests the different diseased population on which Amniotic Membrane is being used depending on the region.
References
Burman S, Tejwani S, Vemuganti GK, Gopinathan U, Sangwan VS (2004) Ophthalmic applications of preserved human amniotic membrane: a review of current indications. Cell Tissue Bank 5:161–175. doi:10.1023/B:CATB.0000046067.25057.0a
Busch MP et al (2005) A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion 45:254–264. doi:10.1111/j.1537-2995.2004.04215.x
Cirman T, Beltram M, Schollmayer P, Rozman P, Kreft ME (2014) Amniotic membrane properties and current practice of amniotic membrane use in ophthalmology in Slovenia. Cell Tissue Bank 15:177–192. doi:10.1007/s10561-013-9417-6
Dominguez-Lopez A, Bautista de Lucio VM, Serafin-Lopez J, Robles-Sanchez E, Garfias Y (2014) Amniotic membrane modulates innate immune response inhibiting PRRs expression and NF-kappaB nuclear translocation on limbal myofibroblasts. Exp Eye Res 127:215–223. doi:10.1016/j.exer.2014.08.002
Dua HS, Gomes JA, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv Ophthalmol 49:51–77
Esther Martinez-Pardo M, Lourdes Reyes-Frias M (2003) The tissue bank at the national nuclear research institute in Mexico. Cell Tissue Bank 4:163–168. doi:10.1023/B:CATB.0000007038.09698.c2
Garfias Y, Zaga-Clavellina V, Vadillo-Ortega F, Osorio M, Jimenez-Martinez MC (2011) Amniotic membrane is an immunosuppressor of peripheral blood mononuclear cells. Immunol Invest 40:183–196. doi:10.3109/08820139.2010.532266
Holzman C, Senagore PK, Wang J (2013) Mononuclear leukocyte infiltrate in extraplacental membranes and preterm delivery. Am J Epidemiol 177:1053–1064. doi:10.1093/aje/kws351
Laurent R, Nallet A, Obert L, Nicod L, Gindraux F (2014) Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank 15:267–275. doi:10.1007/s10561-014-9437-x
Madhavan HN, Priya K, Malathi J, Joseph PR (2002) Preparation of amniotic membrane for ocular surface reconstruction. Indian J Ophthalmol 50:227–231
Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349:447–458. doi:10.1007/s00441-012-1424-6
Mariappan I et al (2010) In vitro culture and expansion of human limbal epithelial cells. Nat Protoc 5:1470–1479. doi:10.1038/nprot.2010.115
Nava-Castaneda A, Ulloa-Orozco I, Garnica-Hayashi L, Hernandez-Orgaz J, Jimenez-Martinez MC, Garfias Y (2015) Triple subconjunctival bevacizumab injection for early corneal recurrent pterygium: one-year follow-up. J Ocul Pharmacol Ther 31:106–113. doi:10.1089/jop.2014.0060
Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9:41–45
Paolin A, Cogliati E, Trojan D, Griffoni C, Grassetto A, Elbadawy HM, Ponzin D (2015) Amniotic membranes in ophthalmology: long term data on transplantation outcomes. Cell Tissue Bank. doi:10.1007/s10561-015-9520-y
Rahman I, Said DG, Maharajan VS, Dua HS (2009) Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond) 23:1954–1961. doi:10.1038/eye.2008.410
Riau AK, Beuerman RW, Lim LS, Mehta JS (2010) Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 31:216–225. doi:10.1016/j.biomaterials.2009.09.034
Sant’Anna LB, Cargnoni A, Ressel L, Vanosi G, Parolini O (2011) Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant 20:441–453. doi:10.3727/096368910X522252
Stock SJ, Kelly RW, Riley SC, Calder AA (2007) Natural antimicrobial production by the amnion. Am J Obstet Gynecol 196(255):e251–e256. doi:10.1016/j.ajog.2006.10.908
Thomasen H et al (2011) The effect of long-term storage on the biological and histological properties of cryopreserved amniotic membrane. Curr Eye Res 36:247–255. doi:10.3109/02713683.2010.542267
Weusten J, Vermeulen M, van Drimmelen H, Lelie N (2011) Refinement of a viral transmission risk model for blood donations in seroconversion window phase screened by nucleic acid testing in different pool sizes and repeat test algorithms. Transfusion 51:203–215. doi:10.1111/j.1537-2995.2010.02804.x
Yang PT, Sharpen L, Chan A, Chan CC (2014) Indications for amniotic membrane grafts used in Ontario from 2011 to 2012. Can J Ophthalmol 49:399. doi:10.1016/j.jcjo.2014.05.006
Acknowledgments
The authors wish to thank Dr. Mohamed Jarraya for his helpful suggestions in improving this manuscript. This work was supported by CONACYT-SALUD-2004-C01-096, CONACYT-SALUD-2011-C01-160286, Conde de Valenciana Foundation, and the Instituto Nacional de Perinatología Isidro Espinosa de los Reyes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chávez-García, C., Jiménez-Corona, A., Graue-Hernández, E.O. et al. Ophthalmic indications of amniotic membrane transplantation in Mexico: an eight years Amniotic Membrane Bank experience. Cell Tissue Bank 17, 261–268 (2016). https://doi.org/10.1007/s10561-015-9540-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-015-9540-7