Abstract
In cell culture, medium supplemented with fetal bovine serum is commonly used, and it is widely known that fetal bovine serum supplies an adequate environment for culture and differentiation of stem cells. Nevertheless, the use of xenogeneic serum can cause several problems. We compared the effects of four different concentrations of autologous serum (1, 2, 5, and 10 %) on expansion and adipogenic differentiation of adipose-derived stem cells using 10 % fetal bovine serum as a control. The stem cells were grafted on nude mice and the in vivo differentiation capacity was evaluated. The isolation of adipose-derived stem cells was successful irrespective of the culture medium. The proliferation potential was statistically significant at passage 2, as follows: 10 % autologous serum >10 % fetal bovine serum = 5 % autologous serum >2 % autologous serum = 1 % autologous serum. The differentiation capacity appeared statistically significant at passage 4, as follows: 10 % fetal bovine serum >10 % autologous serum = 5 % autologous serum >2 % autologous serum = 1 % autologous serum. Ten percent autologous serum and 10 % fetal bovine serum had greater differentiation capacity than 1 and 2 % autologous serum in vivo, and no significant difference was observed between the groups at ≥5 % concentration at 14 weeks. In conclusion, 10 % autologous serum was at least as effective as 10 % fetal bovine serum with respect to the number of adipose-derived stem cells at the end of both isolation and expansion, whereas 1 and 2 % autologous serum was inferior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose-derived stem cells (ASCs) are easy to harvest because of their abundant availability, excellent expansion capacity, and their differentiation potential (Aust et al. 2004; De Ugarte et al. 2003; Zuk et al. 2002). Human adipose tissue in particular is easily obtained from patients undergoing plastic surgery. Therefore, ASCs are considered to be an attractive alternative to bone marrow-derived mesenchymal stem cells (BMSCs) (De Ugarte et al. 2003).
For application of stem cells to clinical studies, such as cell therapy and tissue engineering, a large number of stem cells are needed (Baksh et al. 2004). Most isolation and expansion protocols for clinical-scale production of mesenchymal stem cells use a medium supplemented with fetal bovine serum (FBS). However, use of xenogeneic serum can cause several problems. Viral or bacterial infections and prion transmission can arise (Dedrick 1997; Will et al. 1996; Klein and Dumble 1993). The immunologic reactions to fetal serum albumin have been reported (Kievits et al. 1988; Mackensen et al. 2000).
A chemically-defined, standardized, xenogeneic antigen- and serum-free media is ideal. An alternate formula to FBS with artificially created growth factors and substances has been developed (Brunner et al. 2010; Falkner et al. 2006). Moreover, a serum-free 3D culture system that allows the expansion of ASCs as floating spheres in a defined medium was introduced (Dromard et al. 2011). However, the switch to serum-free media still demands a time-consuming literature survey and a manufacturer search for appropriate medium formulations, because of production problems, formulation upgrades or simply the associated costs (Dromard et al. 2011; Brunner et al. 2010; Falkner et al. 2006). Therefore, considerable effort has been directed toward searching for possible FBS alternatives. Recently, human blood supplements have been identified as promising substitutes, and include autologous (Honmou et al. 2011; Pérez-Ilzarbe et al. 2009; Nimura et al. 2008; Kobayashi et al. 2005; Shahdadfar et al. 2005; Stute et al. 2004; Koller et al. 1998) and allogeneic serum (Kocaoemer et al. 2007; Shahdadfar et al. 2005; Kuznetsov et al. 2000; Koller et al. 1998), and platelet derivatives (Pérez-Ilzarbe et al. 2009; von Bonin et al. 2009; Kocaoemer et al. 2007).
There have been studies comparing expansion and osteogenic differentiation of BMSCs with autologous serum (AS) to FBS, and studies which have determined the best concentration of AS as the medium for expanding BMSCs (Pérez-Ilzarbe et al. 2009; Kobayashi et al. 2005; Shahdadfar et al. 2005; Stute et al. 2004; Koller et al. 1998). There have been few studies comparing AS to FBS as the culture medium for ASCs or demonstrating a difference in vivo. Therefore, we compared the effects of four different concentrations of AS (1, 2, 5, and 10 %) on the expansion and adipogenic differentiation of ASCs using 10 % FBS as the control, and ASCs which were cultivated and differentiated in each media were grafted on nude mice, and the in vivo differentiation capacity was evaluated.
Methods
Donor
Of patients who underwent liposuction or transverse rectus abdominis myocutaneous flaps at Seoul National University Hospital, 10 patients with a voluntary agreement for written informed consent were included in this study. All were females under 60 years of age, with a body mass index less than 30 kg/m2. Discarded adipose tissues or lipoaspirates were harvested from the abdomen, flank and thigh regions, and 100 ml of blood were obtained from the each patient. This study was conducted after obtaining approval by the Institutional Review Board of Seoul National University (H-0605-032-174).
Autologous serum
Venous whole blood was drawn into blood bags without anticoagulant and stored at 4 °C overnight. The blood was centrifuged at 3,000 rpm for 10 min, the serum was aliquoted into Eppendorf tubes (Eppendorf, Hamburg, Germany), and stored at −20 °C until use. Heat inactivation of the serum was not performed because growth factors and nutrients necessary for cell culture may be temperature-sensitive. Four concentrations of AS were tested (1, 2, 5, and 10 %).
Isolation and expansion of ASCs
Minced adipose tissues or lipoaspirates were washed with phosphate buffer solution (Gibco-BRL, Grand Island, NY, USA), and digested with 0.5 % collagenase type I (Worthington Biochemical Corp., Lakewood, NJ, USA) under gentle agitation for 60 min at 37 °C. The digested tissues were centrifuged at 470 g for 5 min to obtain a pellet. The supernatant discarded and the cell pellet resuspended in 160 mM ClNH4 to eliminate red blood cells. After 10 min at 37° C, the cells were again centrifuged, resuspended in Dulbecco’s modified Eagle’s media containing 0.2 mM ascorbic acid and 10 % FBS, and were filtered through a 100-μm nylon cell strainer (BD Biosciences, Bedford, MA, USA). The cells were counted on a hemocytometer (Superior, Marienfeld, Germany), and 3 × 104 cells per well were seeded in 24-well plates (Nunc, Roskilde, Denmark) with Dulbecco’s modified Eagle’s media with 10 % FBS, and 1, 2, 5, and 10 % AS at 37 °C in a humidified atmosphere containing 5 % CO2. After 3 days, the non-adherent cells were discarded and the plastic adherent cells were further expanded with the medium described above and fed every 3 days. At 70–80 % confluency, cells were trypsinized and reseeded in other wells. At passage 2, the cells were counted on a hemocytometer and the number of cells between each group was compared.
Adipogenic differentiation
ASCs were plated at a concentration of 3 × 104 cells per well. Adipogenic differentiation medium was added after 1–2 days following confluence for 48 h, as follows: 0.5 mM isobutyl-methylxanthine (Sigma, St. Louis, MO, USA), 0.1 μM dexamethason (Sigma), 0.1 % insulin transferrin selenium (ITS), and 0.1 mM indomethacin (BD Biosciences, Bedford, MA, USA). Then, the same medium with only insulin added was used for 1–2 days. The differentiation was conducted for 14 days. The differentiation was assessed at passage 4 using Oil Red O stain as an indicator of intracellular lipid accumulation. The cells were washed with phosphate buffered solution, then fixed in 10 % formalin for 10 min and stained with 0.3 % Oil Red O (Sigma) in a mixture of isopropanol and water (3:2) and extracted with 4 % Nonidet (Sigma)/isopropanol for quantification. Absorbance was measured using a spectrophotometer (Molecular Device Co., Sunnyvale, CA, USA) at 490 nm. The mean ± SD of the absorbance of five groups (1, 2, 5, and 10 % AS, and 10 % FBS) was calculated.
In vivo transplants
Absorbable gelatin sponge (Gelform; Pfizer, New York, NY, USA) was used to deliver cells (McCarty et al. 2010). The carrier was cut with a 7-mm punch into uniform cylinder blocks. The cells of each group were suspended in culture medium at a concentration of 5 × 105 cells in 25 μl, and seeded in the carrier. A total of 10 BALB/c nude mice (weight 20–25 g) were used. All animal procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (Seoul, Korea). Briefly, animals were anesthetized using inhaled isofluorane. Six small incisions were performed on the back. The surrounding soft tissues between the pannuculus carnosus and deep fascia of the muscles were dissected using small Metzembaum scissors. After pocketing, the absorbable gelatin sponges containing the cells of each group (1, 2, 5, and 10 % AS, and 10 % FBS) and the control group were transplanted into each pocket. The control group was defined as having no cell but the absorbable gelatin sponge. Skin was then closed and sutured with 6-0 blue nylon. Fourteen weeks after transplantation, biopsies of the grafts were performed, and the volumes were measured by the graduated ruler under the operating microscope. The greatest longitudinal diameter (length) and the greatest transverse diameter (width) were determined. Graft volume was calculated by the modified ellipsoidal formula: Tumor volume = 1/2(length × width 2) (Euhus et al. 1986; Tomayko and Reynolds 1989). The specimens were formalin-fixed and paraffin-embedded. Four-micrometer sections were deparaffinized and stained with hematoxylin and eosin. To identify the engraftment of ASCs, the monoclonal mouse antihuman nuclei (1:100; Chemicon) was used as a marker.
Statistical analysis
All data were used to calculate the group means ± standard deviation. The Kruskal–Wallis test and the Mann–Whitney U test for post hoc test were used to compare the values of cell numbers after expansion, absorbance after adipogenic differentiation, and graft volume among groups. The non-parametric methods were used to compare the whole of the group means. Differences were considered to be significant at a p < 0.05. All statistical tests were performed using the SAS software package (version 9; SAS Institute, Cary, NC, USA).
Results
Isolation and expansion potential
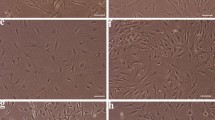
The isolation of ASCs was successful irrespective of the culture medium. For comparing the effect of different serum conditions on the proliferative capacity of ASCs, the number of cells was counted. The cells were observed grossly by light microscopy on day 5 of primary culture. At this time, the highest cell number was generated with 10 % AS (Fig. 1). The difference between each group was more remarkable at passage 2. The number of cells in each group at passage 2 was an average of 219,000 ± 60,452 with 1 % AS, 264,000 ± 47,187.57 with 2 % AS, 374,000 ± 64,325.56 with 5 % AS, 538,000 ± 76,274.36 with 10 % AS, and 428,000 ± 26,677.55 with 10 % FBS (Fig. 2). The proliferation potential was statistically significant, as follows: 10 % AS >10 % FBS = 5 % AS >2 % AS = 1 % AS. The connected single-donor data points were shown in Fig. 3.
Expansion potential of ASCs cultured in 1, 2, 5, and 10 % AS and 10 % FBS at passage 2. There were significant differences among groups by Kruskal–Wallis test (p < 0.001). There was also a significant difference between each of the 2 groups (*p < 0.05). No significant differences were observed between 1 % AS and 2 % AS, and 5 % AS and 10 % FBS. 10 % AS showed the most prominent expansion characteristics. T-bars represent SD from the mean. AS autologous serum, FBS fetal bovine serum
Adipogenic differentiation capacity
We observed the formation of lipid vacuoles in all groups. The mean absorbance at each group on passage 4 was 0.027097 ± 0.008717 with 1 % AS, 0.034835 ± 0.014123 with 2 % AS, 0.0433 ± 0.019894 with 5 % AS, 0.255267 ± 0.086207 with 10 % AS, and 0.5086 ± 0.126216 with 10 % FBS (Fig. 4). The differentiation capacity appeared statistically significant, as follows: 10 % FBS >10 % AS = 5 % AS >2 % AS = 1 % AS. The differentiation capacity depended on the serum concentration between the AS groups. We observed that 10 % FBS had a significantly higher differentiation capacity than 10 % AS when comparing 10 % FBS with 10 % AS (p = 0.0004). The connected single-donor data points were shown in Fig. 5.
Adipogenic differentiation of ASCs cultured in 1, 2, 5, and 10 % AS and 10 % FBS at passage 4. There were significant differences among groups by Kruskal–Wallis test (p < 0.001). There was also a significant difference between each of the 2 groups (*p < 0.05). No significant differences were observed between 1 % AS and 2 % AS, and 2 % AS and 5 % AS. The differentiation capacity depended on the serum concentration between AS groups. Ten percent FBS had a significantly higher differentiation capacity than 10 % AS when compared with 10 % FBS with 10 % AS (p = 0.0004). AS autologous serum, FBS fetal bovine serum
Gross and histologic measurement after in vivo transplants
At 14 weeks, the transplanted gelatin sponge had disappeared and the light yellow soft mass-like adipose tissue was found (Fig. 6). Because there was little subcutaneous tissue in the backs of the nude mice, the graft was clearly distinguished from the surrounding subcutaneous tissue and easily separated. The biopsies with hematoxylin and eosin stain showed typical adipose cells (Fig. 7, left). Through the use of the anti-human antibodies, persistent transplanted human cells could be detected in sections at the time of sacrifice (Fig. 7, right). The volume of grafts at each group was an average of 0.005 ± 0.002236 mm3 with 1 % AS, 0.0045 ± 0.0035 mm3 with 2 % AS, 0.0085 ± 0.006344 mm3 with 5 % AS, 0.012 ± 0.006782 mm3 with 10 % AS, and 0.0115 ± 0.007089 with 10 % FBS (Fig. 8). Ten percent AS and 10 % FBS had greater differentiation capacity than 1 and 2 % AS in vivo, and no significant difference was observed between the groups at a concentration of ≥5 %. The connected single-donor data points were shown in Fig. 9.
In vivo transplantation of adipogenic differentiated stem cells. a Immediate transplantation. b After 14 weeks of the transplantation. Light yellow colored fat-like tissues were clearly distinguished from the surrounding subcutaneous tissue. AS autologous serum, FBS fetal bovine serum. (Color figure online)
Histologic results after 14 weeks of in vivo transplantation. (left) Hematoxylin and eosin staining shows droplets of fat and signet ring sign, which are distinctive findings of adipose tissue. One bar means 500 μm. (right) After treatment with anti-human nuclei antibodies, intense brown labelling of the cell nuclei was detected, indicating that the biopsy tissue is of human origin. One bar means 100 μm
Discussion
Considerable efforts have been directed toward searching for possible FBS alternatives. Human blood supplements have been identified as promising substitutes, which have a similar effect on cell culture compared with FBS and simultaneously are free from ethical concerns and infection (Kocaoemer et al. 2007; Kobayashi et al. 2005; Shahdadfar et al. 2005; Stute et al. 2004). Human blood supplements include autologous and allogeneic serum, and platelet derivatives. The effectiveness of human serum was based mainly on studies using AS and most studies have suggested that AS is similar or better than FBS with respect to the isolation, expansion, and differentiation of stem cells. Shahdadfar et al. (2005) reported that BMSCs were expanded rapidly and with stable gene expression in AS than FBS. Kobayashi et al. (2005) noted that AS might provide sufficient ex vivo expansion of human BMSCs possessing multi-differentiation potential than FBS. Stute et al. (2004) noted that 10 % AS appeared at least as good as 10 % FBS with respect to both isolation and expansion of human mesenchymal stem cells, while 1 % and 3 % AS was inferior. Because the amount of AS is limited, further research of allogeneic serum is being conducted. Thus far, allogeneic serum has been insufficient to substitute for FBS because of inconsistent results, which have been effective in expanding ASCs (Kocaoemer et al. 2007), but not in expanding BMSCs (Shahdadfar et al. 2005; Koller et al. 1998; Kuznetsov et al. 2000). Koller et al. (1998) described more favorable effects of animal sera (20 % FBS, 20 % horse serum, or a mixture of both) on colony-forming unit fibroblast (CFU-F) formation in comparison with 20 % allogeneic human plasma. Kuznetsov et al. (2000) tested the proliferation and colony-forming efficiency of human BMSCs in α-MEM until two passages in four donors and bone formation in vivo with 20 % FBS, which were better than culture in 20 % human AB-serum.
There have been several studies comparing the expansion and osteogenic differentiation of BMSCs with AS to FBS, or which concentration of AS was the best medium for expanding BMSCs (Pérez-Ilzarbe et al. 2009; Kobayashi et al. 2005; Shahdadfar et al. 2005; Stute et al. 2004; Koller et al. 1998). However, the effectiveness of AS as the culture medium for mesenchymal stem cells may depend on the origin of the mesenchymal stem cells, and there has been little research involving ASCs. Therefore, we compared the effects of four different concentrations of AS (1, 2, 5, and 10 %) on the expansion and adipogenic differentiation of ASCs using 10 % FBS as the control. Because in vitro expansion and differentiation which were controlled under an independent environment to the exclusion of several interferences and changes were different from in vivo, ASCs which were cultivated and differentiated in each media were grafted on nude mice, and the in vivo differentiation capacity was evaluated. Our study did not include concentrations >10 %. Because the amount of AS is limited, and Spees et al. (2004) reported that calf serum protein was detected in cultures of mesenchymal stem cells with 20 % FBS and caused strong immunologic reactions. We evaluated the cell expansion potential at passage 2 and differentiation capacity at passage 4. Because there was a significant difference between the groups on day 11 in the expansion of BMSCs using human serum, platelet-rich plasma, and FBS (Kocaoemer et al. 2007), and the difference in adipogenic differentiation potential at passage 4 was significant (Stute et al. 2004). In vitro 10 % AS appeared to be at least as good as 10 % FBS with respect to expansion of ASCs, while 1, 2, and 5 % AS were inferior. It can be safely assumed that higher concentrations of AS contain higher amounts of mitogenic growth factors. For adipogenic differentiation, 10 % FBS was more effective than AS regardless of the concentration. It appeared identical to the results reported by Stute et al. (2004) and Oreffo et al. (1997). This may be because 10 % FBS has a higher degree of adipogenic factors and lipid content (Stute et al. 2004). In in vivo transplants, no significant difference in adipogenic differentiation capacity was observed between 10 % AS and 10 % FBS. We grafted ASCs into nude mice on day 6 of adipogenic differentiation. At the timing of grafting there were some differences in adipogenic differentiation according to each concentration or material. However, except for initial days on the process of in vitro differentiation before grafting on nude mice, the rest of the conditions were similar. Therefore, it cannot help being affected insufficiently from each concentration or material, which might have resulted in a difference of differentiation capacity between in vitro and in vivo. Just like our results, Nimura et al. (2008) noted that the in vitro chondrogenic potential of human synovial MSCs was higher with 20 % FBS than with 10 % AS, but the in vivo chondrogenic potential of rabbit synovial MSCs was similar between two groups following the grafting of undifferentiated rabbit synovial MSCs into rabbits.
With respect to the number of ASCs at the end of both isolation and expansion, 10 % AS appeared at least as effective as a 10 % FBS, whereas 1 and 2 % AS were inferior. Therefore, it can be safely assumed that 10 % AS contains a sufficient amount of the growth factors and substances that are necessary for the isolation and expansion of ASCs, and the cellular yield is high enough to generate ASCs on a clinical scale without the addition of growth factors. However, AS has limited availability. Kobayashi et al. (2005) noted that the addition of basic fibroblast growth factor (bFGF) to AS enhanced the proliferation rate of BMSCs significantly. It can be safely assumed that the amount of AS necessary for cell culture may be reduced by the addition of bFGF. However, Kobayashi et al. (2005) did not compare AS with bFGF to that without bFGF, and in the results from a study by Pérez-Ilzarbe et al. (2009), the addition of bFGF to 10 % AS do not cause a significant difference in the expansion of BMSCs when compared to 10 % FBS. Therefore, further studies will be necessary to confirm the effect of bFGF.
Up until just a few years ago, there had been a number of clinical trials exploring the use of mesenchymal stem cells for treatment of various diseases, most of which used FBS as the culture medium (Quarto et al. 2001; Vacanti et al. 2001; Wakitami et al., 2002; Le Blanc et al. 2004; Bang et al. 2005). However, most recently, clinical trials of mesenchymal stem cells using human blood supplements as the culture medium have been conducted. von Bonin et al. (2009) evaluated BMSCs expanded in human platelet lysates in patients with refractory graft versus host disease. Honmou et al. (2011) introduced the BMSCs, expanded in AS, into stroke patients. However, there has been few clinical trials involving ASCs expanded in human blood supplements. To elucidate the effectiveness of other human blood supplements as well as AS as a culture medium for ASCs, further clinical trials of ASCs need to be performed with a supplement free of xenogeneic proteins to ensure adherence to GMP and the delivery of mesenchymal stem cells as a clinically safe cell product (Dimarakis and Levicar 2006).
References
Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6:7–14
Baksh D, Song L, Tuan RS (2004) Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8:301–316
Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57:874–882
Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G (2010) Serum-free cell culture: the serum-free media interactive online database. ALTEX 27:53–62
De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109
Dedrick VA (1997) Determining the safety of medical devices containing animal tissues: the new European standards. J Regul Affairs Prof Soc 2:20
Dimarakis I, Levicar N (2006) Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells 24:1407–1408
Dromard C, Bourin P, André M, De Barros S, Casteilla L, Planat-Benard V (2011) Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Exp Cell Res 317:770–780
Euhus DM, Hudd C, LaRegina MC, Johnson FE (1986) Tumor measurement in the nude mouse. J Surg Oncol 31:229–234
Falkner E, Appl H, Eder C, Losert UM, Schöffl H, Pfaller W (2006) Serum free cell culture: the free access online database. Toxicol In Vitro 20:395–400
Honmou O, Houkin K, Matsunaga T et al (2011) Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134:1790–1807
Kievits F, Boerenkamp WJ, Ivanyi P (1988) H-2-dependent binding of xenogeneic beta 2-microglobulin from culture media. J Immunol 140:4253–4255
Klein R, Dumble LJ (1993) Transmission of Creutzfeldt-Jakob disease by blood transfusion. Lancet 341:768
Kobayashi T, Watanabe H, Yanagawa T et al (2005) Motility and growth of human bone-marrow mesenchymal stem cells during ex vivo expansion in autologous serum. J Bone Joint Surg Br 87:1426–1433
Kocaoemer A, Kern S, Kluter H, Bieback K (2007) Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25:1270–1278
Koller MR, Maher RJ, Manchel I, Oxender M, Smith AK (1998) Alternatives to animal sera for human bone marrow cell expansion: human serum and serum-free media. J Hematother 7:413–423
Kuznetsov SA, Mankani MH, Robey PG (2000) Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation 70:1780–1787
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363:1439–1441
Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A (2000) Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother 49:152–156
McCarty RC, Xian CJ, Gronthos S, Zannettino AC, Foster BK (2010) Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J 23:204–210
Nimura A, Muneta T, Koga H, Mochizuki T, Suzuki K, Makino H, Umezawa A, Sekiya I (2008) Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthr Rheum 58:501–510
Oreffo RO, Virdi AS, Triffitt JT (1997) Modulation of osteogenesis and adipogenesis by human serum in human bone marrow cultures. Eur J Cell Biol 74:251–261
Pérez-Ilzarbe M, Diez-Campelo M, Aranda P, Tabera S, Lopez T, del Cañizo C, Merino J, Moreno C, Andreu EJ, Prósper F, Pérez-Simón JA (2009) Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion 49:1901–1910
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344:385–386
Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE (2005) In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 23:1357–1366
Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ (2004) Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cell for cell and gene therapy. Mol Ther 9:747–756
Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR (2004) Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol 32:1212–1225
Tomayko MM, Reynolds CP (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24:148–154
Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J (2001) Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med 344:1511–1514
von Bonin M, Stölzel F, Goedecke A, Richter K, Wuschek N, Hölig K, Platzbecker U, Illmer T, Schaich M, Schetelig J, Kiani A, Ordemann R, Ehninger G, Schmitz M, Bornhäuser M (2009) Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant 43:245–251
Wakitami S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M (2002) Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil 10:199–206
Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG (1996) A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921–925
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Acknowledgments
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jaehoon Choi and Jee-Hyeok Chung contributed equally to this paper as first authors.
Rights and permissions
About this article
Cite this article
Choi, J., Chung, JH., Kwon, GY. et al. Effectiveness of autologous serum as an alternative to fetal bovine serum in adipose-derived stem cell engineering. Cell Tissue Bank 14, 413–422 (2013). https://doi.org/10.1007/s10561-012-9341-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-012-9341-1