Abstract
This study was designed to evaluate the effect of autologous bone marrow mesenchymal stem cells (MSCs) seeded into Gelfoam® on structural bone allograft healing. Thirty New Zealand white rabbits were divided into two groups. Segmental bone defect was created on diaphysis of the femur, and the defect was reconstructed with structural bone allograft. In experimental group, structural allograft was wrapped around by Gelfoam® containing autologous MSCs, whereas cells were not included in control group. At 4, 8, 12 weeks, the femur of rabbits underwent radiographic and histologic evaluation for bony union. Bone morphogenic protein-2 (BMP-2), BMP-4, BMP-7, vascular endothelial growth factor (VEGF), and receptor activator of nuclear factor-kappa B ligand (RANKL) were measured within the grafted periosteal tissue. Bony union was not achieved in both groups at 4 and 8 weeks. At 12 weeks, three out of five femurs in experimental group were united, but one out of five in control group was united. Mean Taira scores were significantly different between two groups. The expression of BMP-2 was significantly higher at 4, 8 weeks, the expressions of BMP-4 and BMP-7 were significantly higher at 8 and 12 weeks, and the expression of VEGF and RANKL were significantly higher at all time points in experimental group. Incorporation of the structural bone allograft could be enhanced if allograft is covered with Gelfoam® containing autologous MSCs. MSCs have influence on not only bone formation, but neo-angiogenesis, and bone resorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large segmental bone defect can occur as a result of trauma, revision arthroplasy, and bone tumor resection. Although autologous bone grafts are superior to allografts in healing and remodeling (Garbuz et al. 1998), their use is limited by size and amount of bone that can be harvested, donor site morbidity, and complications of the procedures (Younger and Chapman 1989). As an alternative, structural bone allografts remain a reasonable option for repair and reconstruction of these defects. Structural bone allografts have adequate mechanical strength while retaining osteoconductive properties (Guo et al. 1991). However, the absence of viable cells on processed allografts results in failure to incorporate to the host bone and complications such as fracture and infection (Mankin et al. 1996).
Clinical and experimental studies demonstrate periosteum plays an important role in bone healing and remodeling (Camilli and Penteado 1994). Periosteum contains osteoprogenitor cells that are capable of differentiating into osteoblasts and chondrocytes (Nakahara et al. 1990). In early stage of bone healing, periosteal progenitor cells releases bone morphogenic proteins (BMPs) and cytokines and eventually result in new bone formation leading to incorporate to the host bone (Zhang et al. 2008). Simultaneously, angiogenesis also takes place and vascular ingrowth into the callus is regulated by vascular endothelial growth factor (VEGF) for further progression of the regeneration cascade (Keramaris et al. 2008). However, the exact cellular and molecular mechanisms of periosteal progenitor cells are remained elusive.
Mesenchymal stem cells (MSCs) are multipotent progenitor cells capable of self-renewing and differentiating into various types of cells (Pereira et al. 1995; Prockop 1997). The osteogenic potential of MSCs has been well defined, as evidenced by bone formation following transplantation of MSCs in vivo and in vitro (Kadiyala et al. 1997). Recent studies with coupling MSCs to porous scaffolds have been successful for bone tissue engineering (Eslaminejad et al. 2007; Tanaka et al. 2009), but it is difficult to incorporate MSCs into dense cortical bone allografts. If porous scaffold containing MSCs is used as an artificial periosteum, it can be helpful for incorporating and remodeling of the structural bone allograft.
The purpose of this study was to evaluate the effect of artificial periosteum made by Gelfoam® and autologous MSCs on structural bone allograft healing in rabbit model. We also investigated various osteogenesis-related factors from perioeteal tissue to define the influence of autologous MSCs on bone formation, resorption, and angiogenesis.
Materials and methods
Harvesting and processing of deep frozen allograft
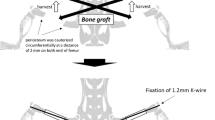
Under sterile condition, diaphyseal bone segment was harvested at the time of surgery during the creation of 1.5 cm femoral defect. All soft tissues including periosteum were carefully removed, and marrow cavity was flushed with distilled water to remove bone marrow tissue. Bones were incubated in distilled water with 0.5 mg/ml gentamicin for 30 min. Bones were then rinsed with 70% ethanol for 1 h, and the process was repeated for 3 times. After drying under laminar flow, processed allografts were secured in sterile triple plastic bags, and stored at −70 °C for more than 4 weeks to be used in the experiment.
Mesenchymal stem cell culture
Bone marrow blood was aspirated from the both iliac crests of rabbits. An average of 1 ml blood was aspirated into the syringe with 2 ml phosphate-buffered saline (PBS) and 5 U/ml heparin. This mixture was centrifuged at 1,800 rpm for 10 min. The supernatant was discarded and buffy coat layer including mononuclear cells was taken out to dilute with PBS. It was added to a culture medium containing 15 ml of Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA) and antibiotics (penicillin 100 U/ml, streptomycin 100 μg/ml). It was seeded to culture flasks at a density of 5 × 105 cells/cm2 and incubated in 5% CO2 at 95% humidity. After 48 h, the culture medium and unattached cells were gently removed. New culture medium was added, and the cells were incubated again for another 48–72 h. MSCs were harvested after reaching 80% confluence, using 0.25% Trypsin and 1 mM EDTA solution (Gibco, Carlsbad, CA), and re-plated for expansion. More than 2 × 106 cells in P2 or P3 generation were transplanted to rabbits in experiment group. The cells were stained with PKH fluorescent (PKH-26; Zynaxis Cell Science, Malvern, PA) that is a red fluorescent dye into lipid regions of the cell membrane to detect MSCs in vivo. Gelfoam® (Pharmacia & Upjohn, Kalamazoo, Michigan) was placed in the culture medium and the composite was incubated and implanted on the same day.
Osteogenic differentiation of MSCs
To evaluate the osteogenic differentiation potential of MSCs, cells were seeded onto a plate at a density of 5 × 103 cells/cm2 and incubated in the control medium for 3 days. Then cells were induced to differentiate into osteoblasts by incubating in a culture medium containing DMEM, 10% FBS, 2 mM l-glutamine, 10 mM β-glycerophosphate, 10nM dexamethasone, 50 μg/ml L-ascorbic acid, 100 U/ml penicillin, 100 μg/ml streptomycin. The cells were cultured for 3 weeks, then fixed in 10% buffered formalin and stained with alkaline phosphatase and von Kossa stains to evaluate new bone formation and calcification.
Surgical procedures
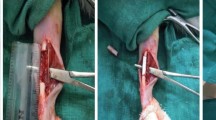
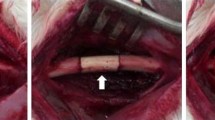
Thirty male New Zealand rabbits weighing 2.5–3.0 kg were used in this study. Rabbits were anesthetized with tiletamine (7.5 mg/kg, IM) and zolazepam (32 mg/kg, IM). The right thigh was shaved and prepared with povidon iodine. An incision was made along the medial thigh and femoral shaft was exposed. Segmental defect, 1.5 cm in length was created on the middle portion of the femur. Processed bone allograft was used to fill the defect and fixed with intramedullary Steinmann pin. The rabbits were divided into two groups. In experimental group (n = 15), allograft was covered by 3 × 2 cm2 sized Gelfoam® (Pharmacia & Upjohn, Kalamazoo, Michigan) containing MSCs. In control group (n = 15), allograft was covered by PBS-soaked Gelfoam® without MSCs. Rabbits were allowed to move freely without immobilization after surgery. Postoperative antibiotics (gentamicin, 6 mg/kg) were administered intramuscularly for 3 days.
Radiologic analysis
Radiographs were taken postoperatively at 4, 8, 12 weeks to evaluate the callus formation, allograft incorporation, and remodeling. Results were evaluated according to the radiographic scoring system suggested by Taira et al (2004) (Table 1). For comparison of the radiographic score between two groups, Mann-Whitney U test was used. To evaluate the radiographic score between each time points, Wilcoxon signed rank test was used. P values less than 0.05 were considered statistically significant.
Histologic analysis
Histologic evaluation was performed on five rabbits of each time points for both groups. Following euthanasia, femoral diaphysis including bone allograft was excised as a block, and periosteal tissue was harvested for evaluation of the growth factors. After being fixed in a 10% phosphate buffered formalin, all bone specimens were decalcified in 10% formic acid solution and dehydrated to be embedded in paraffin. The paraffin sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome to evaluate new bone formation and allograft incorporation histologically. The sections were also observed under a laser scanning confocal microscope (Zeiss LSM 510 META) to determine the presence of transplanted MSCs.
Growth factor analysis
The expression of osteogenesis-related growth factors was determined using the real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted using TRIzol® solution (Life Technologies, Burlington, ON, Canada) according to the manufacture’s recommendation. The RNA concentration and quality were determined by UV spectrophotometer at absorbances of 260 and 280 nm. First strand cDNA was synthesized by random hexamer. The PCR amplification was perform using rabbit BMP2, BMP4, BMP7, VEGF, and receptor activator of nuclear factor-kappa B ligand (RANKL) specific primer. Primers for BMP4 and BMP7 were designed using Primer3 software. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was detected by RT-PCR as an internal control. All primers were described as follows (Table 2). After visualization of PCR products by 2% agarose gel electrophoresis with ethidium bromide staining gel, images were obtained and the densities of the products were quantified using a digital gel imaging analysis system. The relative expression levels were calculated as the density of the product of the respective target gene after normalization with the GAPDH internal control.
Results
All rabbits survived during surgery and postoperative period. Complications such as infection and fixation failure were not found. To evaluate the osteogenic differentiation potential of MSCs, we stained MSCs with alkaline phosphatase and von Kossa stains before and after osteogenic differentiation. We found differentiated MSCs stained with alkaline phosphatase showing extensive deposition of bone materials (Fig. 1a, b). Von Kossa stain showed calcification around differentiated MSCs (Fig. 1c, d).
Radiographic analysis
At 4 weeks, periosteal reactions were found in all animals of experimental group, but only two out of five had minimal periosteal reaction in control group (Fig. 2a, b). Mean Taira score was 2.4 ± 1.1 in experimental group and 0.8 ± 0.8 in control group, which was significantly different (P = 0.041). Periosteal reactions were predominant at 8th weeks after surgery in both groups (Fig. 2c, d). Mean Taira score was 5.4 ± 1.1 in experimental group and 3.2 ± 1.5 in control group, which was significantly different (P = 0.033). Bony union or graft remodeling were not found in both groups at 4 and 8 weeks. At 12 weeks, three out of five femurs in experimental group were united with large amount of callus over the allograft, but one out of five in control was united with less amount of callus (Fig. 2e, f). Mean Taira score was 14.4 ± 3.1 in experimental group and 9.2 ± 1.9 in control group, which was also significantly different between two groups (P = 0.021). Mean Taira score was increased with subsequent time points in both groups (P = 0.007, experimental group; P = 0.008, control group).
Histologic analysis
At 4 weeks, callus always came from the host bone and none of them reached to the allograft in control group. Periosteal bone formation was not observed around the allograft (Fig. 3a). In contrast, bone bridging occurred in three out of five specimens in experimental group. New bone formation at the graft-host junction was seen at the surface area of the allograft (Fig. 3b). Callus from the host bone had cartilage matrix which was a characteristic of endochondral ossification, but callus at the periosteal surfaces of the allograft resembled fibrous tissue, which was the pattern of intramembranous ossification. Bridging callus was more abundant at 8 weeks in experimental group, and cartilage matrix became ossified according to the pattern of endochondral ossification (Fig. 3c). At 12 weeks, three out of five specimens achieved solid bony union at the graft-host junctions in experimental group (Fig. 3d). Evidence of graft resorption and remodeling were observed around newly formed bone bridges from 8 weeks in experimental group, and this was more evident at 12 weeks. One of five specimens achieved bony union at both graft-host junctions in control group at 12 weeks. The presence of transplanted MSCs was confirmed under the laser scanning confocal microscope in experimental group (Fig. 4). Although the number of transplanted MSCs was the highest at 4 weeks, cells could be detected up to 8 weeks after implantation.
Histologic findings of allograft-host bone interface with Masson’s Trichrome stains. In control group a, periosteal callus formation is not found on the allograft surface (right side). In experimental group (b at 4 weeks; c at 8 weeks; d at 12 weeks), abundant callus formation and bridging trabeculi are seen. (×40)
Growth factor analysis
The relative expression levels were calculated after normalization with the GAPDH internal control. The mean expressions of BMP-2 total RNA in periosteal tissue were significantly higher at 4, 8 weeks in experimental group (P = 0.008, 4 weeks; P = 0.008, 8 weeks). At 12 weeks, the differences are not significantly different between two groups (P = 0.222; Fig. 5). The expressions of BMP-4 and BMP-7 were not significantly different at 4 weeks (P = 0.310, BMP-4; P = 0.151, BMP-7), they were significantly higher at 8 and 12 weeks in experimental group (P = 0.016 at 8 weeks, P = 0.008 at 12 weeks for BMP-4; P = 0.032 at 8 weeks, P = 0.008 at 12 weeks for BMP-7; Figs. 6, 7). The expression of VEGF and RANKL were significantly higher at all time points in experimental group (P = 0.008 at 4 and 8 weeks, P = 0.032 at 12 weeks for VEGF; P = 0.016 at 4 weeks, P = 0.008 at 8 and 12 weeks for RANKL; Figs. 8, 9).
Discussion
It has been well known that structural bone allografts frequently fail to heal and remodel, and result in many complications (Mankin et al. 1996). These poor long-term results of the structural bone allografts lead to explore new alternatives using tissue engineering technology. In this study, we demonstrated that incorporation of the structural bone allografts could be enhanced if it is covered with Gelfoam® containing autologous MSCs. This approach resulted in robust callus formation surrounding cortical allograft at 4 weeks, and enhanced healing and incorporation of the allograft at 12 weeks after surgery. We also demonstrated that transplanted MSCs could increase growth factors for bone formation, resorption, and neovascularization in periosteal tissue up to 12 weeks.
The repair of a structural bone allograft is similar to fracture healing and involves multistage processes (Garbuz et al. 1998). Fracture healing is characterized by combined endochondral ossification from the host and intramembranous ossification from the periosteum of the graft. Many studies have shown that periosteum has an important role during fracture healing and bone graft repair (Eyre-Brook 1984). As the periosteum doesn’t exist in structural bone allograft, the repair process can be incomplete and dependent only on endochondral ossification at the graft-host junction. We showed an abundant periosteal bone formation on cortical allograft surface in experimental group. Furthermore, callus at the periosteal surface of the allograft resembled fibrous tissue, which was the pattern of intramembranous ossification. It is suggested that Gelfoam® containing MSCs could act as a periosteal tissue in vivo.
MSCs are multipotent progenitor cells that differentiate into many types of mesodermal lineages, including osteogenic differentiation (Mardon et al. 1987). MSCs are easily isolated and cultured in vitro, so they are thought to be a promising candidate as supporting cells for bone reconstruction. MSCs can be harvested from various tissues such as bone marrow (Ashton et al. 1980), periosteum (Uchida et al. 1988), adipose tissue (Zuk et al. 2002) and synovium (De Bari et al. 2001). As recent study showed that MSCs from bone marrow have the greatest osteogenic potential (Hayashi et al. 2008), we chose bone marrow-derived MSCs in this study. Periosteal cells can be an alternative option, but harvesting of periosteal cells is invasive because it involves surgical excision of periosteum. On the other hand, harvesting of MSCs from bone marrow is more practical clinically because it can be done with bone marrow aspiration.
In this study, we demonstrated that PKH fluorescent-labeled MSCs could be detected up to 8 weeks within the bone tissue. This result indicated that these cells remained viable during repair process, and produced bone tissue after osteogenic differentiation. Arinzeh et al (2003) suggested that fluorescent-labeled MSCs could no longer be detected by 8 weeks after implantation because of dilution of the fluorescent during mitosis and subsequent differentiation of the MSCs. We generally agree with their hypothesis. We also demonstrated that transplanted MSCs could increase BMPs, VEGF, and RANKL concentration in periosteal tissue up to 12 weeks. As PKH fluorescent-labeled MSCs were only detected up to 8 weeks after implantation, it is postulated that cytokines released from transplanted MSCs had an effect on migration of the host MSCs, and resulted in increasing growth factors up to 12 weeks.
Expressions of BMPs, VEGF, and RANKL from MSCs are well documented. MSCs produce BMPs during fracture healing, and they stimulate MSCs proliferation and osteogenic differentiation as an autocrine signaling (Sakou 1998). Kagiwada et al. (2008) demonstrated that VEGF was highly expressed in cultures of human MSCs and the high expression level was maintained during prolonged culture periods. As VEGF plays a crucial role in neoangiogenesis, MSCs can be a source of VEGF production and might be effective in healing of bone allograft. MSCs can also express RANKL and they are able to contribute to osteoclastogenesis (Udagawa et al. 1999). In addition to bone formation deficiency, cortical allograft lacks neovascularization and bone remodeling potential. Ito et al. (2005) showed that gene expression of VEGF and RANKL are significantly decreased during structural allograft healing. As transplantated MSCs can increase BMPs, VEGF, and RANKL expression in periosteal tissue, they can have influence on not only bone formation, but neoangiogenesis, and bone resorption during structural bone allograft healing.
We used Gelfoam® as a tissue-engineered scaffold for MSCs. Gelfoam® is a gelatin-based medical device intended for hemostasis. It is purified from skin gelatin, and capable of absorbing up to 45 times its weight of whole blood. As Gelfoam® is porous, biocompatible, biodegradable, and flexible, the potential as a scaffold to support cells has been explored. Ponticiello et al (2000) demonstrated that 3 weeks cultures of MSCs in Gelfoam® resulted in formation of a cartilage-like extracellular matrix. Rohanizadeh et al (2008) showed that Gelfoam® could be good candidate as a scaffold for osteoblast. We also found that the artificial periosteum made by Gelfoam® and autologous MSCs was quite effective on structural bone allograft healing in rabbit model.
There are several experimental trials other than using MSCs. First is a strategy of applying BMPs to bone allografts. Although clinical studies have proved its effects on allograft incorporation, adverse events such as ectopic bone formation, bone resorption or remodeling, hematoma and seroma are also reported (Salkeld et al. 2001; Jones et al. 2006). Large amount of recombinant proteins is necessary to induce sufficient bone formation which will cost too much. Their localized concentration is difficult to maintain in vivo because of their short half-life (Pereira et al. 2000). As an alternative, many groups have been working on gene therapy approaches for skeletal healing. Although gene therapy offers the potential of local, sustained gene expression (Koh et al. 2008), the safety and effectiveness of delivery vector remains to be proved. On the other hand, our strategy can be readily available in clinic. MSCs are already being introduced into clinical medicine in variety of applications and through different ways of administration (Abdallah and Kassem 2008). Gelfoam® is widely used in clinic especially for bleeding control and embolization (Abada and Golzarian 2007).
Our study has several limitations. We didn’t evaluate the bone histomorphometry and biomechanical testing for incorporated allograft. As we only achieved bony union in three out of five animals in experimental group, longer time period or more rigid fixation will be needed to evaluate the strength of united bone. We selected 12 weeks as a survival period because we wanted to see early growth factor changes by autologous MSCs. Although intramedullary fixation has been successful in some studies (Nather and Goh 2000; Zhang et al. 2005), it was used for small animals and for tibial segmental defect which is stabilized by remaining fibula. So it is expected that intramedullary Steinmann pin fixation might not be enough for femoral diaphyseal defect of the rabbit. The mechanical environment created by the different fixation methods are thought to be responsible for cortical bone allograft incorporation (Benevenia et al. 2000). We couldn’t explain the different growth factor level in different time points because no literatures present growth factor changes for more than 4 weeks. Further study will be needed to explain these growth factor changes in length of time.
In conclusion, incorporation of the structural bone allograft could be enhanced if allograft is covered with Gelfoam® containing autologous MSCs. MSCs have influence on not only bone formation, but neo-angiogenesis, and bone resorption.
References
Abada HT, Golzarian J (2007) Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol 10(4):257–260
Abdallah BM, Kassem M (2008) Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther 15(2):109–116
Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K et al (2003) Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am 85-A(10):1927–1935
Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M (1980) Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res (151):294–307
Benevenia J, Zimmerman M, Keating J, Cyran F, Blacksin M, Parsons JR (2000) Mechanical environment affects allograft incorporation. J Biomed Mater Res 53(1):67–72
Camilli JA, Penteado CV (1994) Bone formation by vascularized periosteal and osteoperiosteal grafts. An experimental study in rats. Arch Orthop Trauma Surg 114(1):18–24
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44(8):1928–1942
Eslaminejad MB, Mirzadeh H, Mohamadi Y, Nickmahzar A (2007) Bone differentiation of marrow-derived mesenchymal stem cells using beta-tricalcium phosphate-alginate-gelatin hybrid scaffolds. J Tissue Eng Regen Med 1(6):417–424
Eyre-Brook AL (1984) The periosteum: its function reassessed. Clin Orthop Relat Res (189):300–307
Garbuz DS, Masri BA, Czitrom AA (1998) Biology of allografting. Orthop Clin North Am 29(2):199–204
Guo MZ, Xia ZS, Lin LB (1991) The mechanical and biological properties of demineralised cortical bone allografts in animals. J Bone Joint Surg Br 73(5):791–794
Hayashi O, Katsube Y, Hirose M, Ohgushi H, Ito H (2008) Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int 82(3):238–247
Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J et al (2005) Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med 11(3):291–297
Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX et al (2006) Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am 88(7):1431–1441
Kadiyala S, Young RG, Thiede MA, Bruder SP (1997) Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant 6(2):125–134
Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N, Ohgushi H (2008) Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med 2(4):184–189
Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV (2008) Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury 39(Suppl 2):S45–S57
Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT (2008) Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J Dent Res 87(9):845–849
Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW (1996) Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res (324):86–97
Mardon HJ, Bee J, von der Mark K, Owen ME (1987) Development of osteogenic tissue in diffusion chambers from early precursor cells in bone marrow of adult rats. Cell Tissue Res 250(1):157–165
Nakahara H, Bruder SP, Goldberg VM, Caplan AI (1990) In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop Relat Res (259):223–232
Nather A, Goh JC (2000) Biomechanical strength of large diaphyseal deep-frozen allografts. Cell Tissue Bank 1(3):201–206
Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD et al (1995) Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A 92(11):4857–4861
Pereira RC, Rydziel S, Canalis E (2000) Bone morphogenetic protein-4 regulates its own expression in cultured osteoblasts. J Cell Physiol 182(2):239–246
Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP (2000) Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res 52(2):246–255
Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276(5309):71–74
Rohanizadeh R, Swain MV, Mason RS (2008) Gelatin sponges (gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med 19(3):1173–1182
Sakou T (1998) Bone morphogenetic proteins: from basic studies to clinical approaches. Bone 22(6):591–603
Salkeld SL, Patron LP, Barrack RL, Cook SD (2001) The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg Am 83-A(6):803–816
Taira H, Moreno J, Ripalda P, Forriol F (2004) Radiological and histological analysis of cortical allografts: an experimental study in sheep femora. Arch Orthop Trauma Surg 124(5):320–325
Tanaka T, Hirose M, Kotobuki N, Tadokoro M, Ohgushi H, Fukuchi T et al (2009) Bone augmentation by bone marrow mesenchymal stem cells cultured in three-dimensional biodegradable polymer scaffolds. J Biomed Mater Res A 91(2):428–435
Uchida A, Kikuchi T, Shimomura Y (1988) Osteogenic capacity of cultured human periosteal cells. Acta Orthop Scand 59(1):29–33
Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K et al (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/rankl but not macrophage colony-stimulating factor: receptor activator of nf-kappa B ligand. Bone 25(5):517–523
Younger EM, Chapman MW (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3(3):192–195
Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR et al (2005) Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 20(12):2124–2137
Zhang X, Awad HA, O’Keefe RJ, Guldberg RE, Schwarz EM (2008) A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res 466(8):1777–1787
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Acknowledgments
The authors wish to acknowledge the financial support of the Catholic Institute of Cell therapy Basic Science Programs Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JY., Choi, MH., Shin, EY. et al. Autologous mesenchymal stem cells loaded in Gelfoam® for structural bone allograft healing in rabbits. Cell Tissue Bank 12, 299–309 (2011). https://doi.org/10.1007/s10561-010-9194-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-010-9194-4