Abstract
The family of metastasis-associated (MTA) genes is a small group of transcriptional co-regulators which are involved in various physiological functions, ranging from lymphopoietic cell differentiation to the development and maintenance of epithelial cell adhesions. By recruiting histone-modifying enzymes to specific promoter sequences, MTA proteins can function both as transcriptional repressors and activators of a number of cancer-relevant proteins, including Snail, E-cadherin, signal transducer and activator of transcriptions (STATs), and the estrogen receptor. Their involvement in the epithelial-mesenchymal transition process and regulatory interactions with estrogen receptor activity has made MTA proteins highly interesting research candidates, especially in the field of hormone-sensitive breast cancer and malignancies of the female reproductive tract. This review focuses on the current knowledge about the function and regulation of MTA1 and MTA3 proteins in gynecological cancer, including ovarian, endometrial, and cervical tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gynecological cancer comprises a wide variety of malignancies arising from tissues of the female reproductive tract. It includes completely different histopathological entities with different causative etiologies, ranging from papillomavirus infection-caused squamous cervical cancer to hormone receptor-driven endometrioid adenocarcinomas. Detection and treatment may lead to a good prognosis, such as in the case of endometrial cancer, or to a completely inadequate and disappointing survival rate, as in the case of ovarian cancer.
Worldwide, gynecological cancer entities represent the most frequently diagnosed cancer in females [1]. Tumor progression to metastatic disease has been recognized as one of the most important aspects for the lethal outcome of cancer. Several factors are responsible for the invasion and metastasis of gynecological cancer. Most of these factors are common to other types of cancer and include the expression of extracellular matrix degrading enzymes, such as cathepsins and matrix metalloproteinases [2]; the loss of cell adhesion proteins, such as E-cadherin [3]; the acquisition of a migratory or epithelial-mesenchymal transition (EMT)-like phenotype [4, 5]; and the ability to disseminate via the blood or the lymphatic system [6]. However, the most intriguing aspect of pathophysiology and carcinogenesis is the hormone dependency of most of the female genital tissues. Whereas the ovary is the primary source of estrogens and progesterons, the endometrium is considered to be the classical target tissue for these steroid hormones. The clinical importance of steroid hormones and their mediating receptors is also reflected by the highly effective anti-hormonal treatment of breast cancer [7–9].
Proteins of the metastasis-associated (MTA) gene family have been recently identified as key regulators of the EMT process and also as controllers of E-cadherin expression [10, 11]. MTA proteins, comprising MTA1, MTA2, and MTA3, are components of the nucleosome remodeling and histone deacetylation (NuRD) complex, in which they interact with histone deacetylases to promote chromatin compaction and thereby gene inactivation [10, 11]. Their recruitment to specific promoter regions, or specific interactions with other transcription factors, leads to transcriptional silencing of their target genes. Several of the identified MTA1 target genes are highly relevant for cancer progression. MTA1 has been shown to negatively interfere with the transactivation function of the estrogen receptor at its target sequences, including estrogen receptor-dependent transcription of breast cancer gene 1 (BRCA1) [12] and MTA3 [10, 11, 13]. MTA3 itself has been described as a transcriptional repressor of the E-cadherin transcription factor Snail [13], thus leading to a transcriptional regulation cascade from MTA1 to MTA3 to E-cadherin, with the final consequence of a suppressive function of MTA1 on E-cadherin expression [13].
MTA protein expression is not restricted to malignancies but can also be found in normal tissues [10, 14–17]. Much seminal work on MTA proteins has been performed in the field of breast cancer research and revealed not only a cancer-promoting interplay of MTA proteins, estrogen receptor activity, and metastasis [13, 18] but also a regulatory function of MTA proteins in normal mammary gland development and maturation [18, 19]. Since the development and physiological function of gynecologic tissues rely on female sex hormones, whose internal and external supply is also influencing the progression of some of the most widespread gynecological cancer entities, several research groups have studied the involvement and interaction of MTA proteins and female steroid hormone receptors in gynecological cancer. This review summarizes the current knowledge about the expression and regulation of MTA proteins in both normal gynecological organs and gynecological cancer.
2 MTA expression in ovarian cancer
The ovary primarily fulfills the function of oocyte storage and maturation. Although endowed with a high proliferative potential, the stromal oocytes and their supportive granulocytes are rarely involved in ovarian malignancies and contribute to less than 5 % of all ovarian cancers [20, 21].
Most ovarian cancers (up to 95 %) are categorized as epithelial ovarian cancers (EOC) and can be subdivided into serous, mucinous, endometrioid, and clear-cell EOC [22]. Serous EOC reflects the most common and, unfortunately, the most aggressive type of ovarian cancer [22].
EOC is the most deadly gynecological cancer worldwide (http://www.ovariancancer.org/about-ovarian-cancer/statistics/). The World Health Organization GLOBOCAN database reports a worldwide incidence of more than 190,000 cases of ovarian cancer [23]. Non-detection of specific early symptoms together with the absence of reliable screening strategies often leads to diagnosing ovarian cancer only in advanced stages, resulting in poor overall survival. Moreover, despite multimodal treatment options, high incidences of relapses occur [24–27]. Although improvements in surgical management and advances in cytotoxic therapy have been accomplished in the past decades, the overall 5-year survival rate for women with advanced disease can be as low as 13 % [24, 25, 28, 29].
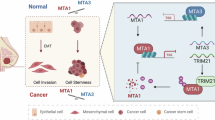
The ovarian surface epithelium is a single-cell layer covering the ovary and has long been renowned as the origin of most epithelial ovarian tumors. The periodical rupture of this cell layer during ovulation, similar to a continuous process of inflammation and wound healing, has led to the “incessant ovulation” theory of the origin of ovarian cancer [30, 31]. However, based on recent histopathological analyses and marker gene expression studies, the epithelium of the oviductal fimbriae at the distal ends of the fallopian tube has also been implicated to play an important role in the genesis of epithelial ovarian cancer [22, 31–33]. According to this theory, EOC originates as a serous tubal intraepithelial carcinoma (STIC) and secondarily metastasizes to the ovarian surface and other peritoneal epithelia [22, 31–33]. The extent to which these different epithelia give rise to EOC is still under debate, but the different histological and developmental origins are of great importance when looking at the aspect of MTA expression. MTA proteins both regulate and are regulated by estrogen receptor activity and are also key regulators of E-cadherin expression (Fig. 1). Notably, fallopian tube epithelial cells (FTE cells) rely on E-cadherin expression for intercellular adhesions, whereas normal ovarian surface epithelial (OSE) cells are devoid of E-cadherin expression ([33]; see also Fig. 2).
Oncogenic pathways involved in MTA expression and regulation. MTA1 expression can directly and indirectly be enhanced by several oncogenic factors, including nuclear c-myc activation and growth-promoting heregulin and TGF-beta cell membrane receptor activation. Elevated MTA1 activity can further lead to transcriptional activation of STAT3 proteins or transcriptional repression of the estrogen receptor alpha leading to reduced MTA3/E-cadherin and BRCA1 expression. Reduced BRCA1 expression, similar to reduced p53 activity as mediated by posttranslational deacetylation of p53 by MTA1, can further promote genomic instability of cancer cells. However, not all malignant transformation steps are common to all gynecological cancers. For example, up to 95 % of all high-grade serous ovarian carcinomas bear p53 mutations and around 40–50 % display direct or indirect BRCA inactivation [22]. E-cadherin expression was found to be absent in ovarian surface epithelial but not fallopian tube epithelial cells. HER2 overexpression and KRAS/BRAF mutations were identified as non-overlapping events occurring primarily in low-grade serous ovarian cancer but not in high-grade serous ovarian cancer [22]. Red letters activated/elevated in malignant cancer cells. Dotted lines indirect effects. HRG heregulin, MMP matrix metalloproteinase, VEGF vascular epithelial growth factor, GRO growth-regulated oncogene
Expression of MTA1 and MTA3 in ovarian epithelial cells and cancer tissues. Non-malignant ovarian and fallopian tube tissues and ovarian cancer tissues were immunohistochemically analyzed for MTA, estrogen receptor, and E-cadherin expression as previously described [35]. Pictures of the ovary depict the single-layered cubic ovarian surface epithelium (OSE), and pictures of the adjacent fallopian tube show the ciliated columnar cells of the oviductal fallopian tube surface epithelium (FSE). Pictures of carcinoma tissues are representatives of various serous epithelial ovarian cancer tissues
Since no information on MTA protein expression in human FTE cells has been published, we have investigated MTA1, MTA3, E-cadherin, and estrogen receptor expression in FTE and OSE cells (Fig. 2). Expression of both MTA1 and MTA3 proteins was detectable in FTE cells and also in normal OSE cells. In contrast, E-cadherin expression was found in FTE cells only, whereas estrogen receptor alpha expression was detectable in both FTE and OSE cells.
It seems contradictory that, and initially is difficult to understand why, two transcription factors of apparent opposite function are expressed together at comparable levels in normal OSE and FTE cells. MTA3 expression is known to be important for sustaining an epithelial phenotype, whereas MTA1 expression is a promoting factor involved in EMT. It can be hypothesized that moderate levels of both MTA1 and MTA3 expression, as found in untransformed ovarian epithelial cells, are needed for an equilibrium or are involved in other as yet unrecognized vital regulatory processes. Under deregulated conditions, however, the impact of MTA protein expression becomes more obvious, as observed in various types of cancer [10, 11, 34]. In a study on 115 serous ovarian cancer samples, we found a significantly increased expression of MTA1 protein in advanced ovarian cancer tissues compared to samples of earlier tumor stages displaying either a lower differentiation grade or a larger extent of peritoneal metastasis and ascites formation [35]. In highly de-differentiated ovarian carcinomas, the expression of nuclear MTA1 protein was found to be nearly twice as high as the median MTA1 expression in well-differentiated ovarian carcinomas [35]. Similar results were obtained in an independent study on 81 ovarian cancer tissues of various histological subtypes [36]. The study also revealed that elevated MTA1 expression was associated with a shortened disease-free survival, although no significant association between MTA1 expression and overall patient survival was observed [36]. Notably, it was also found that elevated MTA1 expression was associated with a poorer response to first-line chemotherapy, in this study most frequently a platinum (plus taxane)-based treatment regimen [36].
In contrast to the pronounced upregulation of MTA1 in advanced and metastatic ovarian cancer tissues, only a slight and statistically non-relevant reduction in MTA3 expression was observed in ovarian cancer [35]. Furthermore, no statistically relevant relation between MTA1 and MTA3 expression as well as E-cadherin expression could be established among the ovarian cancer tissues tested. In single, syngeneic ovarian cancer cell lines, however, it could paradigmatically be shown and be confirmed that ectopic MTA1 overexpression leads to reduced MTA3 and E-cadherin expression in ovarian cancer cells [35]. This indicates that the known intracellular interactions and regulations of MTA proteins in ovarian cancer are probably overlapped by further, yet unidentified, factors that contribute to the expressional regulation of MTA proteins and their downstream targets in ovarian cancer. For example, gene mutation and promoter methylation have been recognized as independent mechanisms of E-cadherin silencing in ovarian cancer [3, 37]. The interaction of tumor cells with stroma cells and infiltrating immune cells has to be considered as well. Several cytokines and growth factors, secreted by fibroblasts, for example, are known to influence EMT of cancer cells. Normal OSE cells have the ability to perform EMT and respond to epithelial growth factor (EGF), transforming growth factor (TGF)-beta, and extracellular matrix proteins [38, 39].
It is also not well understood which mechanisms drive the elevated MTA1 expression observed in advanced ovarian cancer [35, 36]. The identification of MTA1 as a target gene of c-myc has linked a well-studied oncogene to the expression of proto-oncogenic MTA1 [40]. Indeed, a high frequency of c-myc gene amplification was found in epithelial ovarian cancer [41] and may account for elevated MTA1 expression in ovarian cancer cells. However, c-myc amplification is an early event in cancer progression (transformation), and MTA1 overexpression was predominantly observed in advanced cancer stages of ovarian cancer. The heregulin/human epidermal growth factor receptor 2 (HER2) tyrosine kinase receptor pathway has also been identified as an upstream regulator of MTA1 in breast cancer [42]. Enhanced activation of the HER2 pathway occurs in ovarian cancer, but the importance to ovarian cancer progression has been questioned due to a low response of ovarian cancer to HER2-targeted therapies [43]. Therefore, future histological studies could profit from a direct comparison of MTA1 expression with putative oncogenic drivers in ovarian cancer.
Interestingly, a previous immunohistological study on MTA protein expression in ovarian cancer cells revealed an inverse correlation between estrogen receptor beta (ESR2) and MTA1 expression [35]. This supports the notion that the estrogen receptor beta form, in contrast to the growth-promoting estrogen receptor alpha (ESR1), exerts a tumor suppressive function in ovarian cancer [44, 45]. Therefore, although MTA1 has been primarily associated with invasion and metastasis, its expression has also been considered as a cancer cell survival factor during cancer progression. Overexpression of MTA1 has been shown to facilitate anchorage-independent growth of breast [42] and ovarian cancer cells [35, 46]. This has been associated with enhanced expression of the oncogenic cytokine growth-regulated oncogene (GRO) (CXCL-1) in MTA1-overexpressing ovarian cancer cell clones, although the regulation mechanism of GRO by MTA1 remains undetermined [35]. In the p53 wild-type A2780 ovarian cancer cell line, overexpression of MTA1 has recently been shown to enhance expression of the anti-apoptotic survival factor bcl-XL [46].
MTA1 has primarily been regarded as a transcriptional repressor. However, Pakala et al. recently identified MTA1 as a direct positive regulator of STAT3 transcriptional activity in breast cancer cells [47]. Activity of the JAK/STAT (Janus kinase/signal transducer and activator of transcription) signaling pathway is a frequent event in ovarian cancer and contributes to metastasis due to its regulation of pro-angiogenic vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs), and Twist [48–50]. Since Twist has also been identified as a transcriptional repressor of E-cadherin [51], upregulation of Twist by MTA1/STAT3 interaction thus yields another link of MTA1 expression to E-cadherin regulation, invasion, and metastasis (Fig. 1).
3 MTA and endometrial cancer
The inner cell layer of the human uterus, the endometrium, is considered to be the classical target tissue for steroid hormones. However, it is not only a target tissue for hormones but is also an endocrine organ by itself, secreting several mediators that are thought to influence the endometrial function through autocrine and paracrine pathways. Additionally, it is the tissue that develops the most frequent gynecologic malignancy in the Western world: endometrial cancer. The annual incidence is estimated to be 15–20 cases per 100,000 women [52–55]. The lifetime risk of developing endometrial cancer is approximately 2.5 %, while the lifetime probability of death from this cancer is estimated to be 0.52 % [1, 56].
Endometrial cancer is primarily diagnosed in postmenopausal women [57, 58]. Based on the early symptom of postmenopausal bleeding and the subsequent possibility of hysteroscopy, transvaginal ultrasound examination, and endometrial biopsy sampling, endometrial cancer is often diagnosed at early stages and usually can be eliminated by radical hysterectomy and bilateral salpingo-oophorectomy [57, 58].
Endometrial cancer has been described as consisting of two different subgroups depending on pathological and molecular parameters [55, 59–61]. Type I endometrial cancers are mostly well-differentiated endometrioid adenocarcinomas, with a more favorable outcome compared to endometrial cancer of the second group [53, 55, 56, 59, 60, 62]. Type II endometrial cancers are often of the non-endometrioid type, are poorly differentiated, and have a poor prognosis [60, 61, 63]. Most endometrial cancers (80–85 %) can be categorized as type I endometrioid endometrial cancer [57, 58]. Fortunately, this type of endometrioid adenocarcinoma has a good prognosis and accounts for only a minor portion of deaths from gynecologic malignancies. In addition to obesity, an unopposed or excessive estrogen exposure of the glandular epithelium has been identified as a high-risk factor for the origin of endometrioid carcinomas [57, 58]. The 5-year survival rate for serous-papillary histology is approximately 24–34 %, whereas the survival rate for clear-cell adenocarcinoma is estimated to be approximately 42 % [64].
These observations have led to the postulation of a dualistic model for the molecular carcinogenesis in endometrial carcinomas [61]. The carcinogenesis of type I endometrial carcinomas are thought to develop due to estrogenic risk factors [53, 55, 61] and are also characterized by genetic alterations like mutations in phosphatase and tensin homolog (PTEN) and K-ras [61]. Type II cancers more often exhibit p53 mutations [65], HER-2/neu amplification [66], and chromosomal instability [61]. Interestingly, PTEN inactivation and HER2 overexpression have been identified as further cancer-promoting factors in endometrial cancer [57, 67].
Although several prognostic factors have been established [53, 55, 68–70], it is assumed that approximately 20 % of all endometrial cancer patients die of their disease [62]. This is actually an unusual situation for a solid tumor, especially since patients with endometrial cancer are diagnosed at an early stage. Therefore, a better understanding of its pathophysiology and carcinogenesis is urgently needed to help increase life expectancy and optimize therapy for these patients.
Loss of E-cadherin expression has been found in estrogen-dependent endometrial cancers (type I) and also in the more aggressive estrogen-independent non-endometrioid endometrial cancers (type II) [71]. In both type I and type II endometrial cancers, loss of E-cadherin expression was associated with an adverse prognosis and EMT and was also found to be inversely correlated to Snail expression [71, 72]. Being involved in E-cadherin expression and modulated by estrogen receptor expression, it can be assumed that MTA proteins may have an important role in endometrial cancer progression. In fact, Balasenthil et al. [14] observed an expression of MTA1 primarily in glandular and stromal cells of the endometrium in the proliferative phase but only weak MTA1 staining in the secretory phase. In postmenopausal women, the primary risk group for endometrial cancer, a highly variable expression level of MTA1 protein in both stromal and glandular cells, was observed, although the total MTA1 protein expression in postmenopausal tissue extracts was found to be less than in tissue extracts from endometrial cancer. However, among 70 endometrial endometrioid adenocarcinomas tested, no significant association between MTA1 expression and endometrial cancer grade was observed [14].
In contrast to the findings made for MTA1, an expression analysis of MTA3 in 200 endometrioid adenocarcinomas revealed a significant reduction of MTA3 expression in grade III endometrioid adenocarcinomas. However, no statistically relevant association between the expression level of MTA3 and progression-free survival, cause-specific survival, and overall survival was observed [73]. Furthermore, no association was found between MTA3 expression and the expression of the estrogen receptors alpha and beta [73]. Therefore, although the previous immunohistochemical analyses are highly indicative of a function of MTA1 and MTA3 in endometrial cancer progression [14, 73], neither MTA1 nor MTA3 appear to be suitable single markers for a survival prognosis of endometrial cancer patients with endometrioid histology. However, by analyzing uterine non-endometrioid carcinomas (type II), MTA3 expression demonstrated a significant association with FIGO surgical stage, lymph node involvement, and lymphovascular space invasion [74], predisposing the MTA3-overexpressing cell type to high metastatic potential after malignant transformation. Moreover, MTA3 was revealed to be a significant independent prognostic parameter, demonstrating a marked association with patients’ progression-free survival, cause-specific survival, and overall survival [74]. It seems that MTA3 might have a more important and selective role during tumor progression in endometrial cancer type II than observed in type I endometrial cancer and ovarian cancer.
4 MTA and cervical cancer
Since the implementation of screening programs, with the objective to prevent invasive cervical cancer by detecting its precursor cervical lesions, the incidence of this entity has declined in the most developed countries. However, cervical cancer is the second most common malignant disease among women worldwide (http://report.nih.gov/nihfactsheets/viewfactsheet.aspx?csid=76), with more than 500,000 new cancer cases every year [75, 76], and more than 85 % of cases occurring in developing countries [1, 77]. The median age at diagnosis with cervical cancer is 48 years. The disease has two age peaks, one at the age of about 45 and then again at an age beyond 70. Approximately 80 % of cervical cancers arise from squamous cell dysplasias, while 15 % are adenocarcinomas and 5 % clear-cell adenocarcinomas [76, 78]. However, although the Papanicolaou smear is the most cost-effective cancer screening test ever developed, it still can be non-diagnostic or falsely negative in the presence of invasive cancer. Although several risk factors for the development of cervical cancer have been identified, including human papilloma virus (HPV) infection [77, 79–81], the precise carcinogenesis is still unclear and no effective tumor markers are available.
The border of the ecto- and endocervix, called the squamocolumnar junction, is the predominant location of an infection with high-risk HPV, allowing HPV to access the basal cells through minute lesions or abrasions in the cervical epithelium. Being a common infection, most sexually active women will temporarily experience at some time of their life a persistent HPV infection which is the precondition for a high-grade cervical dysplasia, and a previous HPV infection is detectable in 99.7 % of cervical cancers [82]. However, it is quite unclear in which way MTA proteins are involved during viral carcinogenesis. Interestingly, the hepatitis B (HB) virus transactivator protein HBx, a major regulator of cellular responses caused by the HB virus, stimulated the expression of MTA1 in hepatocellular carcinoma cells, involving HBx targeting of transcription factor nuclear factor (NF)-kappaB and the recruitment of HBx/p65 complex to the NF-kappaB consensus motif on the relaxed MTA1 gene chromatin [83]. Therefore, a similar study analyzing the effects of transforming HPV oncogenes might be of interest for our understanding of a possible involvement on MTA1 regulation in cervical cancer.
Studies concerning MTA expression in cervical carcinoma are rare. Rao et al. [84] have shown that MTA1 expression levels in a cell culture model affected migration and invasion of cervical cancer cells. MTA1 protein expression was higher in SiHa cells compared with HeLa cells, which correlated with the potential of migration and invasion. Inhibition of MTA1 expression by RNA silencing impaired cell invasion, migration, and adhesion capabilities. Furthermore, E-cadherin levels were upregulated while beta-catenin levels were downregulated. The authors suppose that an altered E-cadherin/beta-catenin complex could be responsible for the decreased migration and invasion capability.
Liu et al. [85] investigated the effect of MTA1 on survival and lymph node metastasis in cervical carcinoma tissue samples. High levels of MTA1 were shown to be an independent factor for overall survival and disease-free survival. Although normal cervical epithelia showed little or no MTA1 expression in an immunohistochemistry (IHC) assay, cervical carcinoma samples could be divided into a group with low MTA1 expression and a group with high MTA1 expression. High MTA1 expression was associated with high-histologic-grade lymph node metastasis and recurrence. The correlation with lymph node metastasis is an important observation, since cervical cancer metastasizes early through the lymphatic system, a fact that dramatically impairs patients’ survival.
5 Further perspectives in targeting MTA1 and concluding remarks
Due to its pivotal role in cancer progression and metastasis, MTA1 represents a highly interesting target for cancer therapy. Several cell biological studies have applied an siRNA approach for specific knock-down of MTA1 expression and succeeded in reducing cancer cell transformation, proliferation, clonal growth, VEGF expression, invasiveness, and migration [40, 84, 86–89]. Furthermore, at least two microRNAs have been identified that interfere with MTA1 mRNA stabilization and could be used for MTA1 targeting [90, 91]. However, although the genetic targeting approaches revealed promising in vitro results, their clinical usefulness is restricted due to the still encountered limitations of human cancer gene therapy [92, 93]. Therefore, based on the current state-of-the-art, a pharmacological inhibition of MTA1 appears to be more promising.
The growth factor heregulin has been shown to enhance MTA1 protein expression by activating the HER2 pathway [42]. Targeting of HER2 activity by monoclonal antibodies or specific tyrosine kinase inhibitors has proven efficacy in breast cancer but appears less effective in gynecological cancer [43]. Natural polyphenols, such as plant-derived resveratrol and pterostilbene, have been shown to reduce the expression level of MTA1 in prostate cancer cells [94, 95]. Interestingly, this also links the cancer protective effects of a healthy diet to MTA1 expression. However, the clinical usefulness of these cancer-preventive polyphenols for effective cancer therapy is still uncertain [96], and the effects of resveratrol and pterostilbene on MTA1 expression in gynecological cancer cells remains to be evaluated.
Further to the expression regulation of MTA1, the inhibition of its functional activity might be a promising approach to interfere with the cancer-promoting effects of MTA1. Histone deacetylase inhibitors, such as vorinostat and valproic acid, have long been tested for their clinical use in a variety of cancer entities, but they lack specificity due to their many pleiotropic effects [97].
A rational drug design to target MTA1 is therefore urgently needed. Because of the importance of MTA1 in tumor progression and metastasis, a high-resolution analysis of the three-dimensional structure of MTA1 has already insistently been suggested [47]. In fact, a recent crystallographic analysis of MTA1 bound to histone deacetylase 1 (HDAC1) revealed a close association of the ELM2 and SANT1 domain of MTA1 to HDAC1 that further employed an inositol tetraphosphate molecule when shifted to its activated form ([98]; protein database identification number = 4BKX; www.rscb.org/pdb). These observations do not only provide a new function for the old inositol tetraphosphate orphan messenger but also raises completely new aspects and opportunities for pharmacological regulation of histone deacetylase activities by designing inositol derivatives to modulate MTA1 activity either directly or indirectly by modulating the specific regulatory inositol polyphosphate kinase and phosphatase activities.
With such specific inhibitors in hand, a new prospective targeted therapy against MTA1 could valuably support our limited arsenal against several types of highly aggressive, MTA1-overexpressing cancer entities, including several types of gynecological cancer.
References
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61, 69–90.
Deryugina, E. I., & Quigley, J. P. (2006). Matrix metalloproteinases and tumor metastasis. Cancer and Metastasis Reviews, 25, 9–34.
Schmalhofer, O., Brabletz, S., & Brabletz, T. (2009). E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer and Metastasis Reviews, 28(1–2), 151–166.
Bastid, J. (2012). EMT in carcinoma progression and dissemination: facts, unanswered questions, and clinical considerations. Cancer and Metastasis Reviews, 31(1–2), 277–283.
Meng, F., & Wu, G. (2012). The rejuvenated scenario of epithelial-mesenchymal transition (EMT) and cancer metastasis. Cancer and Metastasis Reviews, 31(3–4), 455–467.
Wong, S. Y., & Hynes, R. O. (2006). Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle, 5, 812–817.
Tessel, M. A., Krett, N. L., & Rosen, S. T. (2010). Steroid receptor and microRNA regulation in cancer. Current Opinion in Oncology, 22, 592–597.
Singh, R. R., & Kumar, R. (2005). Steroid hormone receptor signaling in tumorigenesis. Journal of Cellular Biochemistry, 96(3), 490–505. doi:10.1002/jcb.20566.
Miksicek, R. J. (1994). Steroid receptor variants and their potential role in cancer. Seminars in Cancer Biology, 5(5), 369–379.
Toh, Y., & Nicolson, G. L. (2009). The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clinical and Experimental Metastasis, 26(3), 215–227.
Li, D. Q., Pakala, S. B., Nair, S. S., Eswaran, J., & Kumar, R. (2012). Metastasis-associated protein 1/nucleosome remodeling and histone deacetylase complex in cancer. Cancer Research, 72(2), 387–394.
Molli, P. R., Singh, R. R., Lee, S. W., & Kumar, R. (2008). MTA1-mediated transcriptional repression of BRCA1 tumor suppressor gene. Oncogene, 27, 1971–1980.
Fujita, N., Jaye, D. L., Kajita, M., Geigerman, C., Moreno, C. S., & Wade, P. A. (2003). MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell, 113, 207–219.
Balasenthil, S., Broaddus, R. R., & Kumar, R. (2006). Expression of metastasis-associated protein 1 (MTA1) in benign endometrium and endometrial adenocarcinomas. Human Pathology, 37(6), 656–661. doi:10.1016/j.humpath.2006.01.024.
Bruning, A., Makovitzky, J., Gingelmaier, A., Friese, K., & Mylonas, I. (2009). The metastasis-associated genes MTA1 and MTA3 are abundantly expressed in human placenta and chorionic carcinoma cells. Histochemistry and Cell Biology, 132(1), 33–38. doi:10.1007/s00418-009-0595-z.
Li, W., Ma, L., Zhao, J., Liu, X., Li, Z., & Zhang, Y. (2009). Expression profile of MTA1 in adult mouse tissues. Tissue and Cell, 41(6), 390–399. doi:10.1016/j.tice.2009.04.002.
Chen, Y., Miyazaki, J., Nishizawa, H., Kurahashi, H., Leach, R., & Wang, K. (2013). MTA3 regulates CGB5 and Snail genes in trophoblast. Biochemical and Biophysical Research Communications, 433(4), 379–384. doi:10.1016/j.bbrc.2013.02.102.
Singh, R. R., & Kumar, R. (2007). MTA family of transcriptional metaregulators in mammary gland morphogenesis and breast cancer. Journal of Mammary Gland Biology and Neoplasia, 12(2–3), 115–125. doi:10.1007/s10911-007-9043-7.
Zhang, H., Singh, R. R., Talukder, A. H., & Kumar, R. (2006). Metastatic tumor antigen 3 is a direct corepressor of the Wnt4 pathway. Genes and Development, 20(21), 2943–2948. doi:10.1101/gad.1461706.
Pectasides, D., Pectasides, E., & Psyrri, A. (2008). Granulosa cell tumor of the ovary. Cancer Treatment Reviews, 34(1), 1–12. doi:10.1016/j.ctrv.2007.08.007.
Pectasides, D., Pectasides, E., & Kassanos, D. (2008). Germ cell tumors of the ovary. Cancer Treatment Reviews, 34(5), 427–441. doi:10.1016/j.ctrv.2008.02.002.
Kurman, R. J., & Shih Ie, M. (2011). Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Human Pathology, 42(7), 918–931. doi:10.1016/j.humpath.2011.03.003.
International Agency for Research on Cancer, I. (2008). Descriptive Epidemiology Group of IARC (2008) GLOBOCAN 2002 database. http://www-dep.iarc.fr/2.
Longuespee, R., Boyon, C., Desmons, A., Vinatier, D., Leblanc, E., Farre, I., et al. (2012). Ovarian cancer molecular pathology. Cancer and Metastasis Reviews, 31(3–4), 713–732. doi:10.1007/s10555-012-9383-7.
Thibault, B., Castells, M., Delord, J. P., & Couderc, B. (2013). Ovarian cancer microenvironment: implications for cancer dissemination and chemoresistance acquisition. Cancer and Metastasis Reviews. doi:10.1007/s10555-013-9456-2.
Yap, T. A., Carden, C. P., & Kaye, S. B. (2009). Beyond chemotherapy: targeted therapies in ovarian cancer. Nature Reviews Cancer, 9(3), 167–181. doi:10.1038/nrc2583.
Martin, L. P., & Schilder, R. J. (2009). Management of recurrent ovarian carcinoma: current status and future directions. Seminar in Oncology, 36(2), 112–125. doi:10.1053/j.seminoncol.2008.12.003.
Brüning, A., & Mylonas, I. (2011). New emerging drugs targeting the genomic integrity and replication machinery in ovarian cancer. Archives of Gynecology and Obstetrics, 283(5), 1087–1096. doi:10.1007/s00404-010-1757-x.
Heintz, A. P., Odicino, F., Maisonneuve, P., Beller, U., Benedet, J. L., Creasman, W. T., et al. (2003). Carcinoma of the ovary. International Journal of Gynaecology and Obstetrics, 83(Suppl 1), 135–166.
Fathalla, M. F. (1971). Incessant ovulation—a factor in ovarian neoplasia? Lancet, 2(7716), 163.
Dietl, J. (2014). Revisiting the pathogenesis of ovarian cancer: the central role of the fallopian tube. Archives of Gynecology and Obstetrics, 289(2), 241–246. doi:10.1007/s00404-013-3041-3.
Piek, J. M., Verheijen, R. H., Kenemans, P., Massuger, L. F., Bulten, H., & van Diest, P. J. (2003). BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecologic Oncology, 90(2), 491.
Auersperg, N. (2013). Ovarian surface epithelium as a source of ovarian cancers: unwarranted speculation or evidence-based hypothesis? Gynecologic Oncology, 130(1), 246–251. doi:10.1016/j.ygyno.2013.03.021.
Manavathi, B., & Kumar, R. (2007). Metastasis tumor antigens, an emerging family of multifaceted master coregulators. Journal of Biological Chemistry, 282(3), 1529–1533. doi:10.1074/jbc.R600029200.
Dannenmann, C., Shabani, N., Friese, K., Jeschke, U., Mylonas, I., & Bruning, A. (2008). The metastasis-associated gene MTA1 is upregulated in advanced ovarian cancer, represses ERbeta, and enhances expression of oncogenic cytokine GRO. Cancer Biology and Therapy, 7(9), 1460–1467.
Prisco, M. G., Zannoni, G. F., De Stefano, I., Vellone, V. G., Tortorella, L., Fagotti, A., et al. (2012). Prognostic role of metastasis tumor antigen 1 in patients with ovarian cancer: a clinical study. Human Pathology, 43(2), 282–288. doi:10.1016/j.humpath.2011.05.002.
Bhagat, R., Premalata, C. S., Shilpa, V., Pallavi, V. R., Ramesh, G., Vijay, C. R., et al. (2013). Altered expression of beta-catenin, E-cadherin, and E-cadherin promoter methylation in epithelial ovarian carcinoma. Tumour Biology, 34(4), 2459–2468. doi:10.1007/s13277-013-0797-9.
Kruk, P. A., Uitto, V. J., Firth, J. D., Dedhar, S., & Auersperg, N. (1994). Reciprocal interactions between human ovarian surface epithelial cells and adjacent extracellular matrix. Experimental Cell Research, 215(1), 97–108. doi:10.1006/excr.1994.1320.
Ahmed, N., Thompson, E. W., & Quinn, M. A. (2007). Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. Journal of Cellular Physiology, 213(3), 581–588. doi:10.1002/jcp.21240.
Zhang, X. Y., DeSalle, L. M., Patel, J. H., Capobianco, A. J., Yu, D., Thomas-Tikhonenko, A., et al. (2005). Metastasis-associated protein 1 (MTA1) is an essential downstream effector of the c-MYC oncoprotein. Proceedings of the National Academy of Sciences of the United States of America, 102(39), 13968–13973. doi:10.1073/pnas.0502330102.
Dimova, I., Raitcheva, S., Dimitrov, R., Doganov, N., & Toncheva, D. (2006). Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. European Journal of Cancer, 42(5), 674–679. doi:10.1016/j.ejca.2005.11.022.
Mazumdar, A., Wang, R. A., Mishra, S. K., Adam, L., Bagheri-Yarmand, R., Mandal, M., et al. (2001). Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nature Cell Biology, 3(1), 30–37. doi:10.1038/35050532.
Sheng, Q., & Liu, J. (2011). The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. British Journal of Cancer, 104(8), 1241–1245. doi:10.1038/bjc.2011.62.
Burns, K. A., & Korach, K. S. (2012). Estrogen receptors and human disease: an update. Archives of Toxicology, 86(10), 1491–1504. doi:10.1007/s00204-012-0868-5.
Haring, J., Schuler, S., Lattrich, C., Ortmann, O., & Treeck, O. (2012). Role of estrogen receptor beta in gynecological cancer. Gynecologic Oncology, 127(3), 673–676. doi:10.1016/j.ygyno.2012.09.006.
He, X., Zhou, C., Zheng, L., & Xiong, Z. (2013). Overexpression of MTA1 promotes invasiveness and metastasis of ovarian cancer cells. Irish Journal of Medical Science. doi:10.1007/s11845-013-1034-7.
Pakala, S. B., Rayala, S. K., Wang, R. A., Ohshiro, K., Mudvari, P., Reddy, S. D., et al. (2013). MTA1 promotes STAT3 transcription and pulmonary metastasis in breast cancer. Cancer Research, 73(12), 3761–3770. doi:10.1158/0008-5472.CAN-12-3998.
Rosen, D. G., Mercado-Uribe, I., Yang, G., Bast, R. C., Jr., Amin, H. M., Lai, R., et al. (2006). The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer, 107(11), 2730–2740. doi:10.1002/cncr.22293.
Colomiere, M., Ward, A. C., Riley, C., Trenerry, M. K., Cameron-Smith, D., Findlay, J., et al. (2009). Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. British Journal of Cancer, 100(1), 134–144. doi:10.1038/sj.bjc.6604794.
Kamran, M. Z., Patil, P., & Gude, R. P. (2013). Role of STAT3 in cancer metastasis and translational advances. Biomedical Research International, 2013, 421821. doi:10.1155/2013/421821.
Yang, J., Mani, S. A., Donaher, J. L., Ramaswamy, S., Itzykson, R. A., Come, C., et al. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell, 117(7), 927–939. doi:10.1016/j.cell.2004.06.006.
Abeler, V. M., & Kjorstad, K. E. (1991). Endometrial adenocarcinoma in Norway. A study of a total population. Cancer, 67(12), 3093–3103.
Amant, F., Moerman, P., Neven, P., Timmerman, D., Van Limbergen, E., & Vergote, I. (2005). Endometrial cancer. Lancet, 366(9484), 491–505.
Chan, J. K., Wu, H., Cheung, M. K., Shin, J. Y., Osann, K., & Kapp, D. S. (2007). The outcomes of 27,063 women with unstaged endometrioid uterine cancer. Gynecologic Oncology, 106(2), 282–288. doi:10.1016/j.ygyno.2007.05.033.
Prat, J. (2004). Prognostic parameters of endometrial carcinoma. Human Pathology, 35(6), 649–662.
Gloeckler Ries, L. A., Reichman, M. E., Lewis, D. R., Hankey, B. F., & Edwards, B. K. (2003). Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. The Oncologist, 8(6), 541–552.
Wright, J. D., Barrena Medel, N. I., Sehouli, J., Fujiwara, K., & Herzog, T. J. (2012). Contemporary management of endometrial cancer. Lancet, 379(9823), 1352–1360. doi:10.1016/S0140-6736(12)60442-5.
Trimble, C. L., Method, M., Leitao, M., Lu, K., Ioffe, O., Hampton, M., et al. (2012). Management of endometrial precancers. Obstetrics and Gynecology, 120(5), 1160–1175. doi:10.1097/AOG.0b013e31826bb121.
Deligdisch, L., & Holinka, C. F. (1987). Endometrial carcinoma: two diseases? Cancer Detection and Prevention, 10(3–4), 237–246.
Bokhman, J. V. (1983). Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology, 15(1), 10–17.
Lax, S. F. (2004). Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Archiv, 444(3), 213–223.
Jereczek-Fossa, B., Badzio, A., & Jassem, J. (1999). Surgery followed by radiotherapy in endometrial cancer: analysis of survival and patterns of failure. International Journal of Gynecological Cancer, 9(4), 285–294.
Faratian, D., Stillie, A., Busby-Earle, R. M., Cowie, V. J., & Monaghan, H. (2006). A review of the pathology and management of uterine papillary serous carcinoma and correlation with outcome. International Journal of Gynecological Cancer, 16(3), 972–978.
Dallenbach-Hellweg, G. (1999). Histopathologie und Stadieneinteilung des Endometriumkarzinoms inklusive seiner Präkanzerosen. Der Onkologe, 5, 388–395.
Macwhinnie, N., & Monaghan, H. (2004). The use of P53, PTEN, and C-erbB-2 to differentiate uterine serous papillary carcinoma from endometrioid endometrial carcinoma. International Journal of Gynecological Cancer, 14(5), 938–946.
Villella, J. A., Cohen, S., Smith, D. H., Hibshoosh, H., & Hershman, D. (2006). HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. International Journal of Gynecological Cancer, 16(5), 1897–1902.
Tsikouras, P., Bouchlariotou, S., Vrachnis, N., Dafopoulos, A., Galazios, G., Csorba, R., et al. (2013). Endometrial cancer: molecular and therapeutic aspects. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 169(1), 1–9. doi:10.1016/j.ejogrb.2013.01.018.
Mylonas, I. (2010). Inhibin-alpha, -betaA and -betaB subunits in uterine non-endometrioid carcinomas: prognostic significance and clinical implications. European Journal of Cancer, 46(13), 2485–2493. doi:10.1016/j.ejca.2010.06.001.
Mylonas, I., Worbs, S., Shabani, N., Kuhn, C., Kunze, S., Schulze, S., et al. (2009). Inhibin-alpha subunit is an independent prognostic parameter in human endometrial carcinomas: analysis of inhibin/activin-alpha, -betaA and -betaB subunits in 302 cases. European Journal of Cancer, 45(7), 1304–1314. doi:10.1016/j.ejca.2009.01.008.
Shabani, N., Kuhn, C., Kunze, S., Schulze, S., Mayr, D., Dian, D., et al. (2007). Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. European Journal of Cancer, 43(16), 2434–2444. doi:10.1016/j.ejca.2007.08.014.
Mirantes, C., Espinosa, I., Ferrer, I., Dolcet, X., Prat, J., & Matias-Guiu, X. (2013). Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Human Pathology, 44(10), 1973–1981. doi:10.1016/j.humpath.2013.04.009.
Blechschmidt, K., Kremmer, E., Hollweck, R., Mylonas, I., Hofler, H., Kremer, M., et al. (2007). The E-cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagnostic Molecular Pathology, 16(4), 222–228. doi:10.1097/PDM.0b013e31806219ae.
Bruning, A., Juckstock, J., Blankenstein, T., Makovitzky, J., Kunze, S., & Mylonas, I. (2010). The metastasis-associated gene MTA3 is downregulated in advanced endometrioid adenocarcinomas. Histology and Histopathology, 25(11), 1447–1456.
Mylonas, I., & Bruning, A. (2012). The metastasis-associated gene MTA3 is an independent prognostic parameter in uterine non-endometrioid carcinomas. Histopathology, 60(4), 665–670. doi:10.1111/j.1365-2559.2011.04103.x.
Franco, E. L., Schlecht, N. F., & Saslow, D. (2003). The epidemiology of cervical cancer. Cancer Journal, 9(5), 348–359.
Waggoner, S. E. (2003). Cervical cancer. Lancet, 361(9376), 2217–2225. doi:10.1016/S0140-6736(03)13778-6.
Elfstrom, K. M., Herweijer, E., Sundstrom, K., & Arnheim-Dahlstrom, L. (2014). Current cervical cancer prevention strategies including cervical screening and prophylactic human papillomavirus vaccination: a review. Current Opinion in Oncology, 26(1), 120–129. doi:10.1097/CCO.0000000000000034.
Gien, L. T., Beauchemin, M. C., & Thomas, G. (2010). Adenocarcinoma: a unique cervical cancer. Gynecologic Oncology, 116(1), 140–146. doi:10.1016/j.ygyno.2009.09.040.
Munoz, N., Bosch, F. X., de Sanjose, S., Herrero, R., Castellsague, X., Shah, K. V., et al. (2003). Epidemiologic classification of human papillomavirus types associated with cervical cancer. New England Journal of Medicine, 348(6), 518–527. doi:10.1056/NEJMoa021641.
Massad, L. S., Einstein, M., Myers, E., Wheeler, C. M., Wentzensen, N., & Solomon, D. (2009). The impact of human papillomavirus vaccination on cervical cancer prevention efforts. Gynecologic Oncology, 114(2), 360–364. doi:10.1016/j.ygyno.2009.04.005.
zur Hausen, H. (2009). Papillomaviruses in the causation of human cancers—a brief historical account. Virology, 384(2), 260–265. doi:10.1016/j.virol.2008.11.046.
Walboomers, J. M., Jacobs, M. V., Manos, M. M., Bosch, F. X., Kummer, J. A., Shah, K. V., et al. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology, 189(1), 12–19. doi:10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F.
Bui-Nguyen, T. M., Pakala, S. B., Sirigiri, R. D., Xia, W., Hung, M. C., Sarin, S. K., et al. (2010). NF-kappaB signaling mediates the induction of MTA1 by hepatitis B virus transactivator protein HBx. Oncogene, 29(8), 1179–1189. doi:10.1038/onc.2009.404.
Rao, Y., Wang, H., Fan, L., & Chen, G. (2011). Silencing MTA1 by RNAi reverses adhesion, migration and invasiveness of cervical cancer cells (SiHa) via altered expression of p53, and E-cadherin/beta-catenin complex. Journal of Huazhong University of Science and Technology. Medical Sciences, 31(1), 1–9. doi:10.1007/s11596-011-0141-9.
Liu, T., Yang, M., Yang, S., Ge, T., Gu, L., & Lou, G. (2013). Metastasis-associated protein 1 is a novel marker predicting survival and lymph nodes metastasis in cervical cancer. Human Pathology, 44(10), 2275–2281. doi:10.1016/j.humpath.2013.05.009.
Qian, H., Yu, J., Li, Y., Wang, H., Song, C., Zhang, X., et al. (2007). RNA interference of metastasis-associated gene 1 inhibits metastasis of B16F10 melanoma cells in a C57BL/6 mouse model. Biology of the Cell, 99(10), 573–581.
Du, B., Yang, Z. Y., Zhong, X. Y., Fang, M., Yan, Y. R., Qi, G. L., et al. (2011). Metastasis-associated protein 1 induces VEGF-C and facilitates lymphangiogenesis in colorectal cancer. World Journal of Gastroenterology, 17(9), 1219–1226. doi:10.3748/wjg.v17.i9.1219.
Jiang, Q., Zhang, H., & Zhang, P. (2011). ShRNA-mediated gene silencing of MTA1 influenced on protein expression of ER alpha, MMP-9, CyclinD1 and invasiveness, proliferation in breast cancer cell lines MDA-MB-231 and MCF-7 in vitro. Journal of Experimental & Clinical Cancer Research, 30, 60. doi:10.1186/1756-9966-30-60.
Li, Y., Chao, Y., Fang, Y., Wang, J., Wang, M., Zhang, H., et al. (2013). MTA1 promotes the invasion and migration of non-small cell lung cancer cells by downregulating miR-125b. Journal of Experimental & Clinical Cancer Research, 32, 33. doi:10.1186/1756-9966-32-33.
Reddy, S. D., Pakala, S. B., Ohshiro, K., Rayala, S. K., & Kumar, R. (2009). MicroRNA-661, a c/EBPalpha target, inhibits metastatic tumor antigen 1 and regulates its functions. Cancer Research, 69(14), 5639–5642. doi:10.1158/0008-5472.CAN-09-0898.
Zhou, H., Xu, X., Xun, Q., Yu, D., Ling, J., Guo, F., et al. (2012). microRNA-30c negatively regulates endometrial cancer cells by targeting metastasis-associated gene-1. Oncology Reports, 27(3), 807–812. doi:10.3892/or.2011.1574.
Wirth, T., Parker, N., & Yla-Herttuala, S. (2013). History of gene therapy. Gene, 525(2), 162–169. doi:10.1016/j.gene.2013.03.137.
Ibraheem, D., Elaissari, A., & Fessi, H. (2014). Gene therapy and DNA delivery systems. International Journal of Pharmaceutics, 459(1–2), 70–83. doi:10.1016/j.ijpharm.2013.11.041.
Kai, L., Samuel, S. K., & Levenson, A. S. (2010). Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. International Journal of Cancer, 126(7), 1538–1548. doi:10.1002/ijc.24928.
Li, K., Dias, S. J., Rimando, A. M., Dhar, S., Mizuno, C. S., Penman, A. D., et al. (2013). Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS ONE, 8(3), e57542. doi:10.1371/journal.pone.0057542.
Gescher, A., Steward, W. P., & Brown, K. (2013). Resveratrol in the management of human cancer: how strong is the clinical evidence? Annals of the New York Academy of Sciences, 1290, 12–20. doi:10.1111/nyas.12205.
Benedetti, R., Conte, M., & Altucci, L. (2014). Targeting histone deacetylases in diseases: where are we? Antioxidants and Redox Signaling. doi:10.1089/ars.2013.5776.
Millard, C. J., Watson, P. J., Celardo, I., Gordiyenko, Y., Cowley, S. M., Robinson, C. V., Fairall, L., & Schwabe, J. W. (2013). Class I HDACs share a common mechanism of regulation by inositol phosphates. Molecular Cell, 51(1), 57–67.
Acknowledgments
We gratefully appreciate the support by the German Cancer Science Foundation (Bonn, Germany) for previous work on MTA proteins and the current support by the Wilhelm-Vaillant Stiftung (Munich, Germany) for future studies on MTA proteins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brüning, A., Blankenstein, T., Jückstock, J. et al. Function and regulation of MTA1 and MTA3 in malignancies of the female reproductive system. Cancer Metastasis Rev 33, 943–951 (2014). https://doi.org/10.1007/s10555-014-9520-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9520-6