Abstract
Cardiovascular disease (CVD) is the leading cause of end-stage mortality in chronic kidney disease (CKD) patients. However, CVD and CKD are inextricably linked, as microalbuminuria is an independent risk factor for CVD. Herein, we investigated changes in cardiac function and its risk factors in CKD patients who had different urine albumin-to-creatinine ratios (UACRs) and estimated glomerular filtration rates (eGFRs). We prospectively enrolled 182 CKD patients, classified into three groups based on UACRs and eGFRs. Fifty healthy volunteers were included as controls. Changes in clinical and echocardiographic parameters were assessed in each group, and factors independently associated with strain parameters were further analyzed. Compared with those in the control group, the albuminuria but unimpaired renal function (ALB-CKD G1-2), albuminuria and impaired renal function (ALB-CKD G3), and normoalbuminuric CKD (NACKD) groups had decreased left ventricular (LV), right ventricular (RV), and left atrial (LA) strains, the LA contractile strain being the only statistically comparable parameter. Stepwise multiple linear regression analysis revealed varying factors independently correlating with the LV global longitudinal strain. The LA reservoir and conduit strains independently correlated with LV diastolic function in stage 3 CKD associated with comorbid albuminuria or normoalbuminuria. LV function was a partial determinant of LA and RV function in the ALB-CKD G3 group, whereas ventricular and atrial function were independent of each other in the ALB-CKD G1-2 and NACKD groups. Clinical intervention should focus on specific factors affecting cardiac function in patients to reduce the risk of CVD-related death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of chronic kidney disease (CKD) is progressively increasing globally, with multiple comorbid complications, the most serious of which is cardiovascular disease (CVD) [1]. A hallmark of CKD is left ventricular (LV) myocardial hypertrophy, which is mainly due to factors including insulin resistance, accumulation of uremic toxins, and endogenous cardiotonic steroids [2]. Left ventricular hypertrophy is considered to be one of the strongest risk factors for all-cause mortality in patients with advanced CKD, and the pathogenesis of left ventricular hypertrophy is multifactorial [3, 4]. Sympathetic hyperactivity, hypertension, hypoproteinemia, anemia, and accumulation of endogenous nitric oxide synthase inhibitors have been proposed as pathogenic mechanisms of left ventricular hypertrophy [5,6,7]. Norepinephrine, a marker of sympathetic nerves in patients with advanced CKD, promotes cardiomyocyte hypertrophy [8]. Left ventricular hypertrophy reduces coronary artery reserve and induces myocardial ischemia, which in turn promotes myocardial infarction and fatal arrhythmia [9]. There is evidence that increased sympathetic activity is also present in patients with mild to moderate renal insufficiency. Diffuse myocardial interstitial fibrosis has developed in patients with mild to moderate CKD, resulting in abnormal intracardiac blood flow [10]. The presence of protein in the urine increases CVD morbidity and mortality, and is independently associated with traditional risk factors for CVD. Even a urine protein level lower than the microalbuminuria threshold is associated with an increased risk of cardiovascular events [11]. Penno et al. suggested that the risk of CVD was significantly higher in patients with normoalbuminuria and impaired renal function than in patients with normal eGFR but with albuminuria [12]. Epicardial adipose tissue (EAT) is located between the myocardium and the visceral layer of the pericardium. EAT secretes cardioprotective cytokines under normal conditions and pro-inflammatory or pro-atherosclerotic cytokines in obese people [13]. One study reported that EAT volume correlates with the severity of coronary atherosclerosis, demonstrating the unique versatility of EAT [14]. Several studies have shown that patients with CKD and a preserved LV ejection fraction (LVEF) have already developed LV strain, left atrial (LA) strain, and LV diastolic function abnormalities, and it has been reported that LA parameters are the best predictors of adverse cardiovascular outcomes in CKD patients [15,16,17]. Most current studies on the right ventricle in CKD patients have focused on patients who underwent renal replacement therapy. There is limited data on the right ventricle in patients at the stage of compensated renal failure. Myocardial strains assessed using speckle-tracking technology can sensitively detect abnormalities in myocardial motions, which is important in both CVD diagnosis and risk stratification. This study aimed to analyze cardiac function and EAT in populations with different UACRs and eGFRs to determine whether there is an interaction between left and right ventricular (RV) function. We also aimed to identify factors independently correlated to cardiac function in populations with different urine albumin-to-creatinine ratios (UACRs) and estimated glomerular filtration rates (eGFRs) to provide a theoretical basis for clinical intervention in relation to cardiac function at different stages. We hypothesized that cardiac function in different groups of CKD patients would be reduced, but that the degree of reduction may vary with changes in eGFR and UACR.
Methods
Patient population
We prospectively enrolled 182 CKD patients who attended the Department of Nephrology, First Affiliated Hospital, Jinzhou Medical University, from May 2021 to March 2023 as the study population. The inclusion criteria comprised patients with the following CKD diagnostic criteria: (i) CKD was diagnosed with reference to Kidney Disease: Improving Global Outcomes 2020 Clinical Practice Guidelines [18]; (ii) the eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation, based on the creatinine level [19]; and (iii) based on eGFRs and UACRs, patient groups were classified as follows: albuminuria but unimpaired renal function (ALB-CKD G1-2 [eGFR ≥ 60 mL/min/1.73 m2; UACR ≥ 30 mg/g]; n = 55), albuminuria and impaired renal function (ALB-CKD G3 [eGFR 30–60 mL/min/1.73 m2; UACR ≥ 30 mg/g]; n = 95), and normoalbuminuric CKD (NACKD, [eGFR 30–60 mL/min/1.73 m2; UACR < 30 mg/g]; n = 32). The exclusion criteria comprised patients: (i) who underwent renal replacement therapy, (ii) with renal vascular disease or space-occupying lesions, (iii) with poor insonation conditions, (iv) with incorrectly outlined endocardium due to software failure, and (v) with a history of malignancy.
Fifty healthy volunteers who attended our hospital health check-up center were selected as controls (eGFR, ≥ 60 mL/min/1.73 m2; UACR, < 30 mg/g). Based on electrocardiography, echocardiography, blood pressure, biochemical indicators, and routine urine test results, we confirmed that none of the control group volunteers had heart disease, CKD, hypertension, or diabetes mellitus.
Fasting venous blood and random urine samples were collected in the morning from all participants to test the biochemical indicators and calculate the UACRs. This study was approved by the Ethics Committee of First Affiliated Hospital, Jinzhou Medical University (202,335). All participants provided their written informed consent to participate, and all investigations were conducted in accordance with the principles of the Declaration of Helsinki.
Echocardiography
A Philips Epiq 7c ultrasound system (Philips, Best, The Netherlands) was used to examine all the participants. EAT was observed as a hypoechoic space between the visceral layer of the pericardium and the epicardium of the right ventricle. EAT thickness was measured as the depth of this hypoechoic space. In the parasternal long-axis view at the left ventricle, EAT, interventricular septal thickness, and LV posterior wall thickness were measured on still-frame end-diastolic images while the LA dimension was measured on still-frame end-systolic images. All measurements were obtained in accordance with American Society of Echocardiography recommendations. The LVEF, LV end-diastolic volume (LVEDV), and LV end-systolic volume (LVESV) were measured in apical four- and two-chamber views using the biplane Simpson’s method. Left ventricular mass (LVM) and left ventricular mass index (LVMI) were calculated by the American Society of Echocardiography formula [20]. LVM=0.8 × 1.04× [(IVSd+LVPWd+LVEDD)3 -LVEDD3] + 0.6;LVMI=LVM/ BSA. Early diastolic mitral inflow velocity (E) and late diastolic mitral inflow velocity (A) were measured at the mitral orifice using pulsed wave Doppler. Peak early diastolic mitral annular velocity (e’) and peak systolic mitral annular velocity (s’) at the LV lateral wall were measured using tissue Doppler imaging, and E/e’ was calculated. Mitral annular plane systolic excursion and tricuspid annular plane systolic excursion from end-diastole to end-systole were measured using M-mode through placing the sampling line at the roots of the mitral annulus and tricuspid annulus attaching to the LV and RV lateral walls. LA endocardium was outlined in apical four- and two-chamber views using the biplane Simpson’s method, followed by measuring the LA area, LA volume, and LA volume index (LAVI). In the apical 4-chamber views focusing on the right ventricle, end-diastolic RV base diameter, RV mid-diameter, and RV length were measured, and RV fractional area change was obtained through outlining the RV endocardium at end-diastole and end-systole. The sampling frame of the pulse wave Doppler was placed parallel to the direction of blood flow at the tricuspid orifice to obtain the early diastolic tricuspid inflow velocity (E), late diastolic tricuspid inflow velocity (A), and E/A. The tissue Doppler imaging mode was selected and the sampling frame was placed at the intersection of the tricuspid annulus and the RV lateral wall to obtain peak systolic tricuspid annular velocity (s’). Two-dimensional dynamic images of four cardiac cycles in the apical four-, three-, and two-chamber views were acquired and stored within the ultrasound system. Echocardiographic examinations were performed by physicians unaware of the clinical data.
Speckle-tracking echocardiography
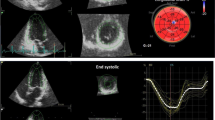
All acquired images were imported into Qlab software (Philips) for offline analysis. The LV myocardial longitudinal strain and the corresponding bull’s-eye diagram were derived from the LV endocardium that was automatically outlined by the system through selecting apical four-, three-, and two-chamber views at the same time as using the Autostrain LV plug-in. Images in the apical four-chamber view were also imported into the Autostrain LA plug-in. The LA reservoir strain (LASr), the LA conduit strain (LAScd), and the LA contractile strain (LASct) were obtained from the LV endocardium automatically outlined by the system using end-diastole as the zero point of strain. Images in the apical four-chamber view focusing on the RV were imported into the Autostrain RV plug-in. The system automatically outlined the RV endocardium and calculated the RV free-wall longitudinal strain (RVFWSL) and the RV four-chamber cardiac strain (RV4CSL). The region of interest generated automatically by the system can be manually modified in all of the above plug-ins until they overlap with the endocardium and epicardium. The final data for each parameter was the mean of three repeated measurements.
Statistical analysis
SPSS 25.0 statistical software was applied and a chi-square test was used for comparing the count data. Analysis of variance was used for comparing the measurement data that conformed to normal distribution, while a Kruskal-Wallis rank sum test was used for comparing the measurement data that did not conform to normal distribution. Pearson’s correlation, point biserial correlation, and Spearman’s rank correlation were used to analyze the correlations between the strain parameters and LV diastolic function, and clinical parameters, as well as EAT, respectively. To identify the factors that independently correlated with the strains in the different groups, stepwise multiple linear regression analyses were performed with the strains being the dependent variable and the clinical parameters, LV diastolic function parameters, and EAT being the independent variables. Univariate linear regression was used to analyze the association between LV global longitudinal strain (LVGLS) and LASr, and LAScd, as well as RVFWSL in different groups. Referring to the control group, receiver operating characteristic (ROC) curves were plotted to evaluate the predictability of the strain parameters for cardiac function. P < 0.05 was considered as statistically significant. Data from 30 participants were randomly selected and analyzed for LVGLS, LASr, and RVFWSL, using Bland-Altman plots, by two physicians skilled in the use of Qlab software.
Results
Clinical characteristics
Figure 1 shows the total number of participants included in this study (n = 232), among whom 50 were in the control group, 55 were in the ALB-CKD G1-2 group, 95 were in the ALB-CKD G3 group, and 32 were in the NACKD group.
As shown in Table 1; Fig. 2, the UACRs of the NACKD and control groups were within the normal range, while microalbuminuria and macroalbuminuria were present in participants in the ALB-CKD G1-2 and G3 groups, with macroalbuminuria being predominant. The ALB-CKD G3 and NACKD groups had similar eGFRs. Differences in eGFRs among the remaining groups were all significant. Participants in the ALB-CKD G3 group had a higher prevalence of hypertension and diabetes mellitus than those in the NACKD group and a higher prevalence of hypertension than those in the ALB-CKD G1-2 group. The prevalence of diabetes mellitus was higher in the G1-2 group than in the NACKD group.
Echocardiographic parameters
As shown in Tables 2and Fig. 3, among the parameters used to assess LV systolic and diastolic function, there was a gradual decrease in LV diastolic function in the ALB-CKD G1-2 group, followed by the NACKD and ALB-CKD G3 groups. LV strain was a superior indicator not only to changes in LVEF but also to changes in peak systolic mitral velocity.
Among the parameters used to assess LA function, the major manifestation of reduced LA function in the ALB-CKD G1-2 group was decreased LA strain, whereas the manifestations of reduced LA function in the NACKD and ALB-CKD G3 groups were decreased LAVI and decreased LA strain.
Among the parameters assessing RV function, only the RVFWSL and RV4CSL of the ALB-CKD G1-2, ALB-CKD G3, and NACKD groups were lower than those of the control group. The remaining RV function parameters remained statistically comparable among the groups.
Stepwise multiple linear regression analysis
In Table 3, the stepwise multiple linear regression analysis of the strain parameters of the ALB-CKD G1-2 group shows that triglyceride level and smoking were correlated with LASr (model 1b, R2 0.190; P = 0.004), while mitral annulus E/e’ and the total cholesterol level were associated with LAScd. In Table 4, the stepwise multiple linear regression analysis of the strain parameters of the ALB-CKD G3 group shows that body mass index (BMI), the level of low-density lipoprotein (LDL), and LVEDV correlated with LVGLS (Model 2a, R2 0.226; P = 0.000), and RVFWSL independently correlated with the level of high-density lipoprotein (HDL). In Table 5, the stepwise multiple linear regression analysis of the strain parameters of the NACKD group shows that LVGLS independently correlated with the serum creatinine level, while the mitral annulus e’ velocity, mitral inflow A velocity, and EAT correlated with LASr (Model 3b, R2 0.508; P = 0.000). RV4CSL of the ALB-CKD G1-2 and NACKD groups and LVGLS of the ALB-CKD G1-2 group did not show independent correlation with clinical parameters.
ROC curve analysis of strain parameters
As shown in Fig. 4, LVGLS had high predictability for LV function in the NACKD group (AUC = 0.987), and LASr and RVFWSL had high predictability for LA and RV function (AUC = 0.860 and AUC = 0.763), respectively, in the ALB-CKD G1-2 group.
Univariate linear regression analysis
As shown in Fig. 5, the LV function in the ALB-CKD G1-2 and NACKD groups was independent of the corresponding LA or RV functions. In the ALB-CKD G3 group, LVGLS had a significant positive effect on LASr, LAScd, and RVFWSL. LV function determined changes in LASr and LAScd to a degree of 12.3%, and changes in RVFWSL to a degree of 7.8%.
Intra- and inter-observer agreement analysis
As shown in Fig. 6, RVFWSL, LASr, and LVGLS had high inter- and intra-observer agreement, with only a few data points scattering outside the 95% confidence interval.
Discussion
Using multimodal echocardiography images, we comprehensively assessed differences in cardiac function among populations with different UACRs and eGFRs and identified factors that independently correlated with different cardiac functions. First, we found that LVGLS in the ALB-CKD G1-2, NACKD, and ALB-CKD G3 groups was significantly lower than that in the control group. Moreover, LVGLS first gradually decreased in the ALB-CKD G1-2 group, followed by the NACKD group, and then the ALB-CKD G3 group. No statistically significant difference was observed among some of the groups; however, we identified trends concerning how the disease progressed. Second, the LASr and LAScd in the ALB-CKD G1-2, NACKD, and ALB-CKD G3 groups were significantly lower than those in the control group. LAScd was comparable among the participants in all four groups. Most studies have focused on populations with reduced eGFRs; however, our study findings suggest that a decrease in LV function could be observed when the eGFR remained at a normal level while abnormal protein appeared in the urine. Third, we analyzed the associations among LV, LA, and RV strains in different groups and found that changes in LV function affected LA and RV functions only in the ALB-CKD G3 group. LV, LA, and RV functions were independent of each other in the other groups. Fourth, reduced RV strain was observed in patients with stage 3 CKD even when they were in a compensated renal failure stage. This was also found in patients with abnormal urine protein levels and normal eGFRs. RV strains were similar among these two groups of patients, suggesting that RV strain is not related to UACR or eGFR in stage 1–3 CKD. Moreover, all three groups of study participants had no statistically significant differences in the RV fractional area change. Fifth, EAT did not correlate with LV, LA, or RV strain in the ALB-CKD G1-2, NACKD, and ALB-CKD G3 groups. It only correlated independently with LASr in the NACKD group. Finally, we also identified the relevant factors affecting cardiac function in patients with different UACRs and eGFRs to provide an appropriate theoretical basis for clinical practice. Two-dimensional speckle tracking technology overcomes the angle dependence, and is not affected by the surrounding segment of the myocardium and the overall motion of the heart, which can be used for real-time continuous observation of cardiac characteristics [21]. Traditional echocardiography can judge the ventricular systolic function of patients by measuring LVEF and RVFAC, which is operator-dependent, and this study showed that LVEF and RVFAC had no significant difference between the four groups of subjects. The ventricular strain obtained by speckle tracking decreased before the change of LVEF and RVFAC, indicating that compared with traditional ultrasound, two-dimensional speckle tracking technology has higher sensitivity in the diagnosis of early myocardial damage in patients with CKD, and can more accurately reflect the strain value of each myocardial stage.
The LVEF of all four groups did not significantly differ and was within the normal range. IVSd, LVPWd, LVEDV, and LVESV increased only in the NACKD and ALB-CKD G3 groups compared with the control and ALB-CKD G1-2 groups. This finding could be a result of LV myocardial hypertrophy due to LV remodeling in CKD patients. Pluta et al. found that patients with stage 2–3 CKD had LV myocardial remodeling, which is similar to the findings of the current study [22]. LVGLS in the NACKD and ALB-CKD G3 groups was lower than that in the ALB-CKD G1-2 group, suggesting that cardiac function in the patients reduced gradually. One possible cause for these findings is that elevated levels of plasma high-sensitivity c-reactive protein and interleukin-6 lead to systolic dysfunction in CKD patients [23]. Another possible cause is the incidence of endotoxemia in CKD patients. Endotoxins and lipopolysaccharide-binding proteins, the important factors contributing to chronic inflammation and inflammatory responses, are both associated with LV dysfunction [24]. Demetgul et al. studied children with CKD who had preserved LVEF and found a reduction in peak systolic longitudinal strain, which is similar to the results of this study [25]. Serum creatinine independently correlated with LVGLS in the NACKD group, while BMI, the LDL level, and LVEDV independently correlated with LVGLS in the ALB-CKD G3 group. One cardiovascular risk factor in CKD patients is obesity. Obesity increases volume, thus, increasing the cardiac burden, and also increases myocardial fat deposition, leading to myocardial fibrosis [1]. Abnormal levels of plasma lipoproteins are equally important causes of CVD in CKD patients. Lee et al. reported that patients with stage 3–5 CKD and an LDL level of ≥ 159 mg/dL had a hazard ratio of 1.261 for adverse cardiovascular events (P < 0.001), suggesting that paying attention to LDL concentrations and implementing early intervention in CKD patients would be beneficial to improving their clinical prognosis [26]. Factors independently correlating with RVFWSL in the NACKD and ALB-CKD G3 groups included LDL and HDL levels, respectively. Therefore, dyslipidemia may affect not only the LV but also the RV function in patients.
The ALB-CKD G1-2, NACKD, and ALB-CKD G3 groups had increased mitral inflow A velocities compared to the control group, while the differences in mitral annulus e’ velocity and E/e’ between the NACKD and ALB-CKD G3 groups, and between the ALB-CKD G1-2 and control groups were statistically significant. These parameters suggest impaired LV diastolic compliance in CKD patients. In CKD patients, myocardial fibrosis and LV hypertrophy progress simultaneously, with a significant increase in extracellular matrix leading to the replacement of functional myocytes with fibrotic scar tissue, which increases LV stiffness and limits LV diastolic filling. We found that changes in LA strain appeared earlier than changes in LAVI and LA dimension in the ALB-CKD G1-2 group, but traditional parameters for assessing LA function and strain all altered in the NACKD and ALB-CKD G3 groups. This suggests that reduction in LA strain had already appeared in the ALB-CKD G1-2 group and that LA was more severely impaired in the NACKD and ALB-CKD G3 groups than in the ALB-CKD G1-2 group. Gan et al. similarly found that changes in LA strain happened before changes in LA volume, and that LA volume might increase as renal function progressively decreases [27]. Our results showed how LA parameter trends varied in patients with CKD at different stages. The activity of angiotensin II is enhanced through upregulation of the renin-angiotensin-aldosterone system in CKD patients. This acts together with other downstream mediators and leads to progressive myocardial fibrosis, affecting the atria in the early stage; thus, driving atrial dysfunction [28]. The factors that independently correlated with LASr and LAScd in the NACKD and ALB-CKD G3 groups were mostly parameters that indicated LV diastolic function, which showed the greater influence of LV diastolic function on LA function from a different perspective.
Most traditional parameters used to assess RV function in the ALB-CKD G1-2, NACKD, and ALB-CKD G3 groups did not differ statistically significantly from those in the control group. However, RVFWSL and RV4CSL were lower in the ALB-CKD G1-2, NACKD, and ALB-CKD G3 groups than in the control group. Some studies have demonstrated that RVFWSL with a threshold of -20% to -21% appears to detect abnormal RV function [29]. Most patients in our study had stage 1–3 CKD. We found that RV function was similar among CKD patients who had different UACRs and eGFRs and was reduced only when compared with RV function of the controls. This suggests that the degree of RV impairment is similar in populations with different UACRs or eGFRs and is independent of disease severity. Further studies are needed to elucidate the specific mechanisms of altered RV strain at different stages in CKD patients. Heart rate independently correlated with RVFWSL in patients in the ALB-CKD G1-2 group. Ohashi et al. reported that heart rate was positively correlated with intrarenal activation of the renin-angiotensin system and that activation of this system could exacerbate renal damage [30]. In addition, they found that increased activation of reactive oxygen species and the renin-angiotensin system in the kidney resulted from activation of reactive oxygen species and the renin-angiotensin system in cardiovascular centers via renal efferent sympathetic nerves [31].
BMI is an inverse predictor of mortality in CKD patients. However, the incidence of ectopic fat deposition associated with coronary atherosclerosis in non-obese CKD patients may suggest accumulated risks [32]. Chen et al. found thickening of EAT in patients with non-dialysis-dependent CKD but found no predictability in relation to changes in EAT for adverse cardiovascular events in such patients [33]. In this study, we found that EAT was increased in the NACKD and ALB-CKD G3 groups compared with the control group, while EAT was similar between the ALB-CKD G1-2 group and the control group. However, neither EAT in the NACKD group nor in the ALB-CKD G3 group correlated with LVGLS, LASr, LAScd, RVFWSL, or RV4CSL. Only EAT in the NACKD group correlated independently with LASr. A possible explanation for these differences is our study population had stage 1–3 CKD, whereas previous studies have mainly focused on patients with stage 3–5 CKD. There have been limited studies on the relationship between EAT and myocardial strain in patients with stage 1–3 CKD. Future large-scale studies are needed to confirm our findings.
Study limitations
This study had some limitations. First, the number of patients in the NACKD and ALB-CKD G1-2 groups was small. During the two-year study period involving patients with stage 3 CKD at our hospital, only 32 patients with a reduced eGFR without an elevated urinary protein level were identified. Thus, our findings are preliminary and exploratory and encourage future evaluation in larger populations. Second, our group did not report natriuretic peptide measurements in this study. Third, we found decreased RV strain in patients with stage 1–3 CKD, but the exact mechanism involved requires further investigation. Finally, Angiotensin-converting enzyme inhibitors were not evenly distributed among groups in this study, which may affect the results of this study.
Conclusion
The ALB-CKD G1-2, ALB-CKD G3, and NACKD groups all had reduced cardiac function, among which the NACKD group had more severely impaired cardiac function than the ALB-CKD G1-2 group. Therefore, clinical attention should be paid to assessments of albuminuria and eGFR.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author upon reasonable request.
References
Matsushita K, Ballew SH, Wang AY et al (2022) Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 18:696–707
Patel N, Yaqoob MM, Aksentijevic D (2022) Cardiac metabolic remodelling in chronic kidney disease. Nat Rev Nephrol 18:524–537
Parfrey PS, Foley RN, Harnett JD et al (1996) Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11:1277–1285
Zoccali C, Benedetto FA, Mallamaci F et al (2001) Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol 12:2768–2774
Middleton RJ, Parfrey PS, Foley RN (2001) Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 12:1079–1084
Zoccali C, Mallamaci F, Tripepi G et al (2002) Norepinephrine and concentric hypertrophy in patients with end-stage renal disease. Hypertension 40:41–46
Zoccali C, Mallamaci F, Maas R et al (2002) Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62:339–345
Simpson P (1983) Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest 72:732–738
Zoccali C, Benedetto FA, Mallamaci F et al (2004) Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int 65:1492–1498
Cao Y, Sun XY, Zhong M et al (2019) Evaluation of hemodynamics in patients with hypertrophic cardiomyopathy by vector flow mapping: comparison with healthy subjects. Exp Ther Med 17:4379–4388
Wu D, Xuan Y, Ruan Y et al (2016) Prevalence of macro- and microvascular complications in patients with type 2 diabetes and kidney disease with or without albuminuria in a single Chinese diabetes centre. Diab Vasc Dis Res 13:21–30
Penno G, Solini A, Bonora E et al (2011) Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 29:1802–1809
Nohara A (2016) Epicardial adipose tissue as a predictor of plaque vulnerability in patients with mild chronic kidney disease. Circ J 2016;80:64 – 6
Hassan M, Said K, Rizk H et al (2016) Segmental peri-coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur Heart J Cardiovasc Imaging 17:1169–1177
Kadappu KK, Abhayaratna K, Boyd A et al (2016) Independent echocardiographic markers of cardiovascular involvement in chronic kidney disease: the value of left atrial function and volume. J Am Soc Echocardiog 29:359–367
Romejko K, Rymarz A, Szamotulska K et al (2022) Serum osteoprotegerin is an independent marker of left ventricular hypertrophy, systolic and diastolic dysfunction of the left ventricle and the presence of pericardial fluid in chronic kidney disease patients. Nutrients 14:2893
Gan GCH, Kadappu KK, Bhat A et al (2021) Left atrial strain is the best predictor of adverse cardiovascular outcomes in patients with chronic kidney disease. J Am Soc Echocardiog 34:166–175
Levey AS, Eckardt KU, Dorman NM et al (2020) Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 97:1117-29
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Mizukoshi K, Takeuchi M, Nagata Y et al (2016) Normal values of left ventricular Mass Index assessed by Transthoracic three-Dimensional Echocardiography. J Am Soc Echocardiogr 29:51–61
Zheng X, Ran H, Ren J et al (2023) Two-dimensional speckle tracking imaging analyses of the correlations between left atrial appendage function and stroke risk in nonvalvular atrial fibrillation patients. Int J Cardiovasc Imaging Published Online December 1
Pluta A, Stróżecki P, Krintus M et al (2015) Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3. Ren Fail 37:1105–1110
Gupta J, Dominic EA, Fink JC et al (2015) Association between inflammation and cardiac geometry in chronic kidney disease: findings from the CRIC Study. PLoS On 10:e0124772
Hassan MO, Duarte R, Dix-Peek T et al (2016) Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin Nephrol 86:131–135
Demetgul H, Giray D, Delibas A et al (2018) 2D-Speckle tracking echocardiography contributes to early identification of impaired left ventricular myocardial function in children with chronic kidney disease. Cardiol Youn 28:1404–1409
Lee Y, Park S, Lee S et al (2020) Lipid profiles and risk of major adverse cardiovascular events in CKD and diabetes: a nationwide population-based study[J]. PLoS ONE 15:e0231328
Gan GCH, Bhat A, Chen HHL et al (2021) Left Atrial Reservoir strain by Speckle Tracking Echocardiography: Association with Exercise Capacity in chronic kidney disease. J Am Heart Asso 10:e017840
Jia G, Aroor AR, Hill MA et al (2018) Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension 72:537–548
Longobardo L, Suma V, Jain R et al (2017) Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr 30:937–946
Ohashi N, Isobe S, Ishigaki S et al (2019) Increased heart rate is associated with intrarenal renin-angiotensin system activation in chronic kidney disease patients. Clin Exp Nephrol 23:1109–1118
Cao W, Li A, Wang L et al (2015) A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol 26:1619–1633
Nohara A (2016) Epicardial adipose tissue as a predictor of plaque vulnerability in patients with mild chronic kidney disease. Circ J 80:64–66
Chen YC, Lee WH, Lee MK et al (2020) Epicardial adipose tissue thickness is not associated with adverse cardiovascular events in patients undergoing haemodialysis. Sci Rep 10:6281
Acknowledgements
We thank all the clinical staff for their support of this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.L drafting the article C.T the conception and design of the study Y. L, Y. L, and L.G: Acquisition of data, or analysis and interpretation of dataY. Li drafting the article C. T revising it critically for important intellectual content, and final approval of the version to be submitted.
Corresponding author
Ethics declarations
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liu, Y., Gao, L. et al. Early impact of albuminuria on cardiac function in patients with chronic kidney disease: a prospective study. Int J Cardiovasc Imaging 40, 873–885 (2024). https://doi.org/10.1007/s10554-024-03056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-024-03056-4