Abstract
Background
A higher heart rate is one of the risk factors for heart failure and cardiovascular disease. Activation of the intrarenal renin–angiotensin system (RAS) plays an important role in the development of hypertension and renal damage. However, the association between heart rate and intrarenal RAS activation is unclear.
Methods
We investigated the relationship between heart rate and urinary angiotensinogen (U-AGT) excretion, a surrogate marker for intrarenal RAS activity, in ten subjects without chronic kidney disease (CKD) and 72 CKD patients who were not taking medications that influence heart rate and RAS blockers (age 50.0 ± 17.4 years, 27 men and 45 women, serum creatinine (sCr) 1.85 ± 2.71 mg/dL, blood pressure 120.5 ± 15.8/72.9 ± 10.1 mmHg, heart rate 67.3 ± 8.9 /min, urinary protein excretion 1.27 ± 2.63 g/day, and U-AGT excretion 747.4 ± 2714.6 µg/day).
Results
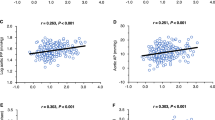
As heart rate is influenced by behavior and emotion, we divided it into daytime and nighttime. Heart rate had a significant positive association with sCr levels during daytime and nighttime in CKD patients but not in non-CKD subjects. Moreover, although heart rate was not associated with U-AGT excretion levels in non-CKD subjects, it was associated with U-AGT excretion levels during daytime (r = 0.23 and p = 0.047) and nighttime (r = 0.45 and p < 0.01) in CKD patients. Multiple linear regression analysis revealed that heart rate had a significant positive association with the U-AGT excretion levels during nighttime, but not daytime, after adjustments for age, sex, body mass index, and sCr (β = 0.31 and p = 0.034).
Conclusion
Heart rate is associated with U-AGT excretion levels, especially during the nighttime, in CKD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that hypertension is one of the risk factors of cardiovascular diseases and end-stage renal failure [1,2,3]. In addition, an elevated heart rate is also known to be associated with cardiovascular morbidity and mortality [4, 5]. Especially, heart rate measurements taken via ambulatory blood pressure (BP) monitoring (ABPM) are useful clinical information to assess cardiovascular risk [6]. Moreover, Eriksen et al. reported that the unadjusted mean rate of glomerular filtration rate (GFR) decline was 0.96 mL/min per year and that 10 bpm higher ambulatory 24-h and daytime heart rate and office heart rate were associated with steeper GFR decline rates of 0.20 to 0.21 mL/min per year (P < 0.01) in multivariable-adjusted linear mixed models. Therefore, they concluded that heart rate may be a useful biomarker to identify persons at risk of accelerated GFR decline [7].
It is well known for many years that the circulating renin–angiotensin system (RAS) plays a critical role in the regulation of arterial pressure and sodium homeostasis. In recent years, the focus of interest on the role of the RAS in the pathophysiology of hypertension and organ injury has changed to the local RAS in specific tissues [8, 9]. In the kidney, all RAS components are present, and it has been clarified that activation of intrarenal RAS plays a critical role in the pathophysiology in some animal models and patients with chronic kidney disease (CKD) or hypertension independent of circulating RAS [10,11,12,13].
However, because the relationship between heart rate and intrarenal RAS activity has not been investigated until now, we have performed this experiment.
Materials and methods
CKD patients and subjects without CKD
This study was approved by the ethics committee of Hamamatsu University School of Medicine (No. 23–193) and adhered to the principles of the Declaration of Helsinki. We recruited 72 patients with CKD who were admitted to our hospital for close investigation and treatment consecutively from February 2012 to November 2016. Written informed consent was obtained from all patients. We excluded arrhythmic patients with or without anti-arrhythmic agents and patients undergoing dialysis (CKD stage 5D). In addition, we excluded patients taking medications that influence heart rate such as β blockers and benzodiazepine class calcium antagonist, and those taking RAS blockers [i.e., angiotensin II (Ang II) receptor blockers, angiotensin-converting enzyme inhibitors, mineralocorticoid receptor blockers, or direct renin inhibitors] that are known to suppress intrarenal RAS activity [10,11,12,13]. However, ten patients taking other antihypertensive drugs including diuretics were included. Subjects aged 20–80 years without CKD were registered as non-CKD subjects.
Study protocols
CKD patients consumed a hospital-served diet containing 10 g/day of salt (standard salt diet) or 6 g/day of salt (low salt diet) under hospitalization. The patients’ diet was determined on the basis of their physicians’ discretion. In non-CKD subjects and CKD patients, we collected urine during the daytime (6:00 am–9:00 pm) and nighttime (9:00 pm–6:00 am). ABPM was conducted at 30-min intervals during the day and night using an automatic device (TM-2431; A and D, Tokyo, Japan). We divided the data collection segments into daytime and nighttime for 24-h ABPM measurement, using sleep and wake times that were recorded in the patients’ behavior records. Blood samples were also drawn at 9:00 pm and 6:00 am the next day, after the patients had rested in the supine position for at least 15 min. The blood samples drawn at 9:00 pm and 6:00 am were considered as the samples at the end of daytime and nighttime, respectively, as described previously [14,15,16,17].
Clinical data
The anthropometric and characteristic data of non-CKD subjects or CKD patients, such as age, sex, height, body weight, and body mass index (BMI), were recorded at the starting day of this study or at the time of admission, respectively. During 24-h ABPM, BP and heart rate were measured noninvasively every 30 min as described above. Daytime BPs were calculated as the average of the readings during the awake hours, whereas nighttime BPs were the average of the remaining values. Serum creatinine concentrations and urinary creatinine, albumin, and protein concentrations were measured in the clinical laboratory of the Hamamatsu University School of Medicine, University Hospital. The levels of urinary angiotensinogen (AGT), known to be a surrogate marker of intrarenal RAS activity, were measured using an enzyme-linked immunosorbent assay (ELISA), as described previously [12, 13, 18]. Plasma Ang II levels, known to be effectors of circulating RAS activity, were determined using radioimmunoassay without special pretreatments (SRL, Tokyo, Japan). Serum creatinine concentrations were measured from blood and the estimated glomerular filtration rate (eGFR) was calculated by the serum creatinine concentrations using the Japanese eGFR equation [19]. The excretion ratios of urinary albumin/creatinine (U-Alb/Cr), urinary protein/creatinine (U-Pro/Cr), and urinary AGT/creatinine (U-AGT/Cr) were calculated during both the daytime and nighttime.
Statistical analyses
The results were expressed as the means ± standard deviation. The significance of differences between daytime and nighttime was determined using the Student’s t test for paired samples. Because the U-Alb/Cr, U-Pro/Cr, and U-AGT/Cr did not show a normal distribution, logarithmic transformation was applied to them and a normal distribution was confirmed by the Kolmogorov–Smirnov test. Thereafter, the Student’s t test was performed. The correlations between heart rate and other clinical parameters were evaluated using Pearson’s product–moment correlation test. Multiple linear regression analyses were conducted to evaluate the relationships between heart rate and U-AGT/Cr. Age, sex, BMI, and serum creatinine were selected as independent variables because these parameters are common to perform multiple linear regression analyses. We selected serum creatinine instead of eGFR, because the absolute value of the correlation coefficient of serum creatinine in the daytime is slightly higher than that of eGFR. Moreover, because eGFR is calculated by age and sex as well as serum creatinine [19], when eGFR is selected as the independent variable, it is possible that multicollinearity is introduced with age and sex. It is suggested that heart rate is positively associated with BP due to common mechanism regulating both of heart rate and BP [20]. In addition, because urinary albumin excretion is a surrogate marker of renal damage and intrarenal RAS activation is known to be associated with renal damage [10,11,12,13], we added systolic BP as well as U-Alb/Cr as an independent variable in this study. We considered a p value of < 0.05 to be statistically significant. Statistical analyses were performed using IBM®SPSS® software, version 23 (IBM Corporation, Armonk, NY, USA).
Results
Characteristics of non-CKD subjects and CKD patients
Seventy-two CKD patients who were admitted to our hospital for close investigation and treatment were included in this study. The causes of CKD were diagnosed as follows: diabetic kidney disease (DKD) was defined by poorly controlled diabetes mellitus that continues for a long time with diabetic retinopathy and/or diabetic neuropathy and relatively massive proteinuria and less hematuria. Most patients with chronic glomerulonephritis (CGN) were diagnosed by renal biopsy. The remaining patients with CGN were diagnosed as having persistent proteinuria and/or hematuria along with hypertension and/or renal insufficiency without performing renal biopsy. Nephrosclerosis was defined by poorly controlled hypertension for a long time with relatively modest proteinuria and an irregular renal surface. CKDs except for DKD, CGN and nephrosclerosis were defined as “others.” Fifty-four CKD patients consumed a standard salt diet and 18 CKD patients consumed a low salt diet in this study. In addition, ten non-CKD subjects were recruited as the control group.
The baseline characteristics are presented in Table 1. Although significant differences were noted in the renal function and the levels of urinary albumin, urinary protein, and urinary AGT, there were no significant differences in age, sex, and BMI and the incidence rate of comorbidities such as diabetes mellitus and hypertension between non-CKD subjects and CKD patients. Most patients were middle aged (50.0 ± 17.4 years). Although 20 patients, including 13 antihypertensive recipients (calcium channel blockers; 11 patients, diuretics; 10 patients and others; 3 patients), suffered from hypertension, BP was well controlled (120.5 ± 15.8/72.9 ± 10.1 mmHg) and heart rate was within the normal range (67.3 ± 8.9/min). Notably, although two non-CKD subjects were diagnosed as hypertensive, they did not take antihypertensive drugs. No significant differences of BP and heart rate were found between non-CKD subjects and CKD patients. The patients’ renal function was as follows: serum creatinine: 1.85 ± 2.71 mg/dL and eGFR: 54.4 ± 27.5 mL/min/1.73 m2, and logarithmic transformation of daily urinary albumin, urinary protein, and urinary AGT excretion levels were 2.45 ± 0.64 mg/day, 2.84 ± 0.43 mg/day, and 2.03 ± 0.77 µg/day, respectively.
Changes of each parameter during daytime and nighttime
Table 2 shows the changes in each parameter between daytime and nighttime in non-CKD subjects and in CKD patients. In non-CKD subjects, systolic and diastolic BPs, heart rate, and the levels of serum creatinine and urinary AGT during the daytime were significantly higher than those during the nighttime, and eGFR during the daytime was significantly lower than that during the nighttime. In addition, the levels of plasma Ang II and urinary albumin and urinary protein did not differ between daytime and nighttime. On the other hand, in CKD patients, systolic and diastolic BPs, heart rate, serum creatinine, and excretion levels of urinary albumin, urinary protein, and urinary AGT were significantly higher during the daytime than during the nighttime, and eGFR during the daytime was significantly lower than that during the nighttime. However, plasma Ang II levels were the same during the daytime and nighttime. These results coincided with our previous data [14, 15, 21].
Relationships between heart rate and clinical parameters including urinary AGT excretion levels for 24 h
We investigated the correlations between heart rate and clinical parameters including urinary AGT excretion levels for 24 h. There were no significant relationships between heart rate and the other clinical parameters including urinary AGT excretion in non-CKD subjects. However, although age, BMI, 24-h BP, plasma Ang II, and daily urinary albumin and protein excretion levels were not correlated with heart rate, daily urinary AGT excretion levels as well as serum creatinine levels were significantly and positively associated with heart rate in CKD patients (r = 0.29, p = 0.015). In addition, eGFR was significantly and negatively associated with heart rate in CKD patients (Table 3).
Relationships between heart rate and clinical parameters including urinary AGT excretion levels during the daytime and nighttime
As it is possible that heart rate is influenced by behavior and emotion, we divided the heart rate in daytime or nighttime periods and compared the clinical parameters including urinary AGT excretion levels (Table 4). No significant relationships were found between heart rate and the other clinical parameters including urinary AGT excretion in non-CKD subjects during the daytime and nighttime. However, a significant and positive relationship was found between heart rate and urinary AGT excretion levels during the daytime and nighttime. Moreover, heart rate was significantly and positively associated with serum creatinine levels or significantly and negatively associated with eGFR levels in the daytime and nighttime. In addition, a significant and positive relationship was found between urinary AGT excretion levels and diastolic BP, urinary albumin, or protein excretion levels in the nighttime but not daytime.
Multiple linear regression analyses of heart rate and the clinical parameters including urinary AGT excretion levels during the daytime and nighttime
Because there were no relationships between heart rate and the other clinical parameters in non-CKD subjects, we performed multiple linear regression analyses to evaluate the relationships between heart rate and the clinical parameters including urinary AGT excretion levels during the daytime and nighttime only in CKD patients.
Significant regression equations were not obtained for the daytime measurements (Model 1; r = 0.36, p = 0.052 and Model 3; r = 0.42, p = 0.052). In addition, a significant relationship was not found between heart rate and urinary AGT excretion levels during the daytime after adjusting for age, sex, BMI, and serum creatinine levels (Model 2; β = 0.18, p = 0.22). On the other hand, there were significant and positive relationships between heart rate and urinary AGT excretion levels during the nighttime, even after adjusting for age, sex, BMI, and serum creatinine, respectively (Model 5; β = 0.46, p < 0.01 and Model 6; β = 0.31, p = 0.034). Moreover, a positive tendency between heart rate and urinary AGT excretion levels even after adjusting for age, sex, BMI, serum creatinine and systolic BP during the nighttime (Model 7; β = 0.30, p = 0.056) was found. In addition, a positive tendency was also found between heart rate and urinary AGT excretion levels even after adjusting for age, sex, BMI, serum creatinine, systolic BP, and urinary albumin excretion during the daytime (Model 4; β = 0.33, p = 0.079) and nighttime (Model 8; β = 0.35, p = 0.061), respectively (Table 5).
Discussion
In the present study, we have clarified that heart rate had a significant positive correlation with intrarenal RAS activation, especially during the nighttime in CKD patients but not in non-CKD subjects.
An increased heart rate is known to be associated with cardiovascular morbidity and mortality. Kannel et al., reported 1876 total deaths and 894 cardiovascular deaths out of 5070 subjects free of cardiovascular disease at entry into the study, and that all-cause, cardiovascular, and coronary mortality rates increased progressively in relation to antecedent heart rates determined biennially [4]. Thereafter, the European Society of Hypertension consensus meeting concluded that there is a large body of evidence showing that heart rate is a strong independent predictor of cardiovascular mortality; this association is present at all ages and in different clinical settings, irrespective of the presence of comorbidities [5].
Recently, the importance of ABPM has been confirmed for evaluating BP levels, circadian rhythm of BPs, and specific cardiovascular events [22,23,24]. In addition to ABPM, ambulatory heart rate has also been shown to be useful. Palatini et al. and Johansen et al. reported the independent predictive value for nighttime heart rate, but not daytime or 24-h heart rate, for cardiovascular, but not all-cause mortality [25, 26]. Moreover, Cheng et al. also confirmed that heart rate may be a potential marker of elevated cardiovascular risk in asymptomatic individuals, prior to the development of clinical hypertension or cardiovascular disease [6]. We did not examine the relationships between heart rate and the markers of cardiovascular events. However, our results indicate that heart rate was associated with urinary albumin or protein excretion levels, a surrogate marker of renal damage, during the nighttime but not daytime, which coincided with the previous studies [6, 25, 26].
Intrarenal RAS plays a role in sodium reabsorption, inflammation, and fibrosis in the kidney and is one of the most important contributors for the pathophysiology of CKD, including IgA nephropathy, diabetic nephropathy, and hypertension [12, 27,28,29]. It is also well known that the activation of intrarenal RAS contributes to renal damage by studying CKD or hypertension in animal models [11, 30,31,32]. In addition, it has been clarified that intrarenal RAS activates sympathetic nervous system. Cao et al. reported that 5/6 nephrectomized rats on a high salt diet demonstrated increased activation of reactive oxygen species (ROS) and RAS in the kidney by renal damage and that their activation in the kidney induced the augmentation of ROS and RAS in the central cardiovascular centers via renal afferent sympathetic nerve activation. Moreover, they showed that increased activation of ROS and RAS in the kidney is caused by the activation of ROS and RAS in the central cardiovascular centers via renal efferent sympathetic nerve activation [33]. In addition to the report by Cao et al., many more studies indicate increased sympathetic nervous system activity due to renal damage in both patients with CKD and animal models. Converse et al. demonstrated that direct recording of neuronal activity from postganglionic sympathetic fibers in the peroneal nerves of patients on chronic dialysis treatment has shown a greater rate of sympathetic nerve discharge than in control subjects [34]. In the animal model of CKD, it was shown that the turnover rate and the secretion of norepinephrine from the posterior hypothalamic nuclei were greater in chronic renal failure than in control rats, and that bilateral dorsal rhizotomy at the level T-10 to L-3 prevented the increase in blood pressure, the increase in norepinephrine turnover in the posterior hypothalamic nuclei, and the progression of renal disease in chronic renal failure rats [35,36,37]. These studies indicate that increased renal sensory impulses generated in the kidney and then transmitted to the central nervous system activate regions in the central nervous system. Moreover, heart rate is an established marker of the sympathetic nervous system activity in various adult patient populations with and without cardiovascular disease [38]. These facts are in line with our data showing that heart rate, a surrogate marker of sympathetic nerve activity, significantly and positively correlated with urinary AGT excretion, a surrogate marker of intrarenal RAS activation in CKD patients, and that heart rate did not correlate with urinary AGT excretion in non-CKD subjects.
The heart rate was modulated by physical and social stress as well as their combination [39]. There are more social and physical stresses during the daytime that have the possibility of influencing heart rate levels compared with those during the nighttime. Therefore, it is difficult to exclude confounding factors between heart rate and urinary AGT excretion during daytime. This is why a significant positive relationship between heart rate and urinary AGT excretion was found during the nighttime but not the daytime in the present study. Although heart rate during the daytime in non-CKD subjects was higher than that in CKD patients, it is possible that physical and social stress at work influenced heart rate in non-CKD subjects compared with that in CKD patients during their hospitalization. However, heart rate during the nighttime in CKD patients was higher than that in non-CKD subjects. In addition, although no significant relationships were found between heart rate and urinary AGT excretion levels during the daytime and nighttime in non-CKD subjects, multiple linear regression analysis revealed that heart rate in CKD patients had a significant positive association with the urinary AGT excretion levels during the nighttime, but not during the daytime. These results coincide with our concepts that heart rate during the nighttime reflects intrarenal RAS activation.
This study has some limitations. First, the sample size was relatively small in our single center cohort. Nevertheless, we could determine that heart rate had a significant and positive relationship with urinary AGT excretion levels during both the daytime and nighttime and that there were significant and positive relationships between heart rate and urinary AGT excretion levels, after adjusting for age, sex, BMI, and serum creatinine during the nighttime in CKD patients but not in non-CKD subjects. However, when systolic BP and urinary albumin excretion levels were added as independent variables, the significant and positive relationship between heart rate and urinary AGT excretion disappeared. Nevertheless, the positive relationship between them was maintained. As it is possible that the relatively small sample size contributed to the results, a larger study is expected to be carried out in the future. Second, we consider that the significant positive relationship between heart rate and urinary AGT excretion was caused by intrarenal RAS activation and activation of sympathetic nerves due to renal damage. Autonomic nerve activity, including sympathetic nerve activity, is generally evaluated by the measurement of plasma catecholamine and thermographic examination. Although the measurement tools can assess immediate sympathetic nerve activity, they cannot assess changes in sympathetic nerve activity over time. The autonomic nervous system has been recently assessed by spectral analysis using electrocardiographic waveform, because the analysis can assess sympathetic nerve activity over time, quantitatively, non-invasively, and easily [40]. However, we could not evaluate direct autonomic nerve activation, because we do not have this system in our hospital. Third, daytime defined as 6:00 am–9:00 pm and nighttime defined as 9:00 pm–6:00 am for urine collection are not exact time periods as demonstrated by examining the data of 24 h-ABPM. However, lights are turned on at 6:00 am and turned off at 9:00 pm at our hospital. Therefore, we collected urine during the daytime and nighttime after patients excreted urine, regardless of a desire to urinate just before turn-off and turn-on times, respectively. Because urine collected during daytime and nighttime can be certainly stored in this method and most of the patients sleep from about 9:00 pm and get up about 6:00 am, we adopted this method, and the time setting for urine has been seen as acceptable up to this point [14,15,16,17]. Fourth, it is possible that blood samples drawn at 9:00 pm and 6:00 am may not reflect daytime and nighttime results, respectively. However, it has not been well established what the representative time should be to estimate daytime and nighttime results. Even though there is no obvious evidence, it is difficult to obtain nighttime blood samples at midnight. Therefore, when we collected urine, we obtained blood samples at 9:00 pm and 6:00 am and defined them as the samples at the end of the daytime and nighttime, respectively. Moreover, because the time setting for blood sample collection has been acceptable up to this point [14,15,16], we adopted this method in the present study. Fifth, it is possible that diuretics influenced the results. However, in general, as it has been reported that diuretics do not influence intrarenal RAS activity, diuretics are frequently used as intrarenal RAS-independent antihypertensives in some animal models [31, 41]. Finally, it is possible that differences in salt loading influenced the results, because we and other researchers have already shown that salt loading aggravates intrarenal RAS activity [33, 42]. We have reanalyzed some of the data using only 54 CKD patients who consumed a standard salt diet. Patients who consumed a low salt diet (6 g/day) suffered from more serious renal damage and were excluded from the analyses. Therefore, it has been difficult to obtain the statistical significance between heart rate and urinary AGT excretion levels. However, a significant relationship has been found between heart rate and urinary AGT excretion levels during the nighttime (r = 0.27, p = 0.046) but not during the daytime (r = 0.011, p = 0.94) (data not shown).
In conclusion, heart rate correlated with urinary AGT excretion levels, especially during the nighttime in CKD patients but not in non-CKD subjects. Heart rate measurement may be a convenient surrogate marker for intrarenal RAS activation in patients with CKD.
References
Ikeda N, Saito E, Kondo N, Inoue M, Ikeda S, Satoh T, Wada K, Stickley A, Katanoda K, Mizoue T, Noda M, Iso H, Fujino Y, Sobue T, Tsugane S, Naghavi M, Ezzati M, Shibuya K. What has made the population of Japan healthy? Lancet. 2011;378:1094–105.
Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, Ueshima H; Observational Cohorts in Japan (EPOCH-JAPAN) Research Group. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res. 2012; 35: 947–53.
Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–5.
Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–94.
Palatini P, Benetos A, Grassi G, Julius S, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Pessina AC, Ruilope LM, Zanchetti A; European Society of Hypertension. Identification and management of the hypertensive patient with elevated heart rate: statement of a European Society of Hypertension Consensus Meeting. J Hypertens 2006; 24: 603–10.
Cheng C, Daskalakis C. Association of ambulatory heart rate and atherosclerosis risk factors with blood pressure in young non-hypertensive adults. Open Heart. 2016;3:e000332.
Eriksen BO, Småbrekke S, Jenssen TG, Mathisen UD, Norvik JV, Schei J, Schirmer H, Solbu MD, Stefansson VTN, Melsom T. Office and ambulatory heart rate as predictors of age-related kidney function decline. Hypertension. 2018;72:594–601.
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–87.
Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22.
Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Activation of reactive oxygen species and the renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:509–15.
Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, Kato A, Miyajima H, Fujigaki Y, Nishiyama A, Yasuda H. Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res. 2016;39:312–20.
Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–50.
Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–54.
Isobe S, Ohashi N, Fujikura T, Tsuji T, Sakao Y, Yasuda H, Kato A, Miyajima H, Fujigaki Y. Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol. 2015;19:231–9.
Ishigaki S, Ohashi N, Isobe S, Tsuji N, Iwakura T, Ono M, Sakao Y, Tsuji T, Kato A, Miyajima H, Yasuda H. Impaired endogenous nighttime melatonin secretion relates to intrarenal renin-angiotensin system activation and renal damage in patients with chronic kidney disease. Clin Exp Nephrol. 2016;20:878–84.
Ohashi N, Isobe S, Ishigaki S, Suzuki T, Motoyama D, Sugiyama T, Nagata M, Kato A, Ozono S, Yasuda H. The effects of unilateral nephrectomy on blood pressure and its circadian rhythm. Intern Med. 2016;55:3427–33.
Fukuda M, Mizuno M, Yamanaka T, Motokawa M, Shirasawa Y, Nishio T, Miyagi S, Yoshida A, Kimura G. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension. 2008;52:1155–60.
Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960960.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–92.
Morcet JF, Safar M, Thomas F, Guize L, Benetos A. Associations between heart rate and other risk factors in a large French population. J Hypertens. 1999;17:1671–6.
Ohashi N, Isobe S, Matsuyama T, Ishigaki S, Tsuji N, Fujikura T, Tsuji T, Kato A, Yasuda H. Night-time activation of the intrarenal renin-angiotensin system due to nocturnal hypertension is associated with renal arteriosclerosis in normotensive IgA nephropathy patients. Hypertens Res. 2018;41:334–41.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–9.
Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–7.
Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–80.
Palatini P, Reboldi G, Beilin LJ, Eguchi K, Imai Y, Kario K, Ohkubo T, Pierdomenico SD, Saladini F, Schwartz JE, Wing L, Verdecchia P. Predictive value of night-time heart rate for cardiovascular events in hypertension. The ABP-International study. Int J Cardiol. 2013;168:1490–5.
Johansen CD, Olsen RH, Pedersen LR, Kumarathurai P, Mouridsen MR, Binici Z, Intzilakis T, Køber L, Sajadieh A. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J. 2013;34:1732–9.
Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–655.
Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–7.
Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–80.
Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–7.
Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:750–5.
Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, Togawa A, Suzuki S, Fujigaki Y, Nakagawa T, Nakamura Y, Suzuki F, Kitagawa M, Hishida A. Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Renal Physiol. 2008;295:F1512–F15181518.
Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, Wilcox CS, Hou FF. A Salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol. 2015;26:1619–33.
Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–8.
Bigazzi R, Kogosov E, Campese VM. Altered norepinephrine turnover in the brain of rats with chronic renal failure. J Am Soc Nephrol. 1994;4:1901–7.
Ye S, Ozgur B, Campese VM. Renal afferent impulses, the posterior hypothalamus, and hypertension in rats with chronic renal failure. Kidney Int. 1997;51:722–7.
Campese VM, Kogosov E, Koss M. Renal afferent denervation prevents the progression of renal disease in the renal ablation model of chronic renal failure in the rat. Am J Kidney Dis. 1995;26:861–5.
Custodis F, Schirmer SH, Baumhäkel M, Heusch G, Böhm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973–83.
von Dawans B, Trueg A, Kirschbaum C, Fischbacher U, Heinrichs M. Acute social and physical stress interact to influence social behavior: The role of social anxiety. PLoS ONE. 2018;13:e0204665.
Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:676–82.
Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–80.
Huang Y, Yamamoto T, Misaki T, Suzuki H, Togawa A, Ohashi N, Fukasawa H, Fujigaki Y, Ichihara A, Nishiyama A, Senbonmatsu T, Ikegaya N, Hishida A. Enhanced intrarenal receptor-mediated prorenin activation in chronic progressive anti-thymocyte serum nephritis rats on high salt intake. Am J Physiol Renal Physiol. 2012;303:F130–F138.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Informed consent
This study was approved by the ethics committee of Hamamatsu University School of Medicine (No. 23–193).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ohashi, N., Isobe, S., Ishigaki, S. et al. Increased heart rate is associated with intrarenal renin–angiotensin system activation in chronic kidney disease patients. Clin Exp Nephrol 23, 1109–1118 (2019). https://doi.org/10.1007/s10157-019-01746-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01746-1