Abstract
Cardiovascular disease is the leading cause of mortality amongst patients with chronic kidney disease (CKD). This is the first study using 3-dimensional echocardiography (3DE) to investigate associations between adverse changes of the left ventricle, and different stages of CKD. Participants were recruited from the Copenhagen CKD cohort study and the Herlev-Gentofte CKD cohort study. Patients were stratified according to GFR category (G1 + 2: eGFR ≥ 60 mL/min/1.73 m2, G3: eGFR = 30–59 mL/min/1.73 m2, and G4 + 5: eGFR ≤ 29 mL/min/1.73 m2), and according to albuminuria (A1: UACR < 30 mg/g, A2: 30–300 mg/g, A3: > 300 mg/g). Echocardiograms were analysed for left ventricular (LV) mass index (LVMi), LV ejection fraction (LVEF), and global strain measures. In adjusted analysis, eGFR groups were adjusted for confounders and albuminuria category, while albuminuria groups were adjusted for confounders and GFR category. The study population consisted of 662 outpatients with CKD and 169 controls. Mean age was 57 ± 13 years, and 61% were males. Mean LVEF and global longitudinal strain (GLS) were increasingly impaired across eGFR groups: LVEF = 60.1%, 58.4%, and 57.8% (p = 0.013), GLS = − 16.1%, − 14.8%, and − 14.6% (p < 0.0001) for G1 + 2, G3, and G4 + 5. LVMi and prevalence of LV hypertrophy increased with albuminuria severity: mean LVMi = 87.9 g/m2, 88.1 g/m2, and 92.1 g/m2 (p = 0.007) from A1-3. Adjusted analysis confirmed reduced LVEF in G3 compared with G1 + 2, and increased LVMi in A3 compared with A1. Increasingly impaired eGFR was associated with adverse changes in LV systolic function, while albuminuria was associated with adverse changes in LV mass assessed by 3DE. Their associations were independent of each other.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2017, the Global Burden of Disease study reported that almost one tenth of the entire global population was afflicted with chronic kidney disease (CKD) [1]. CKD constitutes a significant risk factor for cardiovascular disease (CVD), and it is estimated that 30–40% of patients with CKD suffer from CVD [2, 3], which is the leading cause of death in the patient group [4]. Furthermore, the Global Burden of Disease study estimated that 1.4 million yearly cardiovascular disease-related deaths could be attributed to impaired kidney function [1]. The increased risk of CVD in relation to CKD is only partially explained by the underlying common risk factors, such as hypertension and diabetes mellitus [5]. Thus, the term ‘Cardiorenal Syndrome’ has been established in order to describe the numerous mechanisms of interaction between CKD and CVD [6]. Heart failure (HF) is the most common CVD associated with CKD, owing to several common and uremia-related risk factors, including fluid retention and abnormal calcium-phosphate homeostasis causing arterial stiffness, hypertension, excessive cardiac preload, left ventricular (LV) hypertrophy, and cardiac fibrosis [7].
A highly relevant tool to study the cardiovascular changes associated with CKD is echocardiography. It is a non-invasive, low-cost procedure, widely used clinically and it may provide useful insight into the interaction between CKD and CVD. Most common echocardiographic findings in patients with CKD include LV hypertrophy [8], determined by left ventricular mass index (LVMi), and reduced left ventricular ejection fraction (LVEF), both linked to the subnormal estimated glomerular filtration rate (eGFR) seen in CKD patients [9]. Albuminuria, commonly seen in kidney disease, has also been linked to LV hypertrophy, both in the general population [10] and in kidney patients [11]. Lastly, subclinical impairment of LV systolic function in patients with CKD, assessed by global longitudinal strain (GLS), has been shown in a smaller study. [12].
An echocardiographic method of special interest is three-dimensional echocardiography (3DE), which, unlike two-dimensional echocardiography, does not rely on geometric assumptions about the left ventricle for volume quantifications [13]. The EAE/ASE guidelines recommend the use of 3DE for quantification of LVEF, and also state that the accuracy of 3DE measurements of LV mass is similar to magnetic resonance imaging [14]. With this improved accuracy, 3DE is an appropriate method to further investigate the link between CKD and CVD. Hence, the objective of this study was to investigate whether changes in LV mass and systolic function could be identified at different stages of CKD, assessed both by eGFR and albuminuria, using 3DE. We furthermore sought to elucidate whether eGFR and albuminuria in patients with CKD associated with LV changes independently of one another.
Methods
Population
The Copenhagen CKD Echo study is based on the Copenhagen CKD cohort study (Rigshospitalet, Copenhagen), described elsewhere in detail [15], and the Herlev-Gentofte CKD cohort study (Herlev-Gentofte University Hospital). The total patient population consisted of 825 outpatients with CKD stages 1 to 5, not receiving dialysis treatment, and age 30 to 75 years. Participants were included consecutively from September 2015 to August 2018 at the Departments of Nephrology, Rigshospitalet and Herlev & Gentofte Hospital. Patients who had received a kidney transplant were excluded from the study. The control population was included during the same period in relation to the Copenhagen CKD cohort study and has been described in detail elsewhere [16]. In brief, it consisted of 175 participants matched by age and sex with the CKD cohort, who were included if free of known chronic disease, including CKD. Well-controlled hypertension was acceptable for inclusion, but controls were preferably not using prescription medicine. Control participants with plasma creatinine twice above upper age and sex corrected reference values were excluded. Further exclusion criteria for controls were eGFR < 60 or kidney damage.
Exclusion criteria for both groups included active malignant disease, pregnancy, mental illness, and lack of consent or ability to consent.
The total study population thus consisted of 1000 participants. 169 were excluded due to infeasibility of 3DE, leaving 831 participants for final analysis (169 controls, 662 patients).
Clinical characteristics
Following informed consent from participants, investigators collected baseline data through interview, clinical examinations, and review of medical journals. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the use of antihypertensive medication with hypertension as the indication. With regards to obesity, participants were stratified according to BMI as either normal, overweight, or obese, and cut-off values for BMI were < 25, 25–30, and > 30, respectively. Quantification of eGFR was based on a measured plasma creatinine and the CKD-EPI Creatinine Eq. [17] Interview and review of medical journals provided information on prevalence of diabetes and cardiovascular disease, such as HF, and peripheral artery disease. A clinical diagnosis registered in the medical journal was required for any of these to be registered in the study. Significant valve disease was defined as the presence of moderate or severe regurgitation or stenosis of the mitral or aortic valve assessed echocardiographically.
Definition and staging of CKD was in accordance with KDIGO guidelines [18], and patients were grouped together according to GFR category as follows: G1 + 2 (eGFR ≥ 60 mL/min/1.73 m2), G3 (both G3a and G3b, eGFR of 30-59 mL/min/1.73 m2), and finally G4 + 5 (eGFR ≤ 29 mL/min/1.73 m2). These groups were collectively denoted eGFR groups. The purpose of grouping together GFR categories was to overcome large differences in sample sizes of each individual GFR category. Patients were also stratified according to albuminuria category in accordance with KDIGO guidelines [18]: A1 was defined as a urine albumin to creatinine ratio (UACR) < 30 mg/g, A2 as a UACR between 30 and 299 mg/g, and A3 as a UACR ≥ 300 mg/g. These groups were collectively denoted albuminuria groups.
Echocardiography
All transthoracic echocardiographic examinations were performed with GE Vingmed Ultrasound’s Vivid E9 (Horten, Norway). Participants were placed in the left lateral decubitus position. 3D echocardiograms were obtained using an electrocardiographically triggered multiple-beat technique with volume-stitching over 2 to preferably 6 consecutive heart cycles during breath hold [14]. Patients with irregular heart rhythms, including atrial fibrillation, were excluded from 3DE. All 3D echocardiograms were analysed semi-automatically using EchoPac v. 203, GE Healthcare, by a single investigator who was blinded to all clinical information. 3D echocardiograms used for quantification of LV mass, chamber size, and LVEF, and furthermore 3D speckle tracking echocardiography (3DSTE) were obtained from the transthoracic apical window. A minimum volume rate of 12 volumes per second was required for LVEF to be measured, and 25 volumes per second for 3DSTE to be performed. The LV myocardium was segmented according to the 17 segment model in 3DSTE [19] with tracking of each individual segment. Individual segments with imprecise tracking of the myocardium in 3DSTE could be excluded by the investigator, but the analysis was deemed infeasible if more than three of the 17 segments were excluded. An example of 3DSTE performed in EchoPac can be seen in Fig. 1.
Three-dimensional speckle-tracking echocardiography. The images illustrate the results of speckle-tracking performed on a 3D echocardiogram in the EchoPac software. The column on the left shows the apical 4-, 2-, and 3-chamber projections from top to bottom, while the column in the middle shows cross sections going from apex to basis, also from top to bottom. The curves in the upper right corner illustrate the longitudinal strain value of each of the 17 LV segments throughout one heart cycle. The 17-segment model in the lower left corner illustrates the peak longitudinal strain values for each segment, both numerically and graphically through colour coding. The GLS is printed beside the 17-segment model
LVMi was defined as LV mass indexed to body surface area. The ASE/EAE guidelines currently do not include reference values for LVMi measured by 3DE [19] and definition of LV hypertrophy was therefore based upon the upper limits of normal for LVMi reported in a prior study from 2013 on a healthy Caucasian population [20]. Upper limits were 97 g/m2 for males and 90 g/m2 for females. Reduced LVEF was defined as a value below 52% for both sexes and was based upon reference values found in a population-based study on 3DE. [21].
Finally, impaired GLS was defined as an absolute value below 13.5%, a value based upon the lower reference limit reported in a recent meta-analysis by Truong et al. [22] Intervendor variability in 3DE measurements of LVEF and GLS has been shown to be minimal [22, 23], and 3DE measurements of LVMi are reproducible using magnetic resonance imaging, and these normal ranges of 3DE parameters are therefore applicable regardless of vendor.
Statistical analysis
Clinical and echocardiographic parameters were assessed and compared between the control subjects and patients with CKD, and across eGFR- and albuminuria groups, respectively. Abnormal echocardiographic findings, such as reduced LVEF or GLS and LV hypertrophy, were also compared across eGFR- and albuminuria groups. Gaussian distributed continuous variables were compared with students t-tests and analysis of variance, and results were reported as the mean and standard deviation. Non-gaussian distributed variables were compared using the Wilcoxon rank sum test and the Kruskal–Wallis test and results were reported as the median with interquartile range. Categorical variables were compared using Pearson’s Chi-squared test and results were reported as the total number with percentage. A significance level of α = 0.05 was chosen.
Multivariable adjusted analysis was performed for LVMi, LVEF, and GLS to adjust for the following clinical and echocardiographic confounders: Age, sex, BMI, hypertension, diabetes mellitus, HF, significant valve disease, prior myocardial infarction, peripheral artery disease, and resting heart rate. Furthermore, eGFR groups were adjusted for albuminuria category, while albuminuria groups were adjusted for GFR category. This was to ensure that the resulting associations found for each renal parameter were independent of the other. G1 + 2 served as reference group for eGFR groups, while A1 served as reference for the albuminuria groups. Restricted Cubic Splines were constructed and the optimal number of knots for each model was determined from the lowest akaike information criterion (AIC). However, a maximum of 3 knots was chosen, as to avoid potential overfitting of the splines to our data. Interactions between eGFR and UACR when determining their respective relationships with echocardiographic parameters were examined through multivariable linear regression analysis and resulting p-values were reported. Patients were subdivided according to eGFR group and albuminuria group resulting in nine groups, with the purpose of further determining associations between eGFR group and echocardiographic parameters independently of albuminuria group, and vice versa. p-values for trend were calculated across the three albuminuria groups in each individual eGFR group, and across the three eGFR groups in each individual albuminuria group, which resulted in a total of 6 p-values. Only significant p-values were reported.
Interrater (n = 261) and intrarater (n = 20) variability for measurement of LVMi, LVEF, and GLS was assessed, and results were reported as mean difference and standard deviation (SD). All statistical analyses were performed using R for Windows, version 4.0.3 (R Project for Statistical Computing, Vienna University of Economics and Business Administration, Wien, Austria).
Ethics
This study was approved by the Danish Data Protection Agency (30-0840) and the Regional Committee on Health Research Ethics of the Capital Region of Denmark (H-3-2011-069). The study was conducted in accordance with the Helsinki Declaration.
Results
Clinically, CKD compared with non-CKD was associated with obesity, higher heart rate, and higher prevalence of cardiovascular comorbidities, such as hypertension, diabetes mellitus, and previous myocardial infarction (Table 1). Increasing severity of albuminuria was likewise associated with higher heart rate, higher systolic and diastolic blood pressure, and a lower eGFR (Table 1). Higher eGFR group index was associated with a significant increase in age, systolic and diastolic blood pressure, pulse pressure, UACR, and prevalence of hypertension, diabetes mellitus, peripheral artery disease, and HF (Table 2). The prevalence of HF was roughly three and five times higher in G3 and G4 + 5, compared with G1 + 2. Echocardiographic findings in CKD patients compared with controls included a slight decrease in LVEF and absolute global area strain (Table 1).
Associations of LV mass and function with eGFR
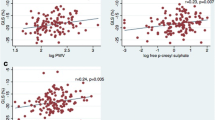
Significant and unadjusted echocardiographic findings across eGFR groups involved LVEF, LVMi, and all absolute global strain measures (Table 2). LVEF and absolute GLS were slightly reduced from G1 + 2 through G4 + 5, while LVMi increased gradually. Absolute global area strain was, similarly to absolute GLS, reduced gradually across eGFR groups, while global radial strain and absolute circumferential strain were most negatively affected in G3 and not G4 + 5, though to a negligible degree. Higher eGFR group index was furthermore associated with higher prevalence of reduced LVEF and absolute GLS (Table 3). The splines in Fig. 2 showed that eGFR associated positively with LVEF, while the association with GLS was increasingly negative. Generally, the association with LVMi was negative, especially in the lower end of eGFR. The scatter plots in Fig. 3b revealed a significant trend of increasing LVMi with eGFR group in patients with severe albuminuria, category A3. LVEF and absolute GLS decreased with eGFR group in patients belonging to A1 + 2 categories. In adjusted analysis, G3 was associated with a poorer LVEF than G1 + 2 independently of albuminuria group and confounding factors (Table 4).
eGFR and UACR vs. echocardiographic parameters. The figure illustrates the relationship between eGFR and UACR, respectively, and echocardiographic parameters, through splines. The UACR is log2-transformed because of the large range of the observations. The splines are natural splines made with the number of knots, that result in the lowest AIC for the models. A maximum of 3 knots was chosen, to avoid potential overfitting. The blue shaded area represents 95% confidence intervals for the spline
Combined associations between eGFR, albuminuria, and echocardiographic parameters. In this figure all patients are stratified both according to eGFR- and albuminuria group, resulting in a total of 9 groups. Figure 3a is a table presenting the sample sizes of each of the 9 groups. Figure 3b presents scatter plots with error bars of echocardiographic parameters across eGFR- and albuminuria group. Dots represent mean values and error bars represent standard errors. The horizontal lines and p-values at the top and bottom of the graph represent p-values for trend across either eGFR- or albuminuria group. The p-values for trend across eGFR groups are further colour-coded according to albuminuria group. Only significant p-values are shown. Figure 3c is a 3-dimensional bar plot further illustrating the combined relationships between eGFR group, albuminuria group, and the echocardiographic parameters. Mean values of the echocardiographic parameters are printed on the 3-dimensional bars. GLS is presented as an absolute value to avoid bars with negative heights for illustrative purposes. All values are crude, i.e., unadjusted values
Associations of LV mass and function with albuminuria
While eGFR associated mostly with LV systolic function (Tables 2, 3, and Fig. 2), albuminuria was to a greater extent associated with increased LVMi and prevalence of LV hypertrophy (Tables 1, 3, and Fig. 2). Splines in Fig. 2 demonstrated a large, positive association between log2-transformed UACR and LVMi. GLS also associated positively with log2-transformed UACR. A significant trend of increasing LVMi with increasing severity of albuminuria in patients within the G3 group was also found (Fig. 3b). In adjusted analysis, A3 was associated with increased LVMi compared to A1 independently of GFR category and confounding factors (Table 4).
Interactions between eGFR and albuminuria
The bar plots in Fig. 3c showed a marked association between increased LVMi and albuminuria at GFR categories 4–5 only. The association between eGFR groups and LVMI were likewise only pronounced at moderate and severe albuminuria stages, A2-3. Thus, increased LV mass was most strongly associated with a combination of decreased eGFR and severe albuminuria. As previously mentioned, both LVEF and absolute GLS were reduced across eGFR groups in A1 and A2, while the association was not present in A3. Furthermore, no significant trends in LVEF and GLS were found across albuminuria groups, suggesting an association between decreased LV systolic function and eGFR, but not albuminuria. Surprisingly, LVEF in G4 + 5 increased with severity of albuminuria (Fig. 3c). No significant interactions between eGFR and UACR were found when determining their respective relationships with LVMi (p-value for interaction = 0.975), LVEF (p = 0.252), and GLS (p = 0.941).
Reproducibility
Intra- and interrater reliability were assessed using Bland Altman plots. For LVMi, intrarater variability analysis showed a mean difference ± SD of 1.34 g/m2 ± 8.78 g/m2, while interrater variability analysis showed 0.10 g/m2 ± 10.76 g/m2. For LVEF, intrarater variability analysis showed a mean difference ± SD of 1.37% ± 6%, while interrater variability analysis showed 2.6% ± 6.11%. Lastly, for GLS, intrarater variability analysis showed a mean difference ± SD of 0.03% ± 1.18%, and interrater variability analysis showed 1.05% ± 1.57%.
Discussion
The present study constitutes the first large-scale use of 3DE in the investigation of adverse cardiac changes associated with CKD. The most significant findings of the study can be briefly summarized as follows: Increase in LVMi and prevalence of LV hypertrophy was more strongly associated with UACR than eGFR. On the contrary, decrease in LVEF and absolute GLS was associated with eGFR, while only the latter was associated with increasing UACR.
LVMi and hypertrophy
Our study found that eGFR and albuminuria were linked to an increase in LVMi, and the latter also to a higher prevalence of LV hypertrophy, which corresponds well with current knowledge [24, 25]. In adjusted analysis, however, only A3 remained significantly associated with an increased LVMi compared to the A1, suggesting a stronger link between LV mass and albuminuria than with eGFR. In patients with kidney disease with an eGFR > 29 mL/min/1.73 m2, corresponding to G4 + 5, the prevalence of LV hypertrophy is estimated to be 16–31% [26], while our study found a prevalence of 37.1%. LV hypertrophy is thought to be a compensatory mechanism that can result, for example, in response to the increased afterload that arises with hypertension [27]. Due to the strong presence of hypertension in relation to CKD, as our study also found, it is thus a likely contributor to the development of LV hypertrophy in CKD patients. Hypertension in kidney disease patients is likely related to the decline in arterial compliance associated with CKD, which is a consequence of extensive calcification of arterial walls [28]. This arterial stiffness would manifest clinically as an increased pulse pressure, which in this study also increased significantly with eGFR group. Furthermore, albuminuria is thought to be a marker of microangiopathy and endothelial dysfunction, which likely plays a role in the high coincidence with LV hypertrophy [29].
eGFR and LV systolic function
Both LVEF and absolute GLS decreased across eGFR groups in unadjusted analysis, while in adjusted analysis, LVEF was also significantly decreased in G3. Importantly, our findings showed that the associations between eGFR and poorer LV systolic function were independent of the severity of albuminuria. Furthermore, we found no significant associations between albuminuria category and LV systolic function. The apparent link between eGFR in patients with CKD and reduced LV systolic function has been demonstrated before. For example, progression from CKD to end-stage renal disease has been linked to a decrease in LVEF [30], while G2-4 have been linked to a subclinical impaired GLS [12]. The reduced LV systolic function is of special interest in relation to HF, the prevalence of which also associates positively with eGFR [31]. This association was also apparent in our study, as the prevalence of HF was increased more than three- and five-fold in G3 and G4 + 5, respectively, compared with G1 + 2. A possible contributing factor to the development of HF in patients with CKD is the increased LV mass seen in relation to advanced impairment of eGFR and albuminuria. According to HF literature, LV hypertrophy represents a precursor state of HF, which over time can lead to LV dysfunction through subendocardial fibrosis and ischemia [32]. Thus, increased LVMi would predispose patients to LV systolic dysfunction.
The KDIGO 2012 CKD guidelines [18] illustrate the risk of cardiovascular mortality in CKD patients stratified according to both GFR and albuminuria. Given the high cardiovascular risk and prevalence of HF associated with late-stage CKD and albuminuria, one would expect poorer values for LVEF and GLS in afflicted patients, since both parameters are proven predictors of cardiovascular mortality [33, 34]. However, our study found a relatively preserved LV systolic function in patients in G4 + 5 and in A2-3. This could reflect a survival bias among patients surviving to reach these late stages of CKD. This could also suggest that the high mortality among patients with advanced CKD and albuminuria, highly attributable to CVD, could obscure the true potential impact of renal impairment on LV systolic function. Furthermore, the antihypertensive diuretic spironolactone has been shown to improve LV systolic function in patients with CKD [35]. A large proportion of the study population had well-controlled hypertension owing to the use of antihypertensive agents and this could potentially have improved the LV systolic function in some patients, since hypertension has been linked with impaired LV systolic function [36].
Clinical perspective and future implications
It would be of interest for future longitudinal studies to investigate whether these echocardiographic parameters could be used in conjunction with eGFR and UACR as predictors of cardiovascular risk to improve upon already established risk stratification of patients with CKD. Additionally, our results imply that decline in eGFR could affect systolic function of the left ventricle to a greater extent than mass, while the opposite is true for albuminuria, and future longitudinal studies are warranted to test this. Should the implication hold true, it could likely contribute to the development of more individually targeted preventive treatments of the cardiovascular symptoms associated with CKD. Furthermore, future works that include echocardiographic measures of diastolic function are warranted.
Limitations
3DE is generally challenging to perform compared with regular 2DE and is therefore feasible in fewer subjects. The ASE/EAE guidelines have yet to establish reference values for LVMi measured by 3DE, and the definition of LV hypertrophy was therefore based upon a study of a healthy, Caucasian population of 226 subjects. A larger and more diverse population would have been more optimal to base reference values upon. Our cohort was mainly of Caucasian ethnicity and the results are therefore possibly not representative of all ethnicities. Lastly, though the study elucidated associations between CKD and changes in LV structure and function, the cross-sectional study design precluded the possibility of establishing causality.
Conclusion
Progressive CKD stage (G1-5, non-dialysis) was associated with reduction in LV systolic function, and increasing severity of albuminuria with an increase in LV mass and higher prevalence of LV hypertrophy. The relationship between eGFR and LV systolic function was independent of albuminuria, traditional risk factors, and confounders. Similarly, the relationship between albuminuria and LVMi was independent of eGFR, risk factors, and confounders.
References
Bikbov B, Purcell CA, Levey AS et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Rahman M, Xie D, Feldman HI et al (2014) Association between chronic kidney disease progression and cardiovascular disease: results from the CRIC study. Am J Nephrol 40(5):399–407. https://doi.org/10.1159/000368915
Martínez-Castelao A, Górriz JL, Portolés JM et al (2011) Baseline characteristics of patients with chronic kidney disease stage 3 and stage 4 in spain: the MERENA observational cohort study. BMC Nephrol 12(1):53. https://doi.org/10.1186/1471-2369-12-53
Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Nally JV (2015) Cause-specific deaths in non–dialysis-dependent CKD. JASN 26(10):2512–2520. https://doi.org/10.1681/ASN.2014101034
Said S (2014) The link between chronic kidney disease and cardiovascular disease. J Nephropathol. https://doi.org/10.12860/jnp.2014.19
Rangaswami J, Bhalla V, Blair JEA et al (2019) Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. https://doi.org/10.1161/CIR.0000000000000664
House AA, Wanner C, Sarnak MJ et al (2019) Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 95(6):1304–1317. https://doi.org/10.1016/j.kint.2019.02.022
Turner N (ed) (2016) Oxford textbook of clinical nephrology, 4th edn. Oxford University Press, Oxford
Mavrakanas TA, Khattak A, Singh K, Charytan DM (2018) Echocardiographic parameters and renal outcomes in patients with preserved renal function, and mild- moderate CKD. BMC Nephrol 19(1):176. https://doi.org/10.1186/s12882-018-0975-5
Lieb W, Mayer B, Stritzke J et al (2006) Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population. Nephrol Dial Transplant 21(10):2780–2787. https://doi.org/10.1093/ndt/gfl364
Matsushita K, Kwak L, Sang Y et al (2017) Kidney disease measures and left ventricular structure and function: the atherosclerosis risk in communities study. J Am Heart Assoc. https://doi.org/10.1161/JAHA.117.006259
Ravera M, Rosa GM, Fontanive P et al (2019) Impaired left ventricular global longitudinal strain among patients with chronic kidney disease and end-stage renal disease and renal transplant recipients. Cardiorenal Med 9(1):61–68. https://doi.org/10.1159/000494065
Wu VC, Takeuchi M (2017) Three-dimensional echocardiography: current status and real-life applications. Acta Cardiol Sin. https://doi.org/10.6515/ACS20160818A
Lang RM, Badano LP, Tsang W et al (2012) EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J: Cardiovasc Imaging 13(1):1–46. https://doi.org/10.1093/ehjci/jer316
Sørensen IMH, Saurbrey SAK, Hjortkjær HØ et al (2020) Regional distribution and severity of arterial calcification in patients with chronic kidney disease stages 1-5: a cross-sectional study of the Copenhagen chronic kidney disease cohort. BMC Nephrol 21(1):534. https://doi.org/10.1186/s12882-020-02192-y
Bjergfelt SS, Sørensen IMH, Hjortkjær HØ et al (2021) Carotid plaque thickness is increased in chronic kidney disease and associated with carotid and coronary calcification. Jandeleit-Dahm K, ed. PLoS ONE 16(11):e0260417. https://doi.org/10.1371/journal.pone.0260417
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408]. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl (2013) 3:1–150
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Muraru D (2013) Comprehensive analysis of left ventricular geometry and function by three-dimensional echocardiography in healthy adults. J Am Soc Echocardiogr 26(6):11
Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R (2012) Population-based reference values for 3D echocardiographic LV volumes and ejection fraction. JACC: Cardiovasc Imaging 5(12):1191–1197. https://doi.org/10.1016/j.jcmg.2012.07.014
Truong VT, Phan HT, Pham KNP et al (2019) Normal ranges of left ventricular strain by three-dimensional speckle-tracking echocardiography in adults: a systematic review and meta-analysis. J Am Soc Echocardiogr 32(12):1586-1597.e5. https://doi.org/10.1016/j.echo.2019.07.012
Muraru D, Cecchetto A, Cucchini U et al (2018) Intervendor consistency and accuracy of left ventricular volume measurements using three-dimensional echocardiography. J Am Soc Echocardiogr 31(2):158-168.e1. https://doi.org/10.1016/j.echo.2017.10.010
Jørgensen PG, Biering-Sørensen T, Mogelvang R et al (2018) Presence of micro- and macroalbuminuria and the association with cardiac mechanics in patients with type 2 diabetes. Eur Heart J: Cardiovasc Imaging 19(9):1034–1041. https://doi.org/10.1093/ehjci/jex231
Wu N, Zhao W, Ye K et al (2014) Albuminuria is associated with left ventricular hypertrophy in patients with early diabetic kidney disease. Int J Endocrinol 2014:1–8. https://doi.org/10.1155/2014/351945
Di Lullo L, Gorini A, Russo D et al (2015) Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med 5(4):254–266. https://doi.org/10.1159/000435838
Devereux RB, Pickering TG, Alderman MH, Chien S, Borer JS, Laragh JH (1987) Left ventricular hypertrophy in hypertension. Prevalence and relationship to pathophysiologic variables. Hypertension. https://doi.org/10.1161/01.HYP.9.2_Pt_2.II53
Briet M, Boutouyrie P, Laurent S, London GM (2012) Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 82(4):388–400. https://doi.org/10.1038/ki.2012.131
Palmieri V, Tracy RP, Roman MJ et al (2003) Relation of left ventricular hypertrophy to inflammation and albuminuria in adults with type 2 diabetes. Diabetes Care 26(10):2764–2769. https://doi.org/10.2337/diacare.26.10.2764
Bansal N, Keane M, Delafontaine P et al (2013) A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol 8(3):355–362. https://doi.org/10.2215/CJN.06020612
Kottgen A, Russell SD, Loehr LR et al (2007) Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18(4):1307–1315. https://doi.org/10.1681/ASN.2006101159
Condorelli G, Morisco C, Stassi G et al (1999) Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl -2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 99(23):3071–3078. https://doi.org/10.1161/01.CIR.99.23.3071
Biering-Sørensen T, Biering-Sørensen SR, Olsen FJ et al (2017) Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: the Copenhagen City heart study. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005521
Wehner GJ, Jing L, Haggerty CM et al (2019) Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J. https://doi.org/10.1093/eurheartj/ehz550
Edwards NC, Ferro CJ, Kirkwood H et al (2010) Effect of spironolactone on left ventricular systolic and diastolic function in patients with early stage chronic kidney disease. Am J Cardiol 106(10):1505–1511. https://doi.org/10.1016/j.amjcard.2010.07.018
Liu H, Wang J, Pan Y, Ge Y, Guo Z, Zhao S (2020) Early and quantitative assessment of myocardial deformation in essential hypertension patients by using cardiovascular magnetic resonance feature tracking. Sci Rep 10(1):3582. https://doi.org/10.1038/s41598-020-60537-x
Acknowledgements
Jacob Christensen was funded by a pregraduate scholarship from the Novo Nordisk Foundation (Grant reference: NNF20OC0065858) received by Tor Biering-Sørensen during preparation of the manuscript.
Funding
The Novo Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Christensen, J., Landler, N.E., Olsen, F.J. et al. Left ventricular structure and function in patients with chronic kidney disease assessed by 3D echocardiography: the CPH-CKD ECHO study. Int J Cardiovasc Imaging 38, 1233–1244 (2022). https://doi.org/10.1007/s10554-021-02507-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02507-6