Abstract
Cardiac sarcoidosis (CS) is known to be associated with ventricular tachycardia (VT); however, most investigations to date have focused on patients with known extra-cardiac sarcoidosis. The presence of CS is typically evaluated using 18F-fluorodeoxyglucose (18F-FDG) uptake on cardiac positron emission tomography (PET) or late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR). In this study, we sought to determine the prevalence of primary CS and the relationship between myocardial 18F-FDG uptake and LGE in patients with VT without known sarcoidosis. We retrospectively identified 67 patients without known sarcoidosis or active ischemic heart disease (i.e. significant ischemic disease that had not been previously revascularized) referred for both CMR and PET for evaluation of VT. Standard cine- and LGE- CMR and cardiac PET protocols were used. Myocardial LGE was defined as signal intensity > 5 SDs above the mean signal intensity of normal myocardium. Cardiac PET images were considered positive if there was focal myocardial 18F-FDG uptake having greater activity than the left ventricular blood pool. 45 patients (67%) had LGE, while only 4 (6%) had myocardial FDG uptake. Nine percent of patients with LGE had FDG-uptake while none without LGE did, and 10% of the cohort had indeterminate FDG uptake presumably from poor dietary preparation. Of those with both FDG uptake and LGE, 3/4 ultimately received a clinical diagnosis of CS. 4.5% of patients without previously known sarcoidosis or active ischemic heart disease presenting with VT have newly diagnosed CS. Detection of CS can be increased using a CMR first approach followed by cardiac PET for patients with non-ischemic LGE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Purpose
Cardiac sarcoidosis (CS) is known to be associated with ventricular tachycardia (VT). The risk of VT is predicted by the presence of late gadolinium enhancement (LGE), representing myocardial scar, on cardiac magnetic resonance (CMR). The myocardial scar is believed to be an important substrate responsible for the generation of reentry circuits that can result in VT. Recently, it has also been suggested that VT can be associated with myocardial inflammation as determined by 18F-fluorodeoxyglucose (18F-FDG) uptake using cardiac positron emission tomography (PET) [1]. The risk of VT has been most extensively studied in patients with known extra-cardiac sarcoidosis, as has the relationship between the presence of LGE and 18F-FDG uptake on cardiac PET [2,3,4]. In addition to predicting a patient’s risk of VT, the burden of 18F-FDG activity is also used to diagnose patients with active CS and guide immunosuppressive therapy [5]. It has also been suggested that there can be both concordant and discordant patterns of scar and inflammation in patients with other types of inflammatory cardiomyopathies and idiopathic VT [1]. In the largest series of 107 patients referred for both CMR and PET to evaluate for cardiac sarcoidosis, of which 35% had a history of VT, CMR and PET provided complementary data used in determining a clinical diagnosis [6]. To date, very little is known about the prevalence of primary CS and the relationship between LGE and 18F-FDG uptake in patients with ventricular arrhythmias who do not have a history of sarcoidosis or active ischemic heart disease.
We hypothesize there is an under-recognized patient population with ventricular arrhythmias due to primary cardiac sarcoidosis. In this study, we sought to determine the prevalence of primary CS and the relationship between myocardial 18F-FDG uptake and presence of LGE in patients with VT without known sarcoidosis.
Methods
Study population
We retrospectively identified patients without a known history of cardiac or extracardiac sarcoidosis referred for both CMR and PET within a month of each other for the evaluation of ventricular arrhythmias thought not due to ischemia or prior myocardial infarction from June 2016 to February 2018. Patients with active ischemic heart disease (i.e. significant coronary artery disease that had not been previously revascularized) were excluded from the study. The cohort was predominantly comprised of patients presenting with VT to the electrophysiology section at University of Chicago who were pro-actively referred for evaluation with CMR and PET as part of a center-specific clinical care algorithm in which patients hospitalized with VT of unknown etiology receive both imaging modalities along with an electrophysiology consultation (n = 34). Another group of patients presenting with ventricular arrhythmias were initially referred for CMR and if there was an abnormality subsequently referred for a cardiac PET study (n = 33).
Baseline demographics and past medical history were obtained from the electronic medical record. Patients were excluded if they had implantation of mechanical circulatory support or initiation of immunosuppression in the time between the CMR and PET scan.
Myocardial metabolic imaging
18F-FDG PET imaging was performed using a 4-ring LSO-detector, 64-slice PET-CT scanner (Siemens mCT Healthineers, Tarrytown, NY). The low dose CT images were used for attenuation correction and localization. Image acquisition was performed in the supine position and coverage spanned from the upper mediastinum through the diaphragms. The patient was instructed to completely avoid carbohydrate ingestion for the entire day before the scheduled cardiac PET scan and to undergo a complete fast starting at 7 pm the evening prior to the study date. Imaging typically was performed in the late afternoon. The dose of F-18 Fluorodeoxyglucose injected for PET imaging was ~ 10 mCi and cardiac images were obtained 60 min post injection.
Cardiac PET images were considered positive if there was non-diffuse myocardial 18F-FDG uptake qualitatively having greater activity than the left ventricular blood pool. The standardized uptake value (SUV) was calculated using commercial MIM software after image acquisition. Patients with diffuse myocardial 18F-FDG uptake and suspicion for a poor carbohydrate fast were categorized as indeterminate. Patients with no evidence of 18F-FDG uptake were classified as negative.
CMR acquisition and analysis
CMR was performed using a 1.5 T scanner (Achieva, Philips). Steady state free precession cines (TR 2.9 ms, TE 1.5 ms, flip angle 60°, and temporal resolution ~ 40 ms) were acquired in the short axis and in the 2-, 3-, and 4-chamber imaging planes. Ten to fifteen minutes after injection of a gadolinium-based contrast agent (0.1–0.2 mmol/kg of gadobenate dimeglumine or gadoterate meglumine depending on renal function), LGE images were acquired using a T1-weighted gradient-echo pulse sequence with a phase sensitive inversion recovery reconstruction (TR 4.5 ms, TE 2.2 ms, TI 250–300 ms, flip angle 30°, flip angle 5°, voxel size 2 × 2 × 10 mm, SENSE factor 2). The optimal inversion time was selected (typically 200 to 300 ms).
Patients with pacemakers or implanted cardioverter-defibrillators (ICD) underwent a modified CMR protocol [7]. Specifically, pre and post-CMR device interrogation was performed to ensure stability of ICD parameters and inhibit inappropriate tachyarrhythmia therapies during imaging. To reduce the metallic artifact, the arm was raised above the head on the ipsilateral side of the ICD, in an effort to increase the distance between the pulse generator and the heart. Immediately following initial survey views, pre-contrast 4- and 2-chamber scout views were obtained at 3 frequency shifts: − 1500, 0 and + 1500 Hz, and the shift with minimal ICD-related artifact was identified. Ten to fifteen minutes after contrast administration, wideband LGE images were acquired in the 2-, 3-, and 4-chamber views as well as short-axis stack. Wideband LGE images were acquired with the optimal frequency shift and inversion time. The standard inversion pulse bandwidth was increased from 1.5 to 3.8 kHz.
Cardiac volumes and ejection fraction (EF) were manually traced in end-diastole and end-systole using commercially available software (Vital Images). The presence of any myocardial LGE was defined as signal intensity > 5 standard deviations above the mean signal intensity of normal myocardium and present in either two imaging planes, two consecutive slices, or two neighboring myocardial segments.
Image comparison
Cardiac PET and MRI were initially classified as normal, abnormal, or indeterminate in a fashion blinded to the results of the other imaging modality. Once each technique was independently assessed, the two modalities were compared jointly by two reviewers (ARP, KYK) on a side-by-side basis to determine whether regions of abnormality overlapped. For the LGE and FDG positive patients, the pattern was labeled as matched (both LGE and FDG positive areas in exact same location), partial overlap (LGE and FDG positive areas in same location but with FDG positive area extending significantly beyond the LGE area), or mismatched (LGE and FDG positive areas in different locations).
To determine the clinical impact of the CMR and PET imaging data, the electronic medical records were reviewed for ablation and device history, medical therapy, biopsy findings, and repeat cardiac imaging.
Results
There were 67 patients identified, and the median time between CMR and PET was 14 days. The majority of patients were male (n = 50, 75%), and the mean age was 60 ± 12 years. Race data was available in 62 (93%) patients. The majority of patients were Caucasian (n = 40, 60%), followed by African American (n = 20, 30%) and other (n = 2, 3%). With respect to cardiovascular risk factors, 29 patients (43%) had hypertension, 9 (13%) had diabetes, 33 (49%) had hyperlipidemia, 18 (27%) had a history of atrial fibrillation, 12 (18%) had a bundle branch block at baseline, and 1 (1%) had end stage renal disease requiring dialysis. 24 patients (36%) had an ICD in place at the time of evaluation. Approximately half of patients presented with sustained VT (n = 35, 52%) while the other half presented with non-sustained VT (NSVT) or premature ventricular contractions (PVCs) (n = 32, 48%). The majority of patients required hospitalization at the time of evaluation (n = 40, 60%), while the remainder (n = 27, 40%) were managed in the outpatient setting. All patients with sustained VT required hospitalization. Atrioventricular (AV) block was rare with 2nd degree AV block present in one patient (1%) and complete AV block was present in 1 patient (1%). Average baseline QRS duration of the cohort was 112 ± 28 ms. Left ventricular systolic dysfunction, defined as EF by CMR ≤ 50%, was present in 40 patients (60%). Twelve patients (18%) had a history of previously revascularized coronary artery disease—none of these patients had ischemic LGE patterns. The majority of patients were on a beta-blocker (82%) and 37% were on an additional antiarrhythmic drug. Twenty (30%) had a prior ablation procedure to treat frequent PVCs or VT (Table 1).
Definite myocardial 18F-FDG uptake was present in 4 (6%) patients (SUV max 6.7 ± 3.6), and 7 (10%) had a mild, diffuse pattern attributed to poor fast and categorized as indeterminate (Fig. 1). Neither of the 2 patients with AV block had definitive FDG uptake, although one did have LGE. Of the 4 patients with definite myocardial 18F-FDG uptake, 3 also had extra-cardiac pulmonary or mediastinal 18F-FDG uptake. All four patients with definite FDG uptake also had LGE, and had been referred for CMR and PET sequentially (rather than simultaneously as part of a clinical care algorithm).
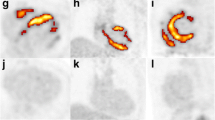
EF and cardiac volumes are outlined in Table 1. LGE was present in 45 (67%) patients. The LGE pattern was non-ischemic pattern in 40 (89%), ischemic in 4 (9%), and mixed ischemic/non-ischemic in 1 (2%). Of the 5 patients with ischemic or mixed pattern of LGE, none had a prior diagnosis of obstructive CAD. None of the patients without LGE (n = 22, 33%) had evidence of definitive FDG uptake; however, 3/22 (14%) had indeterminate FDG uptake. Of the 45 patients with LGE, 4 had definitive FDG uptake (9%) and 4 (9%) had indeterminate FDG uptake. Approximately half of the LGE positive patients had been referred for CMR and PET simultaneously as part of a clinical care algorithm (n = 23, 51%), while the other half (n = 22, 49%) were referred for PET only after CMR demonstrated abnormal findings. For the LGE and definite FDG positive patients, the overlap pattern was matched in 1 patient, had partial overlap with greater extent of FDG positivity in 2 patients; and was unmatched in one patient (Fig. 2, 4 and 5).
Matched late gadolinium enhancement and 18F-fluorodeoxyglucose activity Patient 1′s Cardiac magnetic resonance and cardiac positron emission tomography findings with late gadolinium enhancement the basal anterolateral and basal inferolateral walls (a–d) and matching 18F-fluorodeoxyglucose activity with additional uptake in the bilateral hila (e, f)
In a subgroup that excluded the 20 patients with prior VT ablation (n = 47), 33 were male (70%), and the mean age was 59 ± 12 years. 24 of these patients (51%) presented with sustained VT, and 23 (49%) presented with NSVT or PVCs. Systolic dysfunction was present in 28 patients (60%) in this subgroup, and the average EF on CMR was 42 ± 14%. 28 patients in this subgroup (60%) had LGE present, 3 (6%) had evidence of definitive FDG uptake, and 6 (13%) had FDG uptake categorized as indeterminate.
In the 7 total cases of indeterminate FDG activity, no biopsies were performed and no definitive diagnoses were made. Three patients, all with non-ischemic pattern LGE, were treated with prednisone for suspected inflammatory cardiomyopathy of unknown etiology: the first had no FDG activity on subsequent PET, the second was lost to follow-up, and the third had a VT ablation and one month later presented in cardiogenic shock requiring mechanical circulatory support and died. The other four patients were treated with a combination of medical management, VT ablation, and ICD implantation. None of the indeterminate FDG patients who did not also have LGE were treated with immunosuppressive therapy.
Discussion
In this study, we showed that in a select cohort of patients without a known history of sarcoidosis presenting with ventricular arrhythmias not felt to be secondary to ischemic heart disease: (1) a majority of patients had LGE while only a small proportion of patients had evidence of myocardial FDG uptake; (2) 9% of patients with LGE had definite evidence of FDG-uptake; (3) no patients had a definite abnormal PET in the absence of LGE; and (4) 10% of the cohort had an indeterminate FDG uptake presumably due to poor dietary preparation. Of those with both definite FDG-uptake and presence of LGE, most received a diagnosis of CS and were treated with corticosteroids. For those patients who had indeterminate FDG-uptake in the setting of LGE positivity, the clinical decision appears to have been to still treat with immunosuppressive therapy. Notably, if the indeterminate PET scans were all classified as truly positive, then 16% of the total cohort would have evidence of myocardial inflammation. Conversely, for those patients with indeterminate FDG-uptake in the absence of LGE, the clinical decision appears to have been not to treat with immunosuppressive therapy.
LGE on CMR represents replacement fibrosis and has been implicated as a substrate for ventricular arrhythmias in multiple disease states. In a meta-analysis of patients with non-ischemic cardiomyopathy, LGE was present in 45 ± 15%. Those with LGE had a higher incidence (odds ratio 5.32) of SCD, aborted SCD or appropriate ICD therapy for VT/VF during follow-up [8]. In our cohort, the majority of patients with LGE had no evidence of active inflammation on cardiac PET, therefore management could be focused on arrhythmic and heart failure therapies rather than immunosuppression.
Of the subset with both FDG and LGE, which was approximately 10% of the patients with LGE, 3 of the 4 patients were diagnosed with primary CS without preceding extra-cardiac manifestations. The pattern of LGE in these patients was non-ischemic and in a patchy distribution further increasing suspicion for active CS. This led to major changes in the diagnostic and treatment plan of these patients with focus on first reducing the active inflammation to abate ventricular arrhythmias and prevent progression of heart failure. In patients presenting with ventricular arrhythmias who have evidence of LGE, it is reasonable to pursue imaging with cardiac PET if available to rule out active primary CS. The presentation and management of all 4 patients with both LGE and definitive FDG uptake are described in detail below.
Patient 1 was a 69-year-old man with symptomatic sustained VT, first degree atrioventricular block, and mild non-obstructive CAD on coronary angiogram. His CMR demonstrated mild left ventricular (LV) dilation and normal systolic function (EF 56%). It showed two patches of LGE in the basal anterolateral and basal inferolateral walls (Fig. 2a–d). His cardiac PET showed matching focal FDG (SUV max 3.6) activity in the basal anterolateral and basal inferolateral walls. There was also focal FDG uptake in the bilateral hila (Fig. 2e, f). He was referred for mediastinal lymph node biopsy that showed reactive changes but no evidence of granulomatous disease. He was empirically started on high dose corticosteroids and follow-up imaging showed improvement at 3 months and complete resolution of FDG activity at 9 months. The patient received a diagnosis of likely cardiac sarcoidosis.
Patient 2 was a 38-year-old man presenting with symptomatic NSVT and LV systolic dysfunction. He had a normal coronary angiogram. His CMR demonstrated a moderately dilated left ventricle with severely reduced systolic function (EF 22%). There was a large extent of diffuse patchy, predominately mid-wall LGE present in over half of the myocardial segments (Fig. 3a, b). His cardiac PET showed extensive abnormal markedly hypermetabolic myocardial activity (SUV max = 10.6), compatible with active inflammation with relative sparing of the apex and periapical segments. The area of FDG activity showed partial overlap with the LGE with greater extent of FDG activity. There was also evidence of extracardiac activity with numerous enlarged abnormal hypermetabolic mediastinal lymph nodes (Fig. 3c, d). He was referred for an electrophysiology study and an initially negative endomyocardial biopsy followed by a mediastinoscopy and lymph node biopsy positive for non-necrotizing well-formed granulomas with associated fibrosis, consistent with sarcoidosis. Follow-up imaging showed persistent LGE and marked improvement in FDG activity following administration of high dose corticosteroids. The patient received a clinical diagnosis of cardiac sarcoidosis.
Partial overlap late gadolinium enhancement and 18F-fluorodeoxyglucose activity Patient 2′s Baseline cardiac magnetic resonance and cardiac positron emission tomography findings demonstrating a large extent of diffuse patchy, predominately mid-wall late gadolinium enhancement present in over half of the myocardial segments (a, b) and partially overlapping extensive 18F-fluorodeoxyglucose activity with relative sparing of the apex and periapical segments (c, d)
Patient 3 was a 67-year-old woman with exertional dyspnea and incidentally noted NSVT during a hospitalization. She had a coronary angiogram with no coronary artery disease. CMR showed normal LV size with low-normal systolic function (EF 51%). There was multifocal, patchy LGE in the apical inferior and lateral walls, basal inferior, inferoseptal, and inferolateral walls, and basal to mid anterior walls (Fig. 4a, b). The cardiac PET showed severe uptake of FDG (SUV max 10.1) which was most pronounced in the entire base of the left ventricle, the mid anterior and septal walls, and the apical anterior, lateral, and inferior walls. There were also pulmonary parenchymal opacities and mediastinal lymph nodes which were significantly hypermetabolic (SUV max = 9.3) (Fig. 4c, d). She had an endobronchial biopsy showing non-necrotizing granulomas consistent with sarcoidosis and was subsequently started on corticosteroid therapy. She had a follow-up PET scan 6 months later with significant decrease in size and intensity (SUV max 6.0) of the abnormal uptake of FDG in the left ventricular myocardium. The patient received a clinical diagnosis of cardiac sarcoidosis.
Second case of partial overlap late gadolinium enhancement and 18F-fluorodeoxyglucose activity Patient 3′s Baseline cardiac magnetic resonance and cardiac positron emission tomography findings demonstrating multifocal, patchy late gadolinium enhancement in the apical inferior and lateral walls, basal inferior, inferoseptal, and inferolateral walls, and basal to mid anterior walls (a, b) and partially overlapping 18F-fluorodeoxyglucose activity most pronounced in the entire base of the left ventricle, the mid anterior and septal walls, and the apical anterior, lateral, and inferior walls and additional metabolically active pulmonary parenchymal opacities and mediastinal lymph nodes (c, d)
Patient 4 was a 70-year-old man with non-ischemic cardiomyopathy and frequent PVCs with concern for PVC induced cardiomyopathy. He had a normal coronary angiogram. He was intolerant of beta-blockers due to severe reactive airway disease and was referred to Cardiac Electrophysiology for possible PVC ablation. CMR showed a mildly dilated LV with severely reduced systolic function (EF 29%). There was midwall LGE in the midseptum (Fig. 5a, b). The cardiac PET showed a small area of focal uptake of FDG (SUV max 3.9) which appeared to be in the anterolateral papillary muscle which was unmatched to the LGE (Fig. 5c, d). There was no abnormal extracardiac FDG activity. He had a primary prevention ICD implanted and PVC burden improved with dofetilide therapy. The patient did not undergo endomyocardial biopsy (or other organ biopsy) because it was felt that the procedures would be unlikely to reveal a diagnosis. The patient was presumed not to have CS and was not treated with immunosuppression.
Mismatched late gadolinium enhancement and 18F-fluorodeoxyglucose activity Patient 4′s cardiac magentic resonance and cardiac positron emission tomography findings demonstrating midwall late gadolinium enhancement (a, b) in the midseptum and focal 18F-fluorodeoxyglucose activity of anterolateral papillary muscle (c, d)
Sarcoidosis is a rare disease with an overall prevalence of 10 to 20 per 100,000 in the general population and is up to four times more common in African American patients [9]. However in patients presenting with unexplained VT in the absence of active ischemic heart disease, our study suggests that the prevalence of CS may be considerably higher than in the general population. Further, our results suggest the number needed to image in this population is approximately 20 or fewer patients to identify an individual with active CS in which a disease-targeted treatment plan including immunosuppression can be utilized. However, this number needed to image will need to be demonstrated in larger prospective studies given the single center nature of this study along with the propensity for selection bias related to imaging referral.
Sarcoidosis most commonly affects the lungs and presents with pulmonary symptoms, but up to a third of patients present with organ specific extra-pulmonary manifestations [10]. There is also growing evidence that isolated CS can occur and may present with the onset of cardiac symptoms due to conduction disease, ventricular arrhythmias, and/or significant left ventricular systolic dysfunction [11].
It is important to accurately identify patients with an inflammatory cardiomyopathy such as active CS because the treatment plan would likely be significantly impacted. Prior data suggests those with an inflammatory cardiomyopathy could be missed on CMR but identified with cardiac PET [1]. However, our study suggests that in patients presenting with ventricular arrhythmias, only patients with LGE are likely to be ultimately diagnosed with an inflammatory cardiomyopathy. Possible explanations for the discrepant findings include slight differences in the definition of a positive PET study, regional and racial differences of the study populations, (with a higher proportion of African Americans in our cohort), proportion of patients with AV block (lower in our cohort), and inclusion of patients with NSVT or PVCs. Additionally, in the prior study [1], not all patients had both CMR and cardiac PET. In fact, in our cohort, no patients had definitive evidence of FDG uptake on cardiac PET in the absence of LGE, but we must recognize that a small subgroup of patients without LGE in our cohort did have inconclusive cardiac PET findings.
We propose an algorithmic approach in which patients presenting with ventricular arrhythmias who have evidence of a non-ischemic pattern of LGE be further evaluated with cardiac PET to identify underlying inflammatory cardiomyopathies such as CS (Fig. 6). It remains to be determined how the T1- and T2-mapping techniques now available on CMR might impact this algorithm [12,13,14] since our clinical imaging protocol at the time of the study did not include those sequences. On the other hand, those individuals without LGE should be considered for other diagnoses and cardiac PET may be of limited utility. The low probability of finding myocardial FDG uptake in patients without evidence of LGE is supported by other studies as well [6]. Because the presence of LGE can be due to either inflammation or fibrosis and because many patients have ICDs that can significantly impact CMR image quality, CMR may not be the optimal modality to assess treatment response to corticosteroids in patients with CS. Instead, cardiac PET may be better suited for the assessment of the residual burden of inflammation after the initiation of immunosuppressive therapy.
Limitations
This is a small, single center study with results that may be influenced by regional and demographic factors and by patient referral patterns. This was a retrospective analysis that included a large proportion of patients who required hospitalization for VT along with electrophysiology consultation, CMR, and PET imaging. This increases the possibility of selection bias, which is the largest limitation to this study. However, this study evaluated a systematic approach implemented by the electrophysiology team at our institution, allowing for the patient population to be relatively well defined. Another limitation is the indeterminate cardiac PET scan population, many of which we believe to have represented negative cardiac PET scans. Unfortunately, repeat cardiac PET after an adequate fast to confirm our clinical impression was not routinely performed. Interestingly, when encountering indeterminate cardiac PET scans, treating physicians seemed to have used the presence or absence of LGE to help determine whether or not to initiate corticosteroid therapy.
Conclusions
In our cohort, nearly 5% of patients presenting with ventricular arrhythmias in the absence of active ischemic heart disease or previously known sarcoidosis had active primary CS that was detected using CMR and cardiac PET imaging. The prevalence of CS in this patient cohort appears to be significantly higher than expected in the general population. Based on our data, patients presenting with ventricular arrhythmia without a clear explanation may be good candidates to undergo a diagnostic algorithm which incorporates CMR imaging followed by cardiac PET when LGE is identified to make the diagnosis of active primary CS.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- CS:
-
Cardiac sarcoidosis
- EF:
-
Ejection fraction
- ICD:
-
Implantable cardioverter-defibrillator
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle, left ventricular
- NSVT:
-
Non-sustained ventricular tachycardia
- PET:
-
Positron emission tomography
- PVC:
-
Premature ventricular contraction
- SUV:
-
Standardized uptake value
- VT:
-
Ventricular tachycardia
- 18F-FDG:
-
18F-fluorodeoxyglucose
References
Tung R, Bauer B, Schelbert H, Lynch JP 3rd, Auerbach M, Gupta P, Schiepers C, Chan S, Ferris J, Barrio M, Ajijola O, Bradfield J, Shivkumar K (2015) Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm 12:2488–2498
Dweck MR, Abgral R, Trivieri MG, Robson PM, Karakatsanis N, Mani V, Palmisano A, Miller MA, Lala A, Chang HL, Sanz J, Contreras J, Narula J, Fuster V, Padilla M, Fayad ZA, Kovacic JC (2018) Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging 11:94–107
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF (2014) Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 63:329–336
Bravo PE, Raghu G, Rosenthal DG, Elman S, Petek BJ, Soine LA, Maki JH, Branch KR, Masri SC, Patton KK, Caldwell JH, Krieger EV (2017) Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol 241:457–462
Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R (2014) Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 21:166–174
Vita T, Okada DR, Veillet-Chowdhury M, Bravo PE, Mullins E, Hulten E, Agrawal M, Madan R, Taqueti VR, Steigner M, Skali H, Kwong RY, Stewart GC, Dorbala S, Di Carli MF, Blankstein R (2018) Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging 11:e007030
Singh A, Kawaji K, Goyal N, Nazir NT, Beaser A, O’Keefe-Baker V, Addetia K, Tung R, Hu P, Mor-Avi V, Patel AR (2019) Feasibility of cardiac magnetic resonance wideband protocol in patients with implantable cardioverter defibrillators and its utility for defining scar. Am J Cardiol 123:1329–1335
Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M (2014) Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging 7:250–258
Thomas KW, Hunninghake GW (2003) Sarcoidosis. JAMA 289:3300–3303
Rizzato G, Palmieri G, Agrati AM, Zanussi C (2004) The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 21:119–126
Okada DR, Bravo PE, Vita T, Agarwal V, Osborne MT, Taqueti VR, Skali H, Chareonthaitawee P, Dorbala S, Stewart G, Di Carli M, Blankstein R (2018) Isolated cardiac sarcoidosis: a focused review of an under-recognized entity. J Nucl Cardiol 25:1136–1146
Crouser ED, Ono C, Tran T, He X, Raman SV (2014) Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med 189:109–112
Crouser ED, Ruden E, Julian MW, Raman SV (2016) Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J Investig Med 64:1148–1150
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the society for cardiovascular magnetic resonance (SCMR) endorsed by the European association for cardiovascular imaging (EACVI). J Cardiovasc Magn Reson 19:75
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AP (principal investigator), KK, and AS, designed the study, read MRI’s, and developed the manuscript. SC collected and reviewed clinical data and contributed to the manuscript. RW, DA, and RL read PET studies. EF, JM, and RT helped develop the study concept and performed ablations on several patients. All authors reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The University of Chicago IRB approved with a waiver of consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kebed, K.Y., Carter, S.V., Flatley, E. et al. Prevalence of newly diagnosed sarcoidosis in patients with ventricular arrhythmias: a cardiac magnetic resonance and 18F-FDG cardiac PET study. Int J Cardiovasc Imaging 37, 1361–1369 (2021). https://doi.org/10.1007/s10554-020-02090-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-02090-2