Abstract
Background
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) has shown to be useful in diagnosis, staging and monitoring of cardiac sarcoidosis (CS) but its interpretation is not standardized.
Objectives
We sought to investigate the clinical impact of serial quantitative FDG uptake analysis in patients with CS presenting with ventricular tachycardia (VT) treated by catheter ablation (CA).
Methods
We followed 20 patients (51 ± 9 years, 70% males) with CS and VT who underwent CA, with 92 serial FDG-PET scans (3–10 per patient). Myocardial FDG-avid lesions were quantified using three parameters: maximum standardized uptake value (SUVmax), partial-volume corrected mean standardized uptake value (SUVmean) and partial-volume corrected volume-intensity product [lesion metabolic activity (LMA)]. The volume-intensity product of the entire heart [global cardiac metabolic activity (gCMA)] and the background cardiac metabolic activity (bCMA: difference between gCMA and LMA) were also calculated. The primary end-point was the occurrence of major adverse cardiac events (MACE), including death, heart transplant, hospitalization for heart failure and implantable cardioverter defibrillator (ICD) appropriate interventions. Evolution of echocardiographic parameters over follow-up was also assessed.
Results
During a median follow-up of 35 (20–66) months, 18 MACE (1 death, 2 heart transplants, 12 ICD appropriate interventions, 3 hospitalizations) occurred in 12 (60%) patients. At univariable analysis, lack of PET improvement (defined by decrease in LMA of at least 25%) was the only variable associated with cardiac events during follow-up. In particular, non-responders had a 20-fold higher risk of MACE at follow-up (HR 18.96, 95% CI 2.26–159.27; p = 0.007). Moreover, a significant linear inverse relationship was observed between changes in LMA and changes in left ventricular ejection fraction over follow-up (β = −20.11; p = 0.003).
Conclusions
In patients with CS and VT, temporal change in FDG uptake evaluated by a quantitative approach is associated with parallel change in systolic function. Moreover, reduction in FDG uptake is strongly associated with fewer MACE at long-term follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multisystem disease of unknown etiology, characterized by the presence of non-caseating granulomatous inflammation. The prevalence of cardiac involvement has been estimated in at least 25%, but only 5% of the patients develop a clinically overt cardiac disease [1]. The myocardial injury is driven by active inflammation leading to myocyte loss and reparative fibrosis affecting ventricular function and creating the substrate for reentrant arrhythmias [2]. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has a well-established role in diagnosing cardiac involvement by detecting active inflammation [3]. However, PET interpretation remains largely qualitative or based upon maximum standardized uptake value (SUVmax), with few studies that have attempted to reproducibly quantify the extent and intensity of inflammation [4,5,6,7]. Baseline abnormal myocardial FDG uptake has been associated with increased risk of death and ventricular tachycardia (VT) among patients with cardiac sarcoidosis (CS). Moreover, reduction of inflammation with immunosuppressive therapy has been associated with improvement in systolic function and New York Heart Association (NYHA) functional class [5, 7, 8]. Recently our group demonstrated that the presence of baseline active inflammation as well as its worsening or lack of response to immunosuppressive therapy by qualitative evaluation, significantly increases the risk of VT recurrence in patients with CS undergoing catheter ablation (CA) for VT [9]. A standardized method to quantify the volume and intensity of abnormal FDG uptake may improve treatment response evaluation and identification of high-risk patients with persistent inflammatory activity. In the present study, we sought to investigate the prognostic role of serial quantitative FDG-PET evaluation in patients with active CS undergoing CA for drug-refractory VT.
Methods
Study population
This was a retrospective observational study. The study population was selected from 42 consecutive patients with diagnosis of CS based upon Heart Rhythm Society (HRS) criteria and refractory VT referred for CA to our institution between January 2006 and December 2015 [10]. Only patients who had serial FDG-PET scans performed to assess response to therapy were included. A total of 20 patients followed with 92 scans met the inclusion criteria and were included in the study. Patients gave written informed consent and all the procedures were performed according to the institutional guidelines of the University of Pennsylvania Health System. All the data were entered in a database approved by the institutional review board.
Cardiac PET/CT acquisition protocol
PET/computed tomography (PET/CT) imaging was performed using a Gemini TF PET/CT scanner (Philips Medical System, Tokyo, Japan) following at least 18-h fasting after a low-carbohydrate (<5 g) and high-fat content meal in order to eliminate physiological uptake of FDG from the myocardium [11, 12]. All patients received unfractionated heparin 15 min before FDG injection [13]. The fasting plasma glucose level was measured prior to FDG injection. Images were acquired from the lower neck to the upper abdomen approximately 55 min after intravenous administration of approximately 185 MBq (5.034 mCi) of 18F-FDG. Corresponding CT images were acquired and reviewed alongside the PET images. The low-dose unenhanced CT images were used for attenuation correction and anatomic correlation only. Reconstruction method: BLOB-OS-TF, # iterations: 3, # subsets: 33, matrix size: 144 × 144.
Cardiac PET/CT data analysis
Quantitative analysis was performed on PET images using ROVER software (ABX advanced biochemical compounds GmbH, Radeberg, Germany) and on fused PET/CT images using OsiriX 7.5.1 software (Pixmeo SARL, Bernex, Switzerland) by measuring cardiac SUVmax, partial-volume-corrected SUVmean, partial-volume-corrected lesion metabolic activity (LMA), global cardiac metabolic activity (gCMA) and background cardiac metabolic activity (bCMA) as follow.
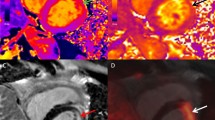
A region of interest (ROI) was placed on the fused axial image encompassing the entire heart (coronal and sagittal images were reviewed to ensure the entire myocardium and no adjacent non-cardiac FDG-avid structures were included and manual editing was performed if necessary). The software automatically calculated uncorrected cardiac SUVmax, partial-volume-corrected SUVmean and partial-volume-corrected LMA (defined as the product of metabolic volume and SUVmean). The metabolic volume (volume of FDG-avid voxels inside the ROI) was automatically calculated using a lower threshold setting of 40% SUVmax with a minimum volume of 1 cm3 as previously described (Fig. 1) [7].
Examples of serial PET scans among patients with cardiac sarcoidosis (CS) responding (a–l) or not (m–r) to immunosuppressive treatment. Each raw shows a single scan in three projections (transverse, coronal and sagittal). Baseline PET scan in a patient with CS involving the mid-basal septum, mid-basal anterior and basal inferior wall of the left ventricle (a–c) that demonstrate complete normalization after 6 months of immunosuppressive therapy (d–f). Baseline PET scan in a patient with CS involving the free wall of the right ventricle, all the septum and the basal anterior and inferior wall of the left ventricle (g–i). Same patient at 6-month control (j–l) no longer showing active inflammation of the myocardium. Patient with CS involving the lateral and anterior wall of the left ventricle as well as the anterolateral papillary muscle at baseline (m–o) and persistent inflammation of the same areas regardless of 6 months of immunosuppressive treatment (p–r)
Global cardiac metabolic activity of the heart was calculated by manually drawing an ROI around the ventricles in all axial fused PET/CT images passing through the whole heart with a slice thickness of 4 mm. The uncorrected SUVmean of each slice was recorded. The volume of each slice was calculated by multiplying the area of each ROI by its slice thickness (4 mm). Then the metabolic activity of each slice was obtained by multiplying the volume by uncorrected SUVmean of that particular slice. Finally, gCMA was generated by summation of all the metabolic activity values over all slices of the heart as previously described [7]. We were unable to separate blood pool activity from the active disease sites in the myocardium with the current version of ROVER software. This was partly due to lower contrast between the FDG uptake sites and the blood pool background activity. Also, cardiac and respiratory motions further degraded the image quality for optimal segmentation by this image analysis technique.
Electroanatomical mapping and catheter ablation
Patients presented to the cardiac electrophysiology laboratory in the fasting state. Catheters were placed into position in the heart using fluoroscopic guidance. A 6-Fr quadripolar catheter with 5-mm interelectrode distance (Bard Inc., Delran, NJ, USA) was placed at the right ventricular (RV) apex. A deflectable 8-Fr mapping/ablation catheter that had a 3.5-mm irrigated tip and a 2-mm ring electrode separated by 1 mm (Thermocool, Biosense Webster, Diamond Bar, CA, USA) was advanced to the RV (transvenous approach), left ventricle (LV; retrograde aortic or transseptal approach), or epicardial space according to the presumed site of origin of the VT or the underlying substrate. Access to the pericardial space and epicardium was obtained using the percutaneous subxiphoid approach described by Sosa et al. [14]. A 64-element phased-array intracardiac echocardiography catheter (AcuNav, Acuson, Mountain View, CA, USA) was used to assist catheter manipulation, radiofrequency (RF) energy delivery, and tissue-catheter contact and monitoring for complications. A high-density (color and surface fill threshold <15 mm), three-dimensional electroanatomical map (EAM - CARTO™; Biosense Webster Inc., Diamond Bar, CA, USA) was created during sinus or paced rhythm to identify low-voltage areas (LVAs) and abnormal electrograms (EGMs) consistent with scarring, as previously reported [15,16,17]. Programmed ventricular stimulation was performed and induced VTs were compared with those occurring spontaneously. Induced VTs were identified as clinical when they matched the cycle length and morphology of stored implantable cardioverter defibrillator EGMs (near-field and far-field) and the 12-lead electrocardiogram (ECG) when available. A combination of activation/entrainment mapping for tolerated VT and/or substrate-based ablation targeting abnormal late/split/fragmented EGMs and sites of best pace mapping within the abnormal substrate for unstable VTs was employed, as previously described [9, 18]. After ablation, repeat programmed stimulation with ≤3 ventricular extrastimuli delivered from 2 different sites at 2 pacing cycle lengths was performed to determine the procedural success, defined as lack of inducibility of any VTs (clinical and non-clinical) with a cycle length > 250 ms.
Clinical follow-up and outcomes
Before the procedure (T0) all patients underwent a diagnostic work-up comprehensive of clinical evaluation, 12-lead ECG, implantable cardioverter defibrillator (ICD) interrogation, trans-thoracic echocardiography and PET scan. A PET scan as well as a comprehensive clinical and echocardiographic evaluation to assess response to immunosuppressive therapy was repeated at 3–6 months (T1). Clinical, echocardiographic and PET data at the last available follow-up (TFUP) were also collected. Basing upon changes of FDG activity on serial PET imaging, patients were categorized into metabolic response (defined by a decrease in the LMA of at least 25%) or non-response (decrease less than 25% or increase). In order to compare LMA to standard calculation of SUVmax and SUVmean as prognostic marker, we used the same criteria to define metabolic response/non-response according to changes in SUVmax or SUVmean. The agreement between different modalities to define metabolic response was assessed using Cohen’s Kappa coefficient. Cardiac death, heart transplant, sustained VT recurrences (defined as any sustained VT on ICD interrogation or 12-lead ECG) and hospitalizations for heart failure were defined as major adverse cardiac events (MACE).
Statistical analysis
Continuous variables were expressed as mean ± SD if normally distributed or median (25th–75th percentile) if not normally distributed. All continuous variables were tested for normal distribution using the one-sample Kolmogorov–Smirnov test. Categorical data were expressed as counts and percentages. Continuous variables were compared using independent-sample parametric (unpaired Student t) or non-parametric (Mann-Whitney U) tests. Categorical variables were compared using the chi-square test or Fisher’s exact test when appropriate. Repeated measures of continuous parameters were compared using paired-sample parametric (paired Student t) or non-parametric (paired Wilcoxon signed rank) tests. Single variable Cox proportional hazards analysis was used to test the association between the outcome event, baseline covariates and metabolic response. A multivariable analysis was not feasible due to the limited number of events, taking into account the number of candidate predictors in a multivariable model has to be inferior to the number of events divided by 10 [19]. The relationship between the changes in LMA and changes in systolic function was estimated by linear regression. Two-tailed tests were considered statistically significant at the 0.05 level. All the analysis was performed using IBM SPSS version 23.0 software (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Twenty consecutive patients (51 ± 9 years, 14 males) with diagnosis of CS based upon HRS criteria were included in the study [10]. All patients were evaluated with PET, echocardiographic and clinical examination at T0, T1 and TFUP. All the patients underwent at least three PET scans (T0, T1 and TFUP), and eight (40%) had more than three serial scans (range 3–10). In total, 92 scans were performed. All the patients were treated with immunosuppressive therapy with prednisone (mean daily dose: 40 ± 13 mg). Methotrexate (mean weekly dose 10 ± 3 mg) was added in 6 cases (30%) as a second-line therapy. Eleven out of 20 (55%) patients had evidence of metabolic response while in the remaining 9 (45%), FDG uptake was unchanged or increased. Clinical data of all patients and comparison between “responders” and “non-responders” at baseline (T0) are summarized in Table 1. No significant differences in baseline characteristics were noted between patients with metabolic response compared to those that did not respond.
Catheter ablation and acute procedural outcomes
All the patients underwent endocardial mapping and ablation while a combined endo/epicardial approach was adopted in in 9 (45%) patients. A median number of 4 [2,3,4,5,6] different VTs were induced with a median cycle length of 360 (320–430) ms. To achieve a good arrhythmia control, 9 (45%) patients underwent a second procedure and in 3 (15%) cases, a third procedure was necessary. At the end of the last procedure, programmed stimulation was performed in 15 (75%) cases and non-inducibility of any VT was reached in 12 (80%) of the cases (Table 1).
Correlation between metabolic response and clinical outcomes
Table 2 and Fig. 2 summarize the time course of quantitative FDG uptake, echocardiographic and electroanatomical data in the study population.
Among patients with metabolic response, at T0, SUVmax was 7 ± 3 g/ml, SUVmean 6 ± 3 g/ml, LMA was 399 (310–1035) g glucose, gCMA was 1858 ± 570 g glucose, bCMA was 1173 (801–1723) and LVEF was 38 ± 11%. At T1, SUVmax and SUVmean were both significantly reduced compared to T0 (both 2 ± 1 g/ml, p < 0.001), as well as LMA [median 0 (0–402) g glucose, p = 0.008)] and gCMA (mean 1473 ± 451 g glucose, p = 0.028). bCMA did not show a significant reduction [median 1195 (1025–1495), p = 0.515] while a significant improvement in systolic function was observed (LVEF 43 ± 11%, p = 0.023). At the last evaluation (TFUP), performed 17 (10–61) months after T1, no further statistically significant change was observed among PET and echocardiographic parameters.
Among non-responder patients, at T0 , SUVmax and SUVmean were both 5 ± 3 g/ml, LMA was 243 (124–508) g glucose, gCMA was 1541 ± 554 g glucose, bCMA was 1221 (878–1591) and LVEF was 43 ± 12%. At T1, none of quantitative PET parameters showed a significant change compared to T0 , while a significant worsening in LVEF was observed (37 ± 11%, p < 0.001). At the last evaluation (TFUP), performed 15 (9–30) months after T1, no statistically significant change was observed among PET parameters except for gCMA that appeared to be increased (2007 ± 924 vs. 1678 ± 706 g glucose, p = 0.029). Systolic function demonstrated a further decrease compared to T1 (33 ± 12%, p = 0.001).
Changes in LMA through serial PET scans showed a significant inverse relationship with changes in LVEF (R2 = 0.215, p < 0.003), as shown in Fig. 3.
The median follow-up of the study population was 35 (20–66) months. During this period, one patient (5%) died, two (10%) had heart transplant, 12 (60%) experienced sustained VT recurrence and 3 (15%) were hospitalized for heart failure worsening. Overall, 12 (60%) patients reached the composite endpoint of MACE; 10 (91%) in the metabolic non-responders group and 2 (22%) in the metabolic responders group (p = 0.005). Metabolic responders had a significantly lower proportion of VT recurrences in comparison to non-responders (22 vs. 91%, p = 0.005). A trend towards better outcomes among responders was observed also for death (0 vs. 9%, p = 1.000) and hospitalizations for heart failure (0 vs. 27%, p = 0.218).
At univariable analysis (Tables 3 and 4), metabolic response (HR 0.05, 95% CI 0.01–0.44, p = 0.007) was the only significant variable associated with less MACE at follow-up (Fig. 4). Only a trend towards worse outcomes was observed for increase of bCMA (HR 1.01, 95% CI 1.00–1.03, p = 0.069; Fig. 4).
Comparison between lesion metabolic activity assessment and SUV
The mean value of SUVmax at T0 was 6 ± 3 and did not significantly differ at T1 (5 ± 3, p = 0.434) and at TFUP (4 ± 3, p = 0.389) while the mean value of SUVmeanwas 6 ± 3 at T0, and 5 ± 2 either at T1 and TFUP (p = 0.529 for comparison with T0). Eleven out of 20 (55%) patients had evidence of metabolic response by SUVmax while 10 (50%) by SUVmean. No significant agreement was found between metabolic response defined by LMA and SUVmax (k = 0.41; p = 0.064) while only a fair agreement was found between LMA and SUVmeank = 0.50; p = 0.025). No significant differences were observed in baseline clinical and procedural characteristics of metabolic responders and non-responders defined by changes in SUVmax and SUVmean (Table 5). Moreover, metabolic responders defined by changes in SUVmax and SUVmean did not present any significant difference in the occurrence of the composite end-point as well as in any of the single outcome events (Table 6). At univariable analysis, neither the metabolic response defined by SUVmax (HR 0.399, 95% CI 0.122–1.303; p = 0.128) nor by SUVmean (HR 0.633, 95% CI 0.197–2.040; p = 0.444) appeared to be correlated to the occurrence of MACE at follow-up, confirming that metabolic response defined by changes in LMA was the only significant variable associated with the outcome event.
Discussion
The present study analyzes the prognostic impact of serial quantitative FDG-PET analysis in patients affected by CS and presenting with drug-refractory VT who underwent CA. The major findings are as follows: (1) a reduction in the extent of inflammation is associated with better long-term clinical outcome; (2) disease progression is associated with persistence of active inflammation as demonstrated by the inverse relationship between changes in inflammatory activity and changes in systolic function; (3) serial quantitative FDG-PET evaluation can reliably guide titration of immunosuppressive therapy and identify high-risk patients who may need early referral to advanced heart failure management/heart transplant evaluation; (4) in about half of the cases, despite use of immunosuppressive therapy, there was no evidence of metabolic response at PET over follow-up.
Evaluation of myocardial inflammatory activity by FDG-PET has an increasing role in the diagnosis and follow-up of patients affected by CS [10]. However, PET analysis is often based on visual-normalized interpretation with only few reports analyzing a quantitative approach and a substantial lack of standardized methods [4,5,6]. Quantitative FDG-PET analysis has been proven to be more sensitive and specific than visual-normalized interpretation in the diagnosis of CS and to be superior in the evaluation of response to immunosuppression [6, 20]. Recent reports have also pointed out how the persistence of active inflammation is crucial for disease progression. In particular, changes in FDG uptake quantified as metabolic volume or metabolic activity have shown an inverse relationship with changes in systolic function [5]. However, there is a substantial lack of knowledge regarding the association of quantitative changes in FDG uptake with clinical outcomes [20].
Our findings show that quantitative assessment of myocardial inflammation is of extreme importance in determining the patients’ response to immunosuppression. In particular, we found that persistence of active inflammation among serial PET scans is strongly associated with disease progression (systolic function worsening) and worse long-term clinical outcomes (higher risk of VT recurrence, higher incidence of hospitalizations for heart failure and higher mortality). Patients without a metabolic response to therapy presented a 20-fold risk of MACE at follow-up. These data support the utility of serial quantitative PET evaluation as a tool to identify high-risk patients that may benefit from more aggressive immunosuppressive therapy and/or early referral to advanced heart failure management/heart transplant evaluation. Of interest, in almost 50% of cases in our series, we found no evidence of improvement in the PET metabolic activity over follow-up despite use of immunosuppression treatment. This finding challenges the common dogma that patients with sarcoidosis and active cardiac inflammation presenting with recurrent VT should be treated only with immunosuppressive therapy [1, 21, 22]. In these cases, CA may be necessary and unavoidable to achieve VT control even in the presence of persistent or worsening cardiac inflammation [9].
Several methods have been proposed to quantify FDG uptake with SUVmax being the most widely used even if affected by several limitations. Motion related to cardiac and respiratory cycles deteriorates the spatial resolution of PET, increasing the partial-volume effect. While cardiac gating has been proposed as a solution to overcome this undesirable phenomenon, issues related to the respiratory cycle are more challenging and cannot be easily addressed with gating approaches [23]. Most of these difficulties can be overcome by measurement of global tracer accumulation in organs or structures. Global activity has been extensively tested in several studies and allows detection of evidence of disease when regional information is of limited value [24, 25]. Previous experiences have demonstrated the superiority of quantitative parameters taking into account the extension of inflammation in terms of volume of lesion showing abnormal FDG uptake and/or product of SUV and lesion volume compared to SUVmax that considers only the point of highest FDG uptake [26]. Our findings confirm the accuracy and reproducibility of the quantitative approach and its superiority to standard calculation of SUVmax and SUVmean as a predictor of treatment response and, most importantly, as predictor of long-term clinical outcomes.
Study limitations
This was a retrospective observational study summarizing a single-center experience with CA in patients with CS and recurrent VT. The main limitation of this study is the relatively small sample size despite being one the largest reported considering the rare nature of this condition. Moreover, the study included only patients with VT and different stages of the disease which may limit the generalizability of the results to patients with non-arrhythmic manifestations of cardiac sarcoidosis. In addition, since patients underwent CA, it is possible that CA influence the good outcome in patients with an improvement in imaging. Finally, a standardized approach to quantify myocardial inflammation and response to treatment does not exist and none of our patients underwent a cardiac biopsy at baseline and/or during follow-up. As such, our results could not be compared against a gold standard methodology. The choice for the specific approach (i.e. the decision to perform a PET/CT scan before the procedure as well as the decision to repeat it and its timing) was not standardized. Therefore, as expected, we are reporting the results from a multiyear study which evolved due to the growing experience in the management of this rare condition and the publication in 2014 of the first consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis by the HRS [10]. As such, all patients underwent a PET/CT scan within 1 week prior to the ablation procedure. Thereafter, patients with positive FDG uptake underwent a repeat study within 3–6 months after the procedure. All patients received immunosuppressive therapy between the two PET/CT studies. The decision to repeat a PET/CT study in patients with a baseline negative scan or to additional repeat PET/CT studies in patients who showed improvement after initial immunosuppressive therapy was mostly driven by the need to exclude disease reactivation as a cause of worsening clinical conditions (i.e. recurrent arrhythmia, new onset/worsening of heart failure). These considerations as well as the long follow-up period (median 35 months) explain the wide range in the number of serial PET/CT scans we performed in each patient.
Conclusions
This is the first systematic report analyzing the prognostic role of serial quantitative FDG-PET analysis in patients with CS presenting with VT and undergoing CA. Our results confirm that changes in quantitative parameters like LMA correlate with disease progression and can accurately and reproducibly identify patients at high risk of cardiovascular events like VT recurrence, worsening of systolic dysfunction, hospitalizations for heart failure and death or transplant. Consequently, serial quantitative FDG-PET analysis may guide immunosuppressive therapy and help to identify a high-risk sub-group of patients that may benefit from early referral to more advanced therapies such as CA and/or advanced heart failure management/evaluation for heart transplant.
References
Birnie DH, Nery PB, Ha AC, Beanlands RSB. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68:411–21.
Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm. Electrophysiol. 2015;8:87–93.
Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8.
Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21:925–39.
Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–74.
Yokoyama R, Miyagawa M, Okayama H, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015;195:180–7.
Lee P-I, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2017;24:19–28.
Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36.
Muser D, Santangeli P, Pathak RK, et al. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9:e004333.
Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1304–23.
Ohira H, Tsujino I, Yoshinaga K. 18F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2011;38:1773–83.
Cheng VY, Slomka PJ, Ahlen M, Thomson LEJ, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: a randomized controlled trial. J Nucl Cardiol. 2009;17:286–91.
Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–43.
Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–6.
Callans DJ, Ren JF, Michele J, Marchlinski FE, Dillon SM. Electroanatomic left ventricular mapping in the porcine model of healed anterior myocardial infarction. Correlation with intracardiac echocardiography and pathological analysis. Circulation. 1999;100:1744–50.
Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–96.
Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808.
Muser D, Santangeli P, Castro SA, et al. Long-term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy. Circ Arrhythm Electrophysiol. 2016;9.
Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Springer; 2015.
Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2016. Available at: http://springerlink.bibliotecabuap.elogim.com/10.1007/s12350-016-0490-7. Accessed September 3, 2016.
Young L, Sperry BW, Hachamovitch R. Update on treatment in cardiac sarcoidosis. Curr Treat Options Cardiovasc Med. 2017;19. Available at: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11936-017-0539-1. Accessed June 14, 2017.
Houston BA, Park C, Mukherjee M. A diagnostic and therapeutic approach to arrhythmias in cardiac sarcoidosis. Curr Treat Options Cardiovasc Med. 2016;18. Available at: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11936-016-0439-9. Accessed June 14, 2017.
Salavati A, Borofsky S, Boon-Keng TK, et al. Application of partial volume effect correction and 4D PET in the quantification of FDG avid lung lesions. Mol Imaging Biol MIB Off Publ Acad Mol Imaging. 2015;17:140–8.
Beheshti M, Saboury B, Mehta NN, et al. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography--a novel concept. Hell J Nucl Med. 2011;14:114–20.
Abdulla S, Salavati A, Saboury B, Basu S, Torigian DA, Alavi A. Quantitative assessment of global lung inflammation following radiation therapy using FDG PET/CT: a pilot study. Eur J Nucl Med Mol Imaging. 2014;41:350–6.
Basu S, Saboury B, Werner T, Alavi A. Clinical utility of FDG–PET and PET/CT in non-malignant thoracic disorders. Mol Imaging Biol. 2010;13:1051–60.
Funding
Pennsylvania Steel Company EP Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosures
The authors declare that there is no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Muser, D., Santangeli, P., Castro, S.A. et al. Prognostic role of serial quantitative evaluation of 18F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging 45, 1394–1404 (2018). https://doi.org/10.1007/s00259-018-4001-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4001-8