Abstract
To assess the image quality and radiation exposure of 320-row area detector computed tomography (320-ADCT) coronary angiography with optimal tube voltage selection with the guidance of an automatic exposure control system in comparison with a body mass index (BMI)-adapted protocol. Twenty-two patients (study group) underwent 320-ADCT coronary angiography using an automatic exposure control system with the target standard deviation value of 33 as the image quality index and the lowest possible tube voltage. For comparison, a sex- and BMI-matched group (control group, n = 22) using a BMI-adapted protocol was established. Images of both groups were reconstructed by an iterative reconstruction algorithm. For objective evaluation of the image quality, image noise, vessel density, signal to noise ratio (SNR), and contrast to noise ratio (CNR) were measured. Two blinded readers then subjectively graded the image quality using a four-point scale (1: nondiagnostic to 4: excellent). Radiation exposure was also measured. Although the study group tended to show higher image noise (14.1 ± 3.6 vs. 9.3 ± 2.2 HU, P = 0.111) and higher vessel density (665.5 ± 161 vs. 498 ± 143 HU, P = 0.430) than the control group, the differences were not significant. There was no significant difference between the two groups for SNR (52.5 ± 19.2 vs. 60.6 ± 21.8, P = 0.729), CNR (57.0 ± 19.8 vs. 67.8 ± 23.3, P = 0.531), or subjective image quality scores (3.47 ± 0.55 vs. 3.59 ± 0.56, P = 0.960). However, radiation exposure was significantly reduced by 42 % in the study group (1.9 ± 0.8 vs. 3.6 ± 0.4 mSv, P = 0.003). Optimal tube voltage selection with the guidance of an automatic exposure control system in 320-ADCT coronary angiography allows substantial radiation reduction without significant impairment of image quality, compared to the results obtained using a BMI-based protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary computed tomography (CT) angiography has been widely used as a primary screening tool for symptomatic patients with suspected coronary artery disease due to its high negative predictive value [1]. Although coronary CT angiography had initially been associated with relatively high radiation exposure levels, various methods such as lower tube voltages, electrocardiography (ECG)-based tube current modulation, and prospective ECG triggering have allowed notable reductions in radiation doses for coronary CT angiography [2]. Automatic exposure control (AEC) systems have also lowered the radiation dose while preserving diagnostic image quality by individually adjusting the tube current according to the patient’s attenuation and body habitus [3]. AEC systems for multi-detector CT scanners are now available from all major scanner manufactures under different names, but there have been some limitations in their use for cardiac imaging due to the technical difficulties in conjunction with ECG-based tube current modulation. To date, there have been few studies that have shown that an AEC system using attenuation-based tube current modulation (CareDose 4D; Siemens Medical Solutions, Erlangen, Germany) can reduce radiation exposure, reporting reductions of 22–50 %, for cardiac CT with preserved image quality [4, 5].
Recently, an AEC system (SUREExposure 3D; Toshiba Medical Systems, Otawara, Japan) that has expanded its function to include cardiac CT has been developed. Among the various ways in which the AEC system operates, SUREExposure adopts the standard deviation (noise) as a measure of image quality and aims to match the image noise to the targeted standard deviation [3]. Considering the diagnostic task at hand, SUREExposure 3D can provide optimal tube current modulation at each different tube voltage so that a user-chosen image noise value can be maintained. Using lower tube voltages has great advantages, especially in cardiac CT, resulting in higher contrast and preserved signal-to-noise ratios (SNR) when appropriately applied [1]. Until recently, many approaches have used body weight or body mass index (BMI) to manually select the tube voltage and tube current for cardiac CT applications [6–9]. However, these approaches have been documented to be limited, as the attenuation and body habitus in the thorax are often discordant with weight and BMI [10]. Considering that AEC systems are adaptive to the attenuation and body profile of the individual patient, we hypothesized that coronary CT angiography with optimal tube voltage selection guided by an image noise-targeted AEC system (SUREExposure 3D) could allow a significant reduction in radiation without a loss of image quality, as compared with that achieved using a BMI-based protocol. Thus, the purpose of our study is to assess the image quality and radiation exposure of 320-ADCT coronary angiography with optimal tube voltage selection and an image noise-targeted AEC system in comparison with those of a BMI-adapted protocol.
Materials and methods
Patients
Our Institutional Review Board approved this retrospective study and waived the requirement for informed consent. From January 2013 to February 2013, 22 consecutive subjects (10 men and 12 women; mean age 61.1 years; range 50–78 years) underwent 320-row area detector CT (320-ADCT) coronary angiography using a built-in AEC system (SUREExposure; Toshiba Medical Systems, Otawara, Japan), which automatically determined the optimal tube current modulation using a targeted noise standard deviation as the image quality index. This modulation considered many of the scanning and reconstruction parameters as well as the diagnostic task at hand.
To establish a control group, a sex- and BMI-matched group of 22 subjects (10 men and 12 women; mean age 62.9 years; range 44–78 years) who underwent 320-ADCT coronary angiography using a BMI-adapted protocol was selected in reverse chronological order from December to October 2012. Table 1 shows the process of establishing the tube voltage and the tube current with this BMI-adapted protocol. BMI matching between the study and control groups was performed using a stringent BMI difference limit with a mean difference of 0.01 ± 1.88 kg/m2.
All of the subjects included in the study were clinically scheduled for cardiac CT for the evaluation of coronary artery diseases. Patients who had previously undergone coronary artery interventions including stenting and/or coronary artery bypass grafts, those who had heart rates higher than 65 beats per minute (bpm) even after beta-blocker premedication, and patients with arrhythmia were excluded. The body weight, height, and BMI were recorded.
CT scanning protocol
All CT examinations were performed using a 320-ADCT scanner (Aquilion ONE; Toshiba Medical Systems, Otawara, Japan). Patients with a pre-scan heart rate of 65 bpm or higher were given 50–100 mg of oral metoprolol (Betaloc; AstraZeneca, Södertälje, Sweden) 45–60 min prior to the CT examination, unless beta blockers were contraindicated. After scanning for calcium scoring, we administrated 0.4 mg of sublingual nitroglycerin (Nitroquick; Ethex, St. Louis, MO). Then, 60 mL of a nonionic contrast medium (Iomeron 400; Bracco Diagnostics, Milan, Italy) was injected into the antecubital vein at 5 mL/sec, followed by 40 mL of normal saline at the same flow rate, with a dual power injector (Stellant; Medrad, Indianola, PA).
In the study group, 22 patients underwent 320-ADCT coronary angiography using the built-in AEC system (SUREExposure), which automatically determined the optimal tube current based on the target noise (standard deviation [SD]) considered as the index for image quality in this system, which was set at 33 for coronary CT angiography, as recommended by the vendor. The tube voltage was selected as the lowest value possible in so far as the tube current stayed within the limit imposed by the restricted heat capacity. In the control group of 22 patients, the tube voltage and tube current were selected according to the patients’ BMI values, as shown in Table 1. All images of both groups were reconstructed using an iterative reconstruction algorithm (adaptive iterative dose reduction 3D, AIDR-3D; Toshiba Medical Systems, Otawara, Japan) with the standard option (Strong: 50 % dose reduction).
The gantry rotation time was 350 ms with the best temporal resolution of 175 ms. As the heart rates of all patients in this study were 67 bpm or less, a single gantry rotation was able to reconstruct all coronary CT angiographic images. Mid-diastolic prospective scanning with an ECG-gated window of 70–80 % of the R–R interval was performed in all subjects.
A field of view (FOV) of a maximal 16 cm in the z-axis, covered by 320 detector rows of 0.5 mm each, permitted the axial volumetric scanning of the range from the mid-ascending aorta to the upper abdomen without table movement. The maximal number of slices was 640 with a 0.5-mm thickness and a 0.25-mm interval using the proprietary double slice technique and cone-beam reconstruction algorithm (coneXact; Toshiba Medical Systems, Otawara, Japan). The number of detectors used for the actual scanning was decided based on the anteroposterior and lateral scanogram images. A medium FOV was selected as it could display all of the relevant cardiac anatomy of every patient within single rotation coverage.
All axial images were reconstructed with a normal soft tissue reconstruction kernel (FC43) with a section thickness of 0.5 mm and a reconstruction interval of 0.5 mm.
CT image analysis
Objective image quality analysis
To analyze the image quality of the coronary arteries objectively, four parameters, the image noise, CT density, SNR, and contrast-to-noise ratio (CNR), were analyzed for each data set by a single reader [11, 12].
Image noise was measured as the standard deviation of CT attenuation measured at the air space outside of the anterior thoracic wall. Subsequently, SNR was calculated by dividing the attenuation of the left main by the image noise. As for CNR, the CT attenuation of the epicardial fat surrounding the artery was measured by placing a region of interest immediately next to the artery and was subtracted from that of the left main coronary artery to find the contrast, which was then divided by the image noise, resulting in the CNR. The four parameters were obtained in the same manner for all images of both groups.
Subjective image quality analysis
For the subjective assessment of image quality, two experienced cardiac radiologists (E.A.P and J.Y.L, having 10 and 3 years of experience, respectively, in cardiac CT) who were blinded to the image groups reviewed the transverse axial CT images in consensus in a randomized order. Decreases in image quality owing to motion and poor gating were not considered in the subjective assessment, as these variables should not be affected by whether the patients belonged in the study group or the control group.
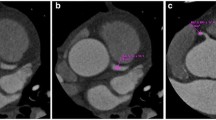
All evaluable coronary artery segments were analyzed using a four-point scale based on the modified American Heart Association 13-segment coronary artery tree [13, 14]; a score of 1 (nondiagnostic) signified impaired image quality with excessive image noise, 2 (adequate) indicated evident limitations in vessel wall definition and in contrast resolution with severe image noise, 3 (good) represented minimal limitations in vessel wall definition and in contrast resolution with moderate image noise, and 4 (excellent) denoted excellent attenuation in the vessel lumen and clear vessel wall definition with barely perceived image noise (Fig. 1).
The thirteen coronary artery segments were categorized into three segmental classes as follows: (1) proximal segments: proximal right coronary artery (RCA), left main coronary artery (LM), proximal left circumflex artery (LCx), and proximal left anterior descending artery (LAD); (2) mid segments: mid RCA, distal RCA, ramus intermedius, obtuse marginalis (OM), first diagonal branch (D1), and mid LAD; and (3) distal segmental classes: posterior descending artery (PDA), distal LCx, second diagonal branch (D2), and distal LAD. Mean scores were calculated for each segmental class as well as for all three segmental classes as a whole.
Estimation of radiation dose
The volume CT dose index (CTDIvol) and the dose-length product (DLP), provided by the scanner system, were documented. The effective dose (mSv) was calculated by multiplying the DLP by a conversion coefficient for the chest (k = 0.014 mSv × mGy−1 × cm−1) as the investigated anatomic region [15].
Statistical analysis
All statistical analyses were performed using a statistical software program (SPSS for Windows, version 17.0; SPSS, Chicago, IL, USA). The results of the two groups were compared with each other using Student’s t test. For all tests, P values less than 0.05 were considered to indicate statistical significance.
Results
There were no significant differences in the patients’ characteristics between the study group and the control group (Table 2). The selected tube voltages used by the AEC-applied and BMI-adapted protocols are listed in Table 3.
Table 4 exhibits the objective and subjective image quality assessment results of the two groups. Although there was a tendency toward higher image noise and vessel attenuation in the study group than in the control group, the differences were statistically insignificant for both image noise (P = 0.111) and vessel attenuation (P = 0.430), as seen in Figs. 2 and 3. Consequently, there were no significant differences in SNR or CNR between the two groups.
CT scans obtained in a 57-year-old woman with a BMI of 24.1 (BMI-adapted group) using window width and level of 1,500 HU and 400 HU, respectively. a Thin-section transverse axial image, b curved multiplanar reformatted image of left anterior descending artery and c three-dimensional volume rendered image. Heart rate was 57 beats per minute. Images were scanned in the axial mode (120 kV and 400 mA) and reconstructed with AIDR 3D. The effective radiation dose was 4.0 mSv. The mean image quality score was 4, the image noise was 5.9 HU, and the left main density was 497 HU. The SNR and CNR were 77.4 and 84.2, respectively
CT scans obtained in a 60-year-old woman with a BMI of 24.1 (AEC-applied group) using a window width and level of 1,500 and 400 HU, respectively. a Thin-section transverse axial image, b curved multiplanar reformatted image of left anterior descending artery and c three-dimensional volume rendered image. Heart rate was 63 beats per minute. Images were scanned in the axial mode (80 kV and 580 mA) and reconstructed with AIDR 3D. The effective radiation dose was 1.1 mSv. The mean image quality score was 4, the image noise was 10.3 HU, and the left main density was 758 HU. The SNR and CNR were 73.6 and 84.3, respectively
The mean values of the total subjective image quality scores showed no significant differences between the two groups (P = 0.960). Furthermore, the mean scores for each segmental class did not significantly differ between the two groups (proximal, 3.57 ± 0.59 vs. 3.64 ± 0.58 for the study group vs. the control group, P = 0.668; mid, 3.47 ± 0.55 vs. 3.59 ± 0.57, P = 0.989; distal, 3.36 ± 0.56 vs. 3.52 ± 0.59, P = 0.593).
Table 4 summarizes the dose parameters of the study and control groups. The mean effective dose was 1.9 ± 0.8 mSv (range 0.2–3.5 mSv) for the study group and 3.6 ± 0.4 mSv (range 2.8–4.1 mSv) for the control group. Radiation exposure was significantly reduced by 42 % in the study group compared with the control group.
Discussion
Our study demonstrated that optimal tube voltage selection guided by an image noise-targeted AEC system (SUREExposure 3D) with iterative reconstruction for 320-ADCT coronary angiography enabled a marked reduction of the mean effective radiation dose by 42 % without significant impairment to either objective or subjective image qualities, as compared to the BMI-adapted protocol with iterative reconstruction.
An AEC system works by modulating the imparted radiation dose via changes in the tube current–time product (mAs) on the basis of each patient’s size and attenuation. AEC systems have a number of potential advantages, including better control of radiation dose, the avoidance of photon starvation artifacts, and a reduced load on the X-ray tube, while being able to maintain image quality in spite of the different attenuation values on CT scans [3]. AEC modulation is performed relatively similarly on most manufacturers’ equipment, although the strength of the clinical performance as well as the definition of how the user specifies a minimum acceptable image quality varies across vendors [3, 16, 17]. In cardiac scanning, two AEC systems are usually considered as commercially available due to the technical difficulties in conjunction with ECG-based tube current modulation: CareDose 4D and SUREExposure 3D. SUREExposure 3D uses a targeted noise standard deviation as the index for image quality, set at 33 for coronary CT angiography, as image noise, when inconsistent, greatly influences diagnostic performance. Image noise depends not only on X-ray intensity but also on the scatter intensity as well as raw data processing parameters. Thus, SUREExposure 3D incorporates many factors, such as reconstruction parameters (e.g., slice thickness and filter kernel), scanning parameters (e.g., tube filter, tube voltage, tube current, and exposure time), and patient parameters (e.g., attenuation and body habitus) to sustain a consistent image noise at the user-chosen level in the axial plane and along the z-axis. It is also equipped with recommended protocol-specific settings so that the tube current can be easily modulated to suit the diagnostic task at hand. In terms of 320-ADCT covering the whole heart in one rotation scan, z-axis tube current modulation is not applied, resulting in a fixed exposure along the z-axis; xy-modulation may play a role in the AEC system. Considering that the body habitus and attenuation distribution along the z-axis would not have much variation through the heart thorax, the z-axis modulation seems not to have much impact on radiation dose reduction. The advantage of an image noise-targeted AEC system (SUREExposure 3D) over a reference mAs-targeted AEC system (CareDose 4D) is the capacity to allow optimal tube current modulation according to each different tube voltage while maintaining constant image noise.
Extensive studies have proven the feasibility and usefulness of empirical tube voltage lowering [1, 18–20]. Although dose reductions are linear with respect to tube current reduction, decreases in tube voltage result in an exponential drop in radiation exposure [21]. Another advantage of a lower tube voltage is that it creates higher attenuation levels of iodinated contrast media, compensating for the higher image noise if applied adequately. In addition, until now, most previous studies have used BMI or body weight to select the proper lower tube voltage for each patient. However, Ghoshhajra et al. [22] recently reported a wide discrepancy (39 % discordance) between BMI and chest size, suggesting that BMI may not be the best parameter for tube voltage selection. They also employed an automated attenuation-based tube voltage selection algorithm (CareKV; Siemens Medical Solutions, Erlangen, Germany), which had been recently introduced in body CT as well as cardiac CT. The CareKV program allows the user to select one of twelve settings depending on the diagnostic task at hand and recommends a tube voltage of 70, 80, 100, 120 or 140 kVp, which provides the tube current profile that has the lowest radiation dose as determined by the attenuation and the body habitus of the patient and the study objective [23]. Ghoshhajra et al. [22] found that this automatic tube voltage selection software improved image quality at a similar radiation dose compared with their standard BMI-based group. In another study, Suh et al. [24] reported that the combination of automatic tube voltage selection with tube current modulation using the iterative reconstruction technique in coronary CT angiography not only improved image quality, but also reduced the radiation dose by up to 30 % on average, while maintaining diagnostic accuracy compared to a BMI-based protocol with filtered back projection. The difference between SUREExposure 3D and CareKV is the target value: SUREExposure 3D performs tube current modulation by keeping the image noise constant according to each different tube voltage and users can select the tube voltage manually. Low tube voltages can be safely used to maintain a constant level of image noise regardless of a patient’s BMI value. On the other hand, CareKV offers tube voltage and tube current modulation to maintain the CNR. Another point of distinction from Suh et al.’s study [24] was that we compared iterative reconstructed images in both groups. Even when compared to a BMI-based protocol with iterative reconstruction, we found that the SUREExposure 3D system reduced the radiation dose by up to 40 % on average without any impairment of image quality.
There were some limitations to our study, with the major limitation being that this was a retrospective study performed in a single institution. Although we attempted to minimize confounding factors by matching the sex and BMI values of the two groups, we were not able to perform an intra-individual comparison of signal and dose parameters, as it was not ethically feasible. In addition, the diagnostic accuracy of measurements for the detection of coronary artery disease was not evaluated. Subsequent studies assessing the diagnostic accuracy of this protocol using the AEC system is warranted.
In conclusion, 320-ADCT coronary angiography with optimal tube voltage selection guided by an image noise-targeted tube current modulation system allowed a marked reduction in radiation dose of 42 %, on average, without significant impairment in image quality compared to that attained using a BMI-based protocol.
Abbreviations
- 320-ADCT:
-
320-Row area detector CT
- 3D:
-
Three-dimensional
- AEC:
-
Automatic exposure control
- AIDR:
-
Adaptive iterative dose reduction
- BMI:
-
Body mass index
- Bpm:
-
Beats per minute
- CNR:
-
Contrast-to-noise ratio
- CT:
-
Computed tomography
- CTDIvol :
-
Volume CT dose index
- D1:
-
First diagonal branch
- D2:
-
Second diagonal branch
- DLP:
-
Dose-length product
- ECG:
-
Electrocardiography
- ED:
-
Effective dose
- FOV:
-
Field of view
- HR:
-
Heart rate
- HU:
-
Hounsfield unit
- LAD:
-
Left anterior descending artery
- LCx:
-
Left circumflex artery
- LM:
-
Left main coronary artery
- OM:
-
Obtuse marginalis
- PDA:
-
Posterior descending artery
- RCA:
-
Right coronary artery
- SNR:
-
Signal-to-noise ratio
References
Park EA, Lee W, Kang JH et al (2009) The image quality and radiation dose of 100-kVp versus 120-kVp ECG-gated 16-slice CT coronary angiography. Korean J Radiol 10(3):235–243
Kurita T, Sakuma H, Onishi K et al (2009) Regional myocardial perfusion reserve determined using myocardial perfusion magnetic resonance imaging showed a direct correlation with coronary flow velocity reserve by Doppler flow wire. Eur Heart J 30(4):444–452
Lee CH, Goo JM, Ye HJ et al (2008) Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics 28(5):1451–1459
Deetjen A, Mollmann S, Conradi G et al (2007) Use of automatic exposure control in multislice computed tomography of the coronaries: comparison of 16-slice and 64-slice scanner data with conventional coronary angiography. Heart 93(9):1040–1043
Francone M, Di Castro E, Napoli A et al (2008) Dose reduction and image quality assessment in 64-detector row computed tomography of the coronary arteries using an automatic exposure control system. J Comput Assist Tomogr 32(5):668–678
Jung B, Mahnken AH, Stargardt A et al (2003) Individually weight-adapted examination protocol in retrospectively ECG-gated MSCT of the heart. Eur Radiol 13(12):2560–2566
Tatsugami F, Husmann L, Herzog BA et al (2009) Evaluation of a body mass index-adapted protocol for low-dose 64-MDCT coronary angiography with prospective ECG triggering. AJR Am J Roentgenol 192(3):635–638
Yu L, Fletcher JG, Grant KL et al (2013) Automatic selection of tube potential for radiation dose reduction in vascular and contrast-enhanced abdominopelvic CT. AJR Am J Roentgenol 201(2):W297–W306
George RT, Arbab-Zadeh A, Miller JM et al (2009) Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging 2(3):174–182
Kitagawa K, Sakuma H, Nagata M et al (2008) Diagnostic accuracy of stress myocardial perfusion MRI and late gadolinium-enhanced MRI for detecting flow-limiting coronary artery disease: a multicenter study. Eur Radiol 18(12):2808–2816
Leipsic J, Labounty TM, Heilbron B et al (2010) Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 195(3):649–654
Bittencourt MS, Schmidt B, Seltmann M et al (2011) Iterative reconstruction in image space (IRIS) in cardiac computed tomography: initial experience. Int J Cardiovasc Imaging 27(7):1081–1087
Chun EJ, Lee W, Choi YH et al (2008) Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr 32(1):86–92
Yoo RE, Park EA, Lee W et al (2013) Image quality of adaptive iterative dose reduction 3D of coronary CT angiography of 640-slice CT: comparison with filtered back-projection. Int J Cardiovasc Imaging 29(3):669–676
The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP 37(2–4):1–332
Raman SP, Johnson PT, Deshmukh S et al (2013) CT dose reduction applications: available tools on the latest generation of CT scanners. J Am Coll Radiol 10(1):37–41
Mulkens TH, Bellinck P, Baeyaert M et al (2005) Use of an automatic exposure control mechanism for dose optimization in multi-detector row CT examinations: clinical evaluation. Radiology 237(1):213–223
Hausleiter J, Martinoff S, Hadamitzky M et al (2010) Image quality and radiation exposure with a low tube voltage protocol for coronary CT angiography results of the PROTECTION II Trial. JACC Cardiovasc Imaging 3(11):1113–1123
Hausleiter J, Meyer T, Hadamitzky M et al (2006) Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation 113(10):1305–1310
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301(5):500–507
Hsieh J (2003) Computed tomography: principles, design, artifacts, and recent advances, 2nd edn. SPIE Press, Bellingham
Ghoshhajra BB, Engel LC, Karolyi M et al (2013) Cardiac computed tomography angiography with automatic tube potential selection: effects on radiation dose and image quality. J Thorac Imaging 28(1):40–48
Siegel MJ, Ramirez-Giraldo JC, Hildebolt C et al (2013) Automated low-kilovoltage selection in pediatric computed tomography angiography: phantom study evaluating effects on radiation dose and image quality. Invest Radiol 48(8):584–589
Suh YJ, Kim YJ, Hong SR et al (2013) Combined use of automatic tube potential selection with tube current modulation and iterative reconstruction technique in coronary CT angiography. Radiology 269(3):722–729
Acknowledgments
This study was supported by Grant no. 06-2012-3020 from the Toshiba Medical Systems Research Fund.
Conflict of interest
Dr Shim is an employee of Toshiba Medical Systems Korea. None of the other authors have disclosed any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, J., Park, EA., Lee, W. et al. Image quality and radiation reduction of 320-row area detector CT coronary angiography with optimal tube voltage selection and an automatic exposure control system: comparison with body mass index-adapted protocol. Int J Cardiovasc Imaging 31 (Suppl 1), 23–30 (2015). https://doi.org/10.1007/s10554-015-0594-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0594-1