Abstract
Purpose
To compare the impact of a 100 kV tube voltage protocol to 120 kV in terms of image quality and radiation dose by a 320 row coronary computed tomography angiography (CCTA) with automatic exposure control (AEC).
Materials and methods
Using a propensity matched analysis we compared a group of 135 patients scanned using a 100 kV tube voltage protocol with a group of 135 subjects scanned employing a 120 kV tube voltage setting. In all subjects the heart rate (HR) was <65 bpm and all CT scans were acquired using a prospective ECG gating and AEC strategy. Mean effective radiation dose and subjective and objective (Noise or N, signal to noise ratio or SNR, contrast to noise ratio or CNR) image quality, were evaluated. Subjective quality was assessed by two experienced radiologists using a 5-point scale (0: non diagnostic—4: excellent) using the 15-segment American Heart Association (AHA) coronary artery classification.
Results
Mean effective dose and noise were non significantly different between the two groups: mean effective dose was 2.89 ± 0.7 mSv in the 100 kV group and 2.80 ± 0.57 mSv in the 120 kV group (p = 0.25) while noise was 28.9 ± 3.3 in the 120 kV group and 29.05 ± 3.6 in the 100 kV group (p = 0.72). Both SNR and CNR were significantly higher in the 100 kV group than in the 120 kV group. This data agrees with the evidence that subjective quality was significantly higher in the 100 kV group in the middle and distal segmental classes.
Conclusion
Our study shows that, in using a 320 row CCTA with AEC strategy it is better to employ a 100 kV tube voltage protocol because compared to 120 kV tube voltage setting, it appears to significantly improve both subjective and objective image quality without decreasing the mean effective radiation dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is the leading cause of death in developed countries [1–3] and, although coronary catheterisation remains the diagnostic gold standard in the evaluation of coronary arteries, several studies demonstrated that CCTA (coronary computed tomography angiography), thanks to its high sensitivity and negative predictive value, represents an effective alternative in the assessment of suspected CAD [3–5]. Concerns over radiation risks of CCTA prompted the CT scanner manufacturers to develop several techniques to lower the radiation exposure, such as ECG- based tube current modulation, prospective ECG gating, noise reduction filters, automatic exposure control systems, low tube voltage protocols and iterative methods of image reconstruction (ASIR, MBIR, IRIS, AIDR, iDose) [3]. All these dose-reducing technologies are widely employed in latest CT scanners, which are multislice and have high rotation tube speed. Several studies showed that a low tube voltage protocol allows to lower mean radiation dose keeping diagnostic image quality.
Although, to the best of our knowledge, there is no study relating the effects of a low tube voltage protocol with the effective radiation dose and the image quality using an automatic exposure control system. In this regard, the main purpose of this study is to evaluate the impact of a 100 kV tube voltage protocol on image quality and radiation dose and compare them with a 120 kV tube voltage setting.
Materials and methods
Patients
In this active control trial, a total of 550 subjects, referred for coronary-CT angiography (CCTA) at San Salvatore Hospital of L'Aquila, were scanned with 320-row CT scanner (Aquilion One, Toshiba Medical Systems). Inclusion criteria were: typical or atypical chest pain, dyspnea, and previous uncertain or inconclusive stress tests. Exclusion criteria were: poor kidney function (serum creatinine >1.5 mg/dl), prior coronary artery surgery, coronary stents, heart rate (HRs) >65 beats per minute (bpm) after beta-blocker treatment. To obtain two groups virtually randomized for important clinical characteristics subjects a matched propensity analysis was employed. The first group was composed of 135 subjects scanned using a standard tube voltage protocol (120 kV). This group was compared with a pool of subjects (135 patients) scanned employing a low tube voltage setting (100 kV). The variables included in the multivariate matched propensity analysis were age, gender, weight, and BMI. In both groups tube current (mA) was adjusted by an automatic exposure control system (Sure Exposure 3D) and images were reconstructed by the adaptive iterative algorithm (AIDR-3D). The images were visually assessed for the presence of significant stenosis defined as the reduction in coronary lumen equivalent to or greater than 50 %. Segments including calcification and stents were excluded. Written informed consent before CT scan was obtained from all individual participants and the study was approved by the San Salvatore Hospital IRB.
Acquisition protocol
All CT scans were performed using a 320 row CT scanner. One hour prior the CT acquisition, blood pressure and heart rate of all subjects were measured and registered. Subjects with a heart rate equivalent to 65 bpm or greater received 5–20 mg of intravenous atenolol (Tenormin®, AstraZeneca, Sweden). All subjects received 5 mg of sublingual nitroglycerin (isosorbidedinitrate, Carvasin, Wyeth Lederle) prior the imaging. Sixty milliliteres of nonionic contrast medium (Visipaque 320; Ge Healthcare Srl, Milan, Italy) was injected into the antecubital vein at a rate of 6 ml/s, followed by 40 mL of saline solution at the same flow rate. A circular region of interest of the bolus tracking technique (SUREStartTM) was placed in the descending aorta with a start scan threshold of 300HU. CT scan was performed in inspiratory apnea. The gantry rotation time was 350 ms, with the best temporal resolution of 175 ms. ECG prospective gating with a mid diastolic acquisition centered at 70–80 % of the R–R was used. The maximum available range along z axis is 16 cm covered by 320 row detectors of 0.5 mm each. AEC (automatic exposure control) adjusts the tube current to keep a user-specified noise level in the image data [3, 6]. SureExposure (Toshiba Medical, Tokyo, Japan), the AEC systems that we employed, supplies both patient-size and z-axis AEC [3, 6]. This system established the tube current (50–500 mA) basing on projection data receiveds from a scanogram [3, 6]. The apt tube current is applied at the maximum water equivalent diameter to achieve the selected standard deviation (noise level) [3, 6]. In this study, we employed one of the latest iterative reconstruction system, the AIDR-3D, which operates in both the raw data and the image domain. The AIDR-3D performs an automatic weighted combination between an original FBP image and an image obtained from the iterative process. In the process of setting the mA, the AEC system considers the AIDR-3D which is incorporated in the current modulation system. The target noise value (expressed as SD, standard deviation) was set as 33 (SD33). For each patient, the system (PhaseXact; Toshiba Medical Systems Corporation, Tochigi, Japan) traced a movement diagram of the sinogram and automatically selected the phase with the least movement artifact. Reconstruction was made with data from a single heartbeat. The maximal number of reconstructed slices was 640 with 0.3 mm thickness and 0.25 mm interval by the means of the proprietary double slice technique and cone-beam reconstruction algorithm (ConeXact; Toshiba Medical Systems, Tochiki-ken, Japan).
Subjective image quality analysis
Subjective assessment of images quality was performed by two experienced cardiac radiologists (EDC and AG), who were blinded to details of CT datasets. Each reviewer evaluated the images independently and in a random fashion. Discordant grades were discussed between the radiologists until a consensus rating was reached. Issues, such as poor gating and movement (respiratory and cardiac pulsation) artifacts were not considered because they could not be attributed to the acquisition protocol. The coronary tree was subdivided into 15 segments according to the modified American Heart Association classification [5, 7]. The intermedial artery, if present, was designated as segment 16 [5, 7]. Each radiologist rated the subjective quality of all evaluable coronary artery segments using a 5-point scoring system graded as follow: 4 (excellent) = excellent wall delineation and opacification of the artery lumen, without movement artifacts and noise-associated blurring; 3 (very good) = very good wall delineation and opacification of the artery lumen, with minimal movement artifacts and image noise; 2 (good) = good wall delineation and opacification of the artery lumen, with moderate movement artifacts and image noise; 1 (adequate) = severely impaired wall delineation and opacification of the artery lumen, because of severe movement artifacts and/or image noise; 0 (non-diagnostic) = poor artery wall delineation (because of severe movement artifact and/or marked image noise- associated blurring) and lack of vessel attenuation [5, 8–10]. The radiologists analyzed internal and external wall delineation, the grade of motion-related artifacts, the differentiation between artery lumen and plaque (calcified and non calcified) [5]. Nevertheless, the end evaluation was an assessment of the general appearance of the artery and the probability of getting a confident diagnosis.
Objective image quality assessment
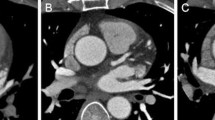
Objective Image Quality assessment of the proximal coronary arteries was performed by two experienced cardiac radiologists (XX and YY), who, as previously indicated, assessed noise (N), CT density (HU), signal to noise ratio (SNR) and contrast to noise ratio (CNR) [5, 8, 11]. The noise image was measured as the standard deviation of CT density which was determined by drawing the largest possible region of interest (ROI) at the aortic root above the left coronary ostium with concern to avoid inclusion of the artery wall (Fig. 1a) [5, 9, 12, 13]. Signal (IV, Internal Vessel) of the proximal coronary arteries was determined as the mean attenuation value (Hounsfield units, HU) within round ROIs placed in the middle part of both left main coronary artery and right coronary artery, with concern to avoid inclusion of the coronary vessel wall (Fig. 1b, c) [5, 13].
CT density of the epicardial fat (EV, External Vessel) around the artery was determined by drawing the ROI just alongside the artery (both left main coronary artery and right coronary artery) [5, 10]. Signal to noise ratio (SNR) and contrast to noise ratio (CNR) were measured as follows: SNR = IV/N, CNR = (IV − EV)/N; SNR was estimated by dividing the density by the image noise and, as for the CNR, CT density of the epicardial fat (EV) was subtracted from signal (IV), which was then divided by the image noise [5, 10].
Estimation of radiation dose
The effective radiation dose of each CCTA study was estimated using an approach suggested by the European Working Group for Guidelines on Quality Criteria in CT [11]. The effective radiation dose was measured multiplying the DLP (Dose Length Product) value by an organ weighting factor for the chest as the examined anatomical structure (k = 0.014 mSv·mGy-1·cm-1), that is deemed to result from the most reliable data set and is balanced between female and male subjects [11].
Statistical analysis
Continuous variables were condensed by the means, standard deviation (SD), standard error (SE) or 95 % CI as appropriate. Differences in continuous variables were analyzed by Student’s t test. Differences in categorical variables were compared with Pearson’s Chi square test with Yates’ continuity correction. Fisher’s exact test was used when data frequencies expected in the contingency tables were less than five. To reduce selection bias and determine technique effectiveness a case control matched propensity analysis was performed [14] using a multivariate logistic regression. Propensity score was arranged using as covariates gender, age, weight and BMI. P values <0.05 were considered statistically significant. Interobserver agreement for image quality was calculated with Cohen k statistic [15], which was interpreted as poor (k < 0.20), fair (k = 0.21–0.40), moderate (k = 0.41–0.60), good (k = 5 0.61–0.80), very good (k = 0.81–0.90) or excellent (k > 0.91). The SPSS® version 13.0 was used for statistical analysis and graphic presentation.
Results
In the final analysis 270 out of 550 subjects were studied. 56 patients were excluded because of prior coronary artery surgery (coronary artery by-pass graft), 31 patients because of a heart rate (HRs) >65 beats per minute (bpm) after beta-blocker treatment and 193 subjects because they did not have overlapping propensity scores.
In Table 1 demographic and clinical features of the study population were given. There were no significant differences between the two groups regarding age, cardiovascular risk factors, clinical presentations, HR, scanning range and length (13.524 cm in 100 kV group and 13.39 cm in 120 kV group). In the 120 kV group, 40 patients (29.6 %) did not have significant stenosis, 47 (34.8 %) had one-vessel disease, 27 (20 %) had two- vessel disease and 21 (15.6 %) had three-vessel disease. In the 100 kV group, 43 patients (31.8 %) did not have significant stenosis, 44 (32.6 %) patients had one-vessel disease, 23 (17 %) patients had 2-vessel disease, 25 patients had 3-vessel disease (18.6 %).
Subjective and objective evaluation of image quality
Among 2160 potentially assessable segments, 449 segments in the 100 kV group and 461 in the 120 kV group, respectively, were deemed to be non assessable because too small (diameter <1.5 mm; 124 and 131 segments, respectively) or entirely occluded (11 and 9 segments, respectively). Interobserver agreement of subjective image quality was deemed as ‘‘very good’’ for both groups (k = 0.88 for the 100 kV group and 0.86 for the 120 kV group). In the 100 kV group, 605 segments (35.4 %) were graded as 4 (=excellent), 985 segments (57.5 %) as 3 (=very good), 90 segments (5.3 %) as 2 (=good), 25 segments (1.5 %) as 1 (= adequate), 6 segments (0.3 %) as 0 (=non-diagnostic). In the 120 kV group, 510 segments (30 %) were graded as 4; 965 segments (56.8 %) as 3; 174 segments (10.2 %) as 2; 34 segments (2 %) as 1; 16 segments (0.9 %) as 0 (Table 2). The rate of non-diagnostic segments (0.9 %) was significantly higher statistically in the 120 kV group (p = 0.032) although the percentage of subjects with at least one non-diagnostic segment did not reach a significant difference (p = 0.26) [4/135 (2.9 %) and 9/135 (6.6 %) in the 100 and 120 kV groups, respectively].
Objective image quality analysis
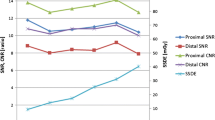
In Table 3 the results of objective image quality analysis were given. Noise was not significantly different between the two group (p = 0.72) while the 100 kV group showed a significant increase of SNR and CNR (Table 3) compared to 120 kV group (Fig. 2). A 0.15 (95 % CI of mean difference −0.68 to 0.72) mean Noise increment, a 1.9 (95 % CI of mean difference −2.72 to 1.07) mean SNR increment and a 2.2 (95 % CI of mean difference −3.0 to −1.4) CNR increment was observed at the level of LM. At the level of RCA, a 1.83 (95 % CI of mean difference −2.53 to −1.31) mean SNR increment and a 2.1 (95 % CI of mean difference −2.86 to −1.34) mean CNR increment (Table 3) was observed. The mean effective radiation dose was 2.89 ± 0.7 mSv in the 120 kV Group and 2.80 ± 0.57 mSv in the 100 kV Group with no significant difference (p = 0.25) (Table 3).
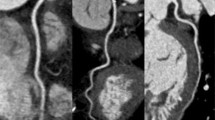
Coronary computed tomography angiography (CCTA) images obtained with an AEC (Automatic Exposure Control) system and, respectively, a 120 kV tube voltage protocol (a, b, c) and a 100 kV tube voltage setting (d, e, f). The two subjects have similar body mass index (BMI): patient in (a, b, c) images has BMI of 28.7 while subject in (d, e, f) images has BMI of 28.3. CCTA images obtained with a 120 kV tube voltage setting show a CT density of 442 HU and a noise of 30.7, while CCTA images acquired with a 100 kV tube voltage protocol present a CT density of 617.7HU and a noise of 31.6. Mean effective dose was similar between the two subjects: 2.9 mSv in the 120 kV protocol and 2.8 mSv in the 100 kV setting
Discussion
Studies conducted over the past years using 64-slices, DS 64-slices and 320-row CT scanners have demonstrated that the use of low tube voltage is an important tool to lower radiation exposure because the latter is proportional to the square of tube voltage [16–18]. Nevertheless, low tube voltage is inevitably accompanied by an increase in image noise and, thus, its use is advisable only for patients with a BMI (Body Mass Index) lower than 25–30 [18–20]. We suggest that BMI is only a rough index of patient attenuation profile because it gives no information about the distribution of body weight. Therefore, we agree with Blankstein et al. [21] who selected the tube voltage (kV) and current (mA) not only on the basis of patient’s BMI but also according to clinical indications, patient’s body shape and, above all, chest wall attenuation. This was performed by both visual examination of the patient prior the scan and by evaluation of the coronal scout image and axial test-bolus image (obtained before the scan) [21]. Blankstein et al. [21] was able, using this approach, to lower substantially the radiation exposure without compromising the image quality. However, as concluded by these authors, this approach is not completely realistic in the clinical routine due to its subjective nature and because it is not always possible to have a physician experienced in cardiac imaging technique at every scan. AEC (automatic exposure control) allows to overcome these issues. Indeed, the operator selects the highest level of acceptable image noise and system sets consequentially the tube current (mA) [3]. The range in tube current is narrow (50–500 mA) and, thus, when the patient attenuation profile requires more than 500 mA, the system increases also the tube voltage to avoid underexposure. The system also works in the same way when the patient attenuation profile requires less than 50 mA and, in this case, reduces the tube voltage to avoid overexposure. Thus, AEC is a system to optimize exposure parameters [3]. Our study, performed with a 320-detector CT scanner, evaluated the effects, on radiation exposure and image quality, of a 100kv protocol in comparison with a conventional 120 kV protocol demonstrating that a 100 kV protocol, in association with AEC, significantly improves the image quality without reducing the radiation exposure. Subjective image quality analysis showed that the rate of non-diagnostic segments was significantly higher in the 120 kV group although this figure did not parallel with the percentage of subjects with at least one non-diagnostic segment. Furthermore, the percentage of segments scored as 4 (excellent) was significantly higher in the 100 kV group for both middle and distal segmental classes. These results were achieved without significantly different radiation exposure, which was similar in both group. To the best of our knowledge, only one previous study [18] evaluated the effects, on radiation exposure and image quality, of a 100kv protocol in comparison with a conventional 120 kV protocol. Zhang et al. [19] reported a 54 % reduction in radiation exposure in 100 kV group without a significantly difference in image quality. Our data may seem to be in contradiction with Zhang’s results [18] but this discrepancy may be explained by a number of different parameters included in our study. In his study, Zhang [19] used a 100 kV protocol only in subjects with BMI <25, while using a 120 kV protocol for overweight patients. Furthermore, he [19] employed no automatic exposure control system but adjusted the tube current settings according to BMI. Our data demonstrated that, using an AEC and selecting the same level of acceptable image noise, a change in the tube voltage (100 or 120 kV) has no effect on the radiation exposure because AEC increases the tube current to maintain the same image quality, but increases signal, signal to noise ratio and contrast to noise ratio (Fig. 2). These results can be explained by the fact that a lower tube voltage translates into lower effective photon energy (effective photon energy is approximately one half of the kV) and, when the effective photon energy is closer to the K-edge of iodine (33.2 keV), CT attenuation increases [21]. Without the use of an automatic exposure control system, a low tube voltage protocol is inevitably accompanied by an increase in image noise, and, thus, without a careful selection of eligible patients, it leads to a lower image quality, which could compromise the diagnostic quality of the images. An increase in image noise is possible also using an AEC but, in this case, only by selecting a higher level of acceptable image noise. We would like to evaluate, in the future, image quality and radiation exposure of protocol with higher level of acceptable image noise to identify the noise level, which offers the best compromise between image quality and radiation exposure. As demonstrated in previous study [22–24], the contrast material has to produce a high intra-coronary attenuation, that allows more reliable visualization of coronary arteries. Nonetheless, in accordance with La Grutta et al. [24], which recently demonstrated that different iodinated CM have an analogous impact on plaque attenuation profile (in terms of SNR and CNR) depending on the iodine load, we employed low concentration CM (320 mgI/mL) with high flow-rate of 6 mL/s and, consequently, high iodine load (IDR = 1.920 gI/s) in both protocols. A focal point of our study is the use of a propensity analysis, which helped us to obtain two groups of subjects virtually randomized for main clinical characteristics and made the results less prone to methodological biases in comparison with other usual statistical methods. Our study has some limitations. First, the image quality scoring system was largely subjective and thus potentially biased. We also did not test the diagnostic accuracy with coronary catheterisation correlation. In our opinion, the latter is the main limitation because we did not assess the diagnostic accuracy of a 100 kV protocol in the evaluation of calcified plaque, as the severity of calcified plaque is the most likely to be overestimated using a 100 kV protocol considering that, theoretically, a low voltage setting may increase blooming artifacts [25, 26]. Nevertheless, unlike Zhang [19], we did not report a higher number of unassessable segments due to calcification in 100 kV group than in 120 kV one.
Conclusion
Our study shows that, using a 320 row CCTA with AEC, it is preferable to use a 100 kV tube voltage setting because compared to 120 kV tube voltage protocol, it seems to significantly improve subjective and objective image quality, without significantly lowering the mean effective radiation dose. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its later amendments.
References
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2010) Executive summary: heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 121(7):948–954. doi:10.1161/CIRCULATIONAHA.109.192666
Einstein AJ, Henzlova MJ, Rajagopalan S (2007) Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 298(3):317–23
Di Cesare E, Gennarelli A, Di Sibio A, Felli V, Splendiani A, Gravina GL, Barile A, Masciocchi C (2014) Assessment of dose exposure and image quality in coronary angiography performed by 640-slice CT: a comparison between adaptive iterative and filtered back-projection algorithm by propensity analysis. Radiol Med 119(8):642–649. doi:10.1007/s11547-014-0382-3
Di Cesare E, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, Di Renzi P, Esposito A, Faletti R, Fattori R, Francone M, Giovagnoni A, La Grutta L, Ligabue G, Lovato L, Marano R, Midiri M, Natale L, Romagnoli A, Russo V, Sardanelli F, Cademartiri F; Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM) (2012) Clinical indications for cardiac computed tomography. From the Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM). Rad Med 117(6):901–938
Di Cesare E, Gennarelli A, Di Sibio A, Felli V, Splendiani A, Gravina GL, Masciocchi C (2015). Image quality and radiation dose of single heartbeat 640-slice coronary CT angiography: A comparison between patients with chronic Atrial Fibrillation and subjects in normal sinus rhythm by propensity analysis. Eur J Radiol 84(4): 631–636. doi: 10.1016/j.ejrad.2014.11.035
Lee CH, Goo JM, Ye HJ, Ye SJ, Park CM, Chun EJ, Im JG (2008) Radiation dose modulationtechniques in the multidetector CT era: from basics to practice. Radiographics 28(5):1451–1459. doi: 10.1148/rg.285075075
Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB (1975). A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51(4 Suppl):5–40
Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, Taylor C, Dunning A, Earls JP (2010) Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 195(3):649–654. doi:10.2214/AJR.10.4285
Park EA, Lee W, Kim KW, Kim KG, Thomas A, Chung JW, Park JH (2012) Iterative reconstruction of dual-source coronary CT angiography: assessment of image quality and radiation dose. Int J Cardiovasc Imaging 28(7):1775–1786. doi:10.1007/s10554-011-0004-2
Chun EJ, Lee W, Choi YH, Koo BK, Choi SI, Jae HJ, Kim HC, So YH, Chung JW, Park JH (2008) Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr 32(1):86–92. doi: 10.1097/rct.0b013e318059befa
Bittencourt MS, Schmidt B, Seltmann M, Muschiol G, Ropers D, Daniel WG, Achenbach S (2011) Iterative reconstruction in image space (IRIS) in cardiac computed tomography: initial experience. Int J Cardiovasc Imaging 27:1081–1087.doi: 10.1007/s10554-010-9756-3
Yoo RE, Park EA, Lee W, Shim H, Kim YK, Chung JW, Park JH (2013) Image quality of adaptive iterative dose reduction 3D of coronary CT angiography of 640-slice CT: comparison with filtered back-projection. Int J Cardiovasc Imaging 29(3):669–676. doi:10.1007/s10554-012-0113-6
Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W (2009) Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol 193(3):764–771. doi: 10.2214/AJR.09.2397
Bongartz G, Golding SJ, Jurik AG et al (2004). European Guidelines for Multislice Computed Tomography. Funded by the European Commission; EUR 16262 EN
Rubin DB (1997) Estimating causal effects from large data sets using propensity scores. Ann Intern Med 127(8 Pt 2):757–763
Cohen J (1960) A coefficient of agreement for nominal scales. EducPsycholMeas 20:37–46
Bischoff B, Hein F, Meyer T, Hadamitzky M, Martinoff S, Schoemig A, Hausleiter J (2009) Impact of a reduced tube voltage on CT angiography and radiation dose: results of the protection I study. JACC Cardiovasc Imaging 2(8):940–946
Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, Alkadhi H, Wildermuth S (2008) Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. EurRadiol 18(9):1809–1817
Zhang C, Zhang Z, Yan Z, Xu L, Yu W, Wang R (2011) 320-row CT coronary angiography: effect of 100-kV tube voltages on image quality, contrast volume, and radiation dose. Int J Cardiovasc Imaging 27:1059–1068. doi:10.1007/s10554-010-9754-5
Alkadhi H, Stolzmann P, Scheffel H, Desbiolles L, Baumuller S, Plass A, Genoni M, Marincek B, Leschka S (2008) Radiation dose of cardiac dual-source ct: the effect of tailoring the protocol to patient-specific parameters. Eur J Radiol 68(3):385–391. doi:10.1016/j.ejrad.2008.08.015
Blankstein R, Bolen MA, Pale R, Murphy MK, Shah AB, Bezerra HG, Sarwar A, Rogers IS, Hoffmann U, Abbara S, Cury RC, Brady TJ (2011) Use of 100 kV versus 120 kV in cardiac dual source computed tomography: effect on radiation dose and image quality. Int J Cardiovasc Imaging 27:579–586. doi:10.1007/s10554-010-9683-3
Szucs-Farkas Z, Verdun FR, von Allmen G, Mini RL, Vock P (2008) Effect of X-ray tube parameters, iodine concentration, and patient size on image quality in pulmonary computed tomography angiography: a chest-phantom-study. Invest Radiol 43(6):374–381. doi:10.1097/RLI.0b013e3181690042
Cademartiri F, Mollet NR, Lemos PA et al (2006) Higher intracoronary attenuation improves diagnostic accuracy in MDCT coronary angiography. AJR Am J Roentgenol 187:W430–W433
LaGrutta L, Galia M, Gentile G et al (2013) Comparison of iodinated contrast media for the assessment of atherosclerotic plaque attenuation values by CT coronary angiography: observations in an ex vivo model. Br J Radiol 86:20120238. doi:10.1259/bjr.20120238
Cademartiri F, La Grutta L, Palumbo A et al (2007) Imaging techniques for the vulnerable coronary plaque. Radiol Med. 112(5):637–659
Makaryus JN, Makaryus AN (2009) Coronary calcification: Achilles’ heel in the assessment for coronary artery disease in patients with symptomatic angina? Int J Cardiovasc Imaging 25(8): 855–857. doi: 10.1007/s10554-009-9535-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Di Cesare, E., Gennarelli, A., Di Sibio, A. et al. 320-row coronary computed tomography angiography (CCTA) with automatic exposure control (AEC): effect of 100 kV versus 120 kV on image quality and dose exposure. Radiol med 121, 618–625 (2016). https://doi.org/10.1007/s11547-016-0643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0643-4