Abstract
The standard protocol in multislice spiral CT (MSCT) angiography for coronary arteries with fixed tube current-time settings leads to an overexposure and thus to an unnecessary high radiation dose in patients with lower weight when compared to heavier patients. The purpose of this study was to estimate the effect of reducing the radiation dose by adapting the tube current-time settings individually. Fifty patients underwent retrospectively ECG-gated MSCT of the heart. In 25 patients (group A1) a standard protocol with constant tube current-time settings was used (4×1-mm collimation, 120 kV, 400 mAseff). Subsequently, artificial image noise was added to the data of these patients simulating a directive for weight-adapted tube current-time settings (group A2). In the other 25 patients (group B) an alternative protocol with individually weight-adapted tube current-time settings was applied. The data of all groups were evaluated by a regression analysis. The image quality was assessed objectively by measuring the CT attenuation in standardised regions of interest and subjectively by three radiologists using a five-point scoring system in a consensus reading. Applying the weight-adapted tube current-time settings the effective radiation dose was reduced by 17.9% for men and 26.3% for women. The standard protocol leads to an overexposure in light patients as seen in the plot of noise vs weight (slope 0.16±0.07 HU/kg). By applying the weight-adapted tube current-time settings a weight-independent, constant image noise is achieved (slope 0.04±0.1 HU/kg). Diagnostic image quality was preserved in all patients. Individually weight-adapted tube current-time settings allow for a substantial dose reduction when performing retrospectively ECG-gated MSCT angiography for coronary arteries without impairment of diagnostic image quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From 1990 to 1992 CT caused 35% of the effective cumulative radiation dose in all radiological examinations, although only approximately 4–6% were CT examinations [1, 2]; hence, in 1998 the European Community classified CT as a high-dose procedure [3].
On the other hand, multislice computed tomography (MSCT) angiography for coronary arteries with subsecond gantry rotation time showed promising results and has gained further clinical acceptance since its introduction in 1998 [4, 5, 6]. This technique allowed for the first time retrospectively ECG-gated MSCT of the heart during a single breath-hold with a reasonable collimation [7].
In the future CT examination numbers and subsequently cumulative radiation dose are expected to increase further [8, 9, 10]; therefore, it is important to keep radiation dose for the individual examination as low as reasonably achievable—a principle often referred to as ALARA.
Several approaches for dose reduction in MSCT were introduced in clinical routine including adaptation of tube current-time settings to biometric data such as weight which already proved to be useful in abdominal and thoracic CT [11, 12, 13, 14, 15]. Furthermore, retrospectively ECG-gated MSCT of the heart can be combined with ECG-controlled tube current modulation leading to a relevant dose reduction [10, 16].
The purpose of this study was to develop a simple directive for dose reduction in retrospectively ECG-gated MSCT angiography of the coronary arteries using individually weight-adapted tube current-time settings.
Patients and methods
The study population consisted of 50 randomly chosen patients (34 men, 16 women; mean age 64.2±10.0 years). All of the patients underwent retrospectively ECG-gated MSCT of the heart due to suspected coronary artery disease. Informed consent was obtained prior to the examination. The patients personal data including weight, height and heart rate during the examination were recorded.
All of the patients were examined with a four-detector-row MSCT scanner (Somatom Volume Zoom, Siemens, Forchheim, Germany) during a single breath-hold of 28- to 50-s duration (mean 39.6 s). The standardised examination protocol included a 4×1-mm collimation, 1.5-mm table feed per rotation (normalised pitch 0.375) and a tube rotation time of 500 ms. Non-ionic contrast material (Ultravist 370, Schering, Berlin, Germany) was administered via an 18-G access in the cubital vein. The scan delay was determined by injection of a 20-ml test bolus with a flow-rate of 2.5 ml/s and repeated scanning every 2 s at the level of the ascending aorta. The time to peak enhancement plus 4 s was chosen as delay time. For vessel and ventricle enhancement 120 ml of contrast material were injected at a flow-rate of 2.5 ml/s. Both injections were followed directly by a 50-ml saline chaser bolus injected at the same flow rate.
For image reconstruction a field of view (FOV) of 180×180 mm with a 512×512 reconstruction matrix and a medium smooth convolution kernel (B30f) were selected. The effective slice thickness was 1.25 mm with a reconstruction increment of 0.8 mm. In patients with a heart rate of 65 beats per minute (bpm) or less at the point of imaging the temporal resolution was 250 ms constantly. At higher heart rates the temporal resolution ranged from 125 to 250 ms depending on the heart rate at the point of imaging using multisector image reconstruction [17]. For this study we reconstructed axial image series at 60% of the RR interval in every patient.
We examined two randomly chosen groups (A and B) with 25 patients each. The groups were comparable regarding the biometric data (Table 1). Group A was examined according to a standard protocol based on the recommendations provided by the suppliers (tube voltage 120 kV, effective tube current-time product 400 mAseff) [18]. To develop a dose reduction directive the raw data of these examinations were reconstructed twice: first with the standard reconstruction procedure (group A1). Afterwards, the raw data were transferred to an external personal computer equipped with an image reconstruction software (CardioRecon 6, Siemens, Forchheim, Germany). For a second image reconstruction (group A2) we added an individually adapted amount of random distributed image noise to the data simulating a reduction of the tube current-time product [19, 20] according to patient weight in kilograms multiplied by four in milliampere seconds [15]. By this procedure we planned to achieve a dose reduction of 25% for an average patient weighing 75 kg compared to a patient weighing 100 kg without a relevant increase of image noise. The other image reconstruction parameters were kept constant.
In general, noise in the CT data is determined by the quantum noise of the tube and thus follows a Poisson distribution. For a measured number n of X-ray quanta the noise is proportional to the square root of n; however, n is dependent on the X-ray attenuation. By adding a count-rate adjusted amount of artificial Poisson-distributed noise to each measured point in the raw data, a reduced tube current-time setting could be simulated; therefore, the noise is adjusted to patient geometry.
The second patient group (group B) was examined with the same scan protocol as group A but according to the results of group A2 with weight-adapted tube current-time settings, i.e. patient weight in kilograms multiplied by four in milliampere seconds as the tube current-time product [15].
Subsequently, the overall quality for all images (group A1, A2 and B) was assessed blinded and randomised by three experienced radiologists (B.J., A.H.M., J.E.W.) in a single consensus reading based on a five-point grading scale as follows: 1=clear delineation of small structures, distinct anatomic detail and sharp vessel; 2=clear anatomic detail with mild increase in image noise; 3=distinct increase in image noise with still unaffected diagnostic image quality; 4=obscured anatomic detail due to deterioration in image quality, extensive blurring and distinct increase in image noise leading to unsure diagnosis or even resulting in an insufficient evaluation of diagnosis; and 5=non-diagnostic [11].
Axial images were assessed in a single consensus reading from hard copies with window settings as follows: width 420 Hounsfield units (HU) and Center 120 HU.
The mean attenuation values in Hounsfield units and the standard deviation were measured from standardised regions of interest (ROI) with an area of 2 cm2, placed in the ascending aorta as well as the pulmonary trunk. The ROIs were placed in large vessels so that beam-hardening artifacts from contrast material in the vena cava could be minimised. The standard deviation of the CT attenuation was used as a measure for the amount of image noise. Image noise levels as well as mean densities were compared to each other with paired (group A1 vs A2) and unpaired (group A vs B) two-tailed Student’s t tests to find statistically significant differences in image noise between the patient groups.
The effective radiation dose according to ICRP 60 was calculated with the computer program WinDose 2.1 (IMP, Erlangen, Germany) [21].
In order to examine the effects of the weight-adapted imaging protocol we plotted the image noise levels against weight, body mass index (BMI) and height for group A1 and B. The linear regressions for weight, height and BMI were analysed. Additionally, we calculated a t test to delineate whether the slope was different from zero. A p value <0.05 was considered to be statistically significant and a dependency between image noise and the biometrical parameter was assumed. Results are given as mean values ± standard deviation.
Results
Relation of image noise in group A1, A2 and B
Image noise was assigned to the standard deviation measured in each ROI in Hounsfield units. From group A1 to A2 the mean image noise level of all patients increased by 18% in the aorta and by 21% in the pulmonary trunk, respectively from group A1 to group B by 12% in the aorta and 17% in the pulmonary trunk.
The level of image noise was statistically different between group A1 and group A2 as well as between group A1 and B, whereas the CT attenuation itself did not show statistically significant differences. On the other hand, significant differences between the image noise levels of group A2 and B could not be ascertained. Results are shown in Table 2.
Assessment of image quality
By calculating the tube-current-time settings equal to the patients weight in kilograms multiplied by four in milliampere seconds (group A2), an optimised dose distribution was achieved while retaining a nearly unimpaired diagnostic image quality. The average image quality according to the consensus assessment was 1.5±0.5 for group A1, 1.6±0.5 for group A2 and 1.7±0.7 for group B. No examination in any of the subgroups was rated as poor (score of 4) or non-diagnostic (score of 5) (Figs. 1, 2).
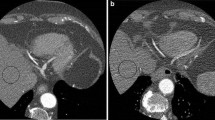
a Curved multiplanar reformation (MPR) of a male patient (50 kg, 1.74 m; group A1). Tube current-time settings: 400 mAseff, tube voltage 120 kV. b Same patient as in Fig. 1a with additionally added image noise (group A2). Simulated tube current-time settings: 200 mAseff, tube voltage 120 kV, simulated dose reduction of 50%
MPR of a male patient (76 kg, 1.69 m; group B). Tube current-time settings: 300 mAseff 120 kV. The left anterior descending coronary artery (LAD) is clearly depicted and unrestrictedly evaluable. After dose reduction, even small diagonal branches of the LAD are still uncompromised accessible (arrows)
Reduction of radiation dose
The tube current-time settings ranged from 220 to 340 mAs (282.9±41.9 mAs) for women and from 200 to 372 mAs (312.0±42.7 mAs) for men in group A2. In group B they ranged from 216 to 360 mAs (298.0±50.5 mAs) for women and from 260 to 392 mAs (334.7±35.5 mAs) for men.
According to ICRP 60 the mean of the calculated effective radiation dose was 6.78±1.41 mSv for men, 8.83±1.29 mSv for women in group A1, and 5.29±1.09 mSv for men, 6.22±0.89 mSv for women in group A2. The weight-adapted protocol used in group B led to an effective radiation dose of 5.57±0.89 mSv for men and 6.51±0.94 mSv for women, corresponding to a reduction by approximately 17.9% in men. For women the reduction amounted to 26.3% with a maximum dose reduction of 46%.
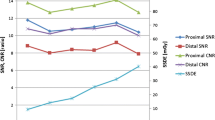
Scatter plots for image noise vs biometrical data
To test for dependencies between image noise and biometrical data several scatter plots were analysed (Figs. 3, 4, 5, 6). The slopes of the linear fits are given in Table 3. The regressions were compared to a function with slope zero using Student’s t test. The p values are given in Table 4. A p value <0.05 was considered statistically significant indicating a dependency between image noise and the biometrical parameter.
Compared to group A1 the slope of the linear fit for weight was reduced in group A2 and was nearly zero in group B (Figs. 3, 4); however, the slope of the linear fit for the BMI in group B was also reduced but still significantly different from zero (Figs. 5, 6). There was a good agreement between the results measured in the aorta and the pulmonary trunk for all patient groups indicating a homogeneous contrast media delivery.
Discussion
Computed tomography is the most important source of ionizing radiation for diagnostic purposes. Beyond, new applications are likely to further increase the radiation dose caused by CT examinations. On the other hand, retrospectively ECG-gated MSCT angiography of coronary arteries shows promising results for risk stratification by detection and quantification of coronary artery calcifications and assessment of the coronary arteries and coronary artery bypass grafts [4, 6, 16, 22, 23, 24, 25]. For this reason MSCT of the heart is increasingly performed.
Retrospectively ECG-gated MSCT angiography requires a relatively high radiation dose with values up to 12.8 mSv [8, 16, 22]. The standard protocol with fixed tube current-time settings leads to an overexposure and thus to an unnecessary high radiation exposure in patients with lower weight when compared to heavier patients.
In this study the data of group A1 was obtained according to a standard protocol as recommended by the supplier [18]; thus, image noise was expected to be correlated to the individual biometrical parameters [26].
As shown in Table 3 the linear fits of image noise vs the patients weight and the BMI are showing non-zero slopes in group A1 indicating a correlation, whereas no correlation could be found for height. As expected, the slopes for BMI and weight are positive (Figs. 3, 5) indicating growing noise levels for increasing patient weight and BMI. Since radiation dose and therefore the image noise level was chosen to be diagnostically sufficient for heavier patients, this led to a relative overexposure of low-weighted patients. In theory this could be corrected by introducing BMI or weight-adapted tube current-time settings; the latter was successfully established for CT of the chest and the abdomen [11, 13]. As BMI depends on two parameters (weight and height), weight-adapted tube current-time settings were used for this study to keep the correction directive as simple as possible.
By adding artificial image noise to the data as in group A2 (patient weight in kilograms multiplied by four in milliampere seconds) the image quality was unimpaired without any examination rated as poor (score of 4) or non-diagnostic (score of 5). Judged by the mean signal level and image noise the quality of the images dropped only slightly with a constant signal level but higher image noise.
The slopes of the linear fits for noise vs weight decreased to nearly zero indicating an improved dose distribution within the patient group A2. This was verified with a t test for a slope of zero where the patients weight and image noise were decoupled so that there was neither an overexposure for patients with low weight nor an underexposure of heavy patients. Regarding the linear adaptation a slope of zero indicating a balanced image noise can be judged as optimal; thus, the simulated dose reduction (group A2) with current-time settings equal to patients weight multiplied by four in milliampere seconds showed an optimised dose usage when compared to the standard scan protocol (group A1); however, it has to be kept in mind that the dose settings and the hereby obtained results are only valid for our particular CT scanner. But it can be assumed that the approach is transferable to other CT scanners.
Applying weight-adapted tube current-time settings to group B led to a smaller but similar increase in image noise (12% in the aorta and 17% in the pulmonary trunk) compared to group A2 while preserving a constant signal level. The mean values of the assessed diagnostic quality for the three groups were nearly equivalent and well within each others standard deviation. The constant high image quality was opposed to a considerable decrease of the applied radiation dose.
The effective radiation dose was reduced by 17.9% in men and 26.3% in women with a maximum of 46% in a single female patient. The mammary gland which is highly sensitive to radiation exposure, lies within the scan range and is considered in the calculation of the effective dose; therefore, any dose reduction will be even more effective in women when compared with men.
True to the simulation the linear fit of image noise and weight in group B showed a slope of nearly zero (p>0.68) indicating an optimised dose distribution in this patient group; hence, the weight-adapted protocol is a practical and easy-to-handle directive for use in clinical routine.
Furthermore, weight-adapted imaging can be combined with other dose-reduction techniques such as ECG-controlled tube current modulation [10, 16]. Both techniques represent different approaches for dose reduction and do not interfere with each other. ECG-controlled tube-current modulation reduces the tube current during the systole while only diastolic images are typically used for image analysis, leading to a dose reduction of up to 48% [10] and 44%, respectively [16]; thus, ECG-controlled tube-current modulation is well suited for a combined use with individual weight-adapted current-time settings which reduce radiation dose during the entire cardiac cycle. The combined use of these techniques would lead to a further reduction of patient radiation exposure and is therefore recommended.
Conclusion
The applied weight-adapted examination protocol reduces the radiation dose by an average of 21% for all patients without a decrease of image quality or a relevant increase in image noise. This technique is easy to handle and can therefore be recommended for use in clinical routine.
References
Schmidt T (2000) A few remarks on radiation exposure in CT. In: Nagel HD (ed) Radiation exposure in computed tomography. European Coordination committee on the radiological and electromedical industries (COCIR), Hamburg, pp 1–4
Kaul A, Bauer J, Bernhardt D, Nosske D, Veit R (1997) Effective doses to members of the public from the diagnostic application of the ionizing radiation in Germany. Eur Radiol 7:1127–1132
Commission of the European Community (1998) Quality criteria for computed tomography. Working document EUR 16262. European Commission, Luxembourg
Kopp AF, Ohnesorge B, Flohr T, Georg C, Schroder S, Kuttner A, Martensen J, Claussen CD (2000) Cardiac multidetector-row CT: first clinical results of retrospectively ECG-gated spiral with optimized temporal and spatial resolution. Fortschr Röntgenstr 172:429–435 (in German)
Nieman K, Oudkerk M, Rensing BJ, van Ooijen P, Munne A, van Geuns RJ, de Feyter PJ (2001) Coronary angiography with multi-slice computed tomography. Lancet 357:599–603
Achenbach S, Ulzheimer S, Baum U, Kachelriess M, Ropers D, Giesler T, Bautz W, Daniel WG, Kalender WA, Moshage W (2000) Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation 102:2823–2828
Ohnesorge B, Flohr T, Becker C, Knez A, Knopp AF, Fukuda K, Reiser MF (2000) Cardiac imaging with rapid, retrospective ECG synchronized multilevel spiral CT. Radiologe 40:111–117 (in German)
McCollough CH (2003) Patient dose in cardiac computed tomography. Herz 28:1–6
Greess H, Nomayr A, Wolf H, Baum U, Lell M, Bowing B, Kalender W, Bautz WA (2002) Dose reduction in CT examination of children by an attenuation-based on-line modulation of tube current (CARE Dose). Eur Radiol 12:1571–1576
Jakobs TF, Becker CR, Ohnesorge B, Flohr T, Suess C, Schoepf UJ, Reiser MF (2002) Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol 12:1081–1086
Wildberger JE, Mahnken AH, Schmitz-Rode T, Flohr T, Stargardt A, Haage P, Schaller S, Günther RW (2001) Individually adapted examination protocols for reduction of radiation exposure in chest CT. Invest Radiol 36:604–611
Donnelly LF, Emery KH, Brody AS, Laor T, Gylys-Morin VM, Anton CG, Thomas SR, Frush DP (2001) Minimizing radiation dose for pediatric body applications of single-detector helical CT: strategies at a large children’s hospital. Am J Roentgenol 176:303–306
Coppenrath E, Schmid C, Brandl R, Szeimis U, Hahn K (2001) Spiral CT of the abdomen: weight-adjusted dose reduction. Fortschr Röntgenstr 173:52–56 (in German)
Greess H, Wolf H, Baum U, Lell M, Pirkl M, Kalender W, Bautz WA (2000) Dose reduction in computed tomography by attenuation-based on-line modulation of tube current: evaluation of six anatomical regions. Eur Radiol 10:391–394
Mahnken AH, Wildberger JE, Simon J, Koos R, Flohr TG, Schaller S, Günther RW (2003) Detection of coronary calcifications: feasibility of dose reduction with a body weight-adapted examination protocol. Am J Roentgenol 181:533–538
Poll LW, Cohnen M, Brachten S, Ewen K, Modder U (2002) Dose reduction in multi-slice CT of the heart by use of ECG-controlled tube current modulation (“ECG pulsing”): phantom measurements. Fortschr Röntgenstr 174:1500–1505 (in German)
Flohr T, Ohnesorge B (2001) Heart rate adaptive optimization of spatial and temporal resolution for electrocardiogram-gated multislice spiral CT of the heart. J Comput Assist Tomogr 25:907–923
Siemens Inc. (2001) SOMATOM volume zoom application guide: special protocols. Software version A40. Siemens, Forchheim, p 28
Frush DP, Slack CC, Hollingsworth CL, Bisset G, Donnelly LF, Hsieh J, Lavin-Wensell T, Mayo JR (2002) Computer-simulated radiation dose reduction for abdominal multidetector CT of pediatric patients. Am J Roentgenol 179:1107–1113
Kalender WA (2000) Computed tomography, 1st edn. Publicis MCD, Munich, pp 84–85
Kalender WA, Schmidt B, Zankl M, Schmidt M (1999) A PC program for estimating organ dose and effective dose values in computed tomography. Eur Radiol 9:552–562
Cohnen M, Poll L, Puttmann C, Ewen K, Modder U (2001) Radiation exposure in multi-slice CT of the heart. Fortschr Röntgenstr 173:295–299
Becker CR, Kleffel T, Crispin A, Knez A, Young J, Schoepf UJ, Haberl R, Reiser MF (2001) Coronary artery calcium measurement: agreement of multirow detector and electron-beam CT. Am J Roentgenol 176:1295–1298
Kopp AF, Kuttner A, Heuschmid M, Schroder S, Ohnesorge B, Claussen CD (2002) Multidetector-row CT cardiac imaging with 4 and 16 slices for coronary CTA and imaging of atherosclerotic plaques. Eur Radiol 12 (Suppl 2):S17–S24
Knez A, Becker C, Ohnesorge B, Haberl R, Reiser M, Steinbeck G (2000) Noninvasive detection of coronary artery stenosis by multislice helical computed tomography. Circulation 101:E221–E222
Sevkurov A, Pratap A, Doss C, Jelnin V, Hoff JA, Kondos GT (2002) Electron-beam tomography imaging of coronary calcium: the effect of body mass index on radiologic noise. J Comput Assist Tomogr 26:592–597
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, B., Mahnken, A.H., Stargardt, A. et al. Individually weight-adapted examination protocol in retrospectively ECG-gated MSCT of the heart. Eur Radiol 13, 2560–2566 (2003). https://doi.org/10.1007/s00330-003-2111-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2111-5