Abstract
Purpose
Breast cancer mortality is higher in Black women than in White women. The prevalence of type 2 diabetes mellitus is also higher, yet data on whether diabetes affects breast cancer mortality in this population are lacking. We investigated the relation of diabetes at the time of breast cancer diagnosis to breast cancer mortality in the Black Women’s Health Study, a prospective cohort study.
Methods
1,621 Black women with invasive breast cancer diagnosed in 1995–2013 were followed by mailed questionnaires and searches of the National Death Index. Multivariable Cox regression analysis was used to compute hazard ratios (HRs) for diabetes in relation to breast cancer mortality and all-cause mortality, with adjustment for age, stage, treatment modality, estrogen receptor (ER) status, and body mass index.
Results
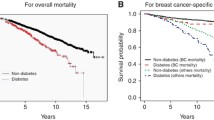
There were 368 deaths during follow-up, of which 273 were due to breast cancer. Breast cancer mortality was significantly increased in women who had been diagnosed with diabetes at least 5 years before breast cancer occurrence, HR 1.86 (95% CI 1.20–2.89), with elevations observed for both ER+ and ER− breast cancer. All-cause mortality was also higher in diabetics, with HRs of 1.54 (95% CI 1.12–2.07) overall and 2.26 (95% CI 1.62–3.15) for ≥5-year duration of diabetes relative to non-diabetics.
Conclusions
Our results present the first solid evidence of a positive association of type 2 diabetes with breast cancer mortality in Black women. Given the higher prevalence and earlier onset of type 2 diabetes in Black women, it is likely that diabetes contributes to racial disparities in breast cancer mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer mortality is 40% higher in US Black women than in US White women [1]. Type 2 diabetes mellitus (T2DM) prevalence is also higher among Blacks in comparison with Whites [2]. Prior studies conducted mainly in White women have found that diabetics with breast cancer have worse overall survival compared to non-diabetics [3–10]. Studies assessing the association of T2DM with breast cancer recurrence and breast cancer death have yielded inconsistent results, with several indicating a positive association [6, 9, 11, 12] and others no association [3, 5, 8]. Despite the greater burden of both T2DM and breast cancer mortality among Black women, only one study included an appreciable number of Black women [9], with little evidence of a positive association in that population group.
We investigated the relation of T2DM to breast cancer mortality among Black women, using data from the Black Women’s Health Study (BWHS). We also assessed the relationship of T2DM with all-cause mortality.
Methods
The BWHS is a prospective cohort study established in 1995 when 59,000 African-American women aged 21–69 enrolled by completing questionnaires mailed to subscribers of Essence magazine (a popular magazine targeted toward Black women) and members of several professional organizations [13]. Of the baseline cohort, 27% lived in the Northeast, 29% in the South, 23% in the Midwest, and 21% in the West [14]. The baseline questionnaire elicited information on demographics, medical history (including physician diagnosis of T2DM), reproductive history, family history of breast and other cancers, height, weight, smoking, and other lifestyle and behavioral characteristics. Biennial follow-up questionnaires update information on new occurrences and recurrences of cancer, other incident diseases, current weight, physical activity, menopausal status, and use of medications, among other factors [13, 15, 16]. Follow-up is complete for approximately 85% of person-years from enrollment through 2013. The study was approved by the institutional review board of the Boston University Medical Campus. Informed consent was obtained from each participant at recruitment into the parent study.
Cohort of breast cancer survivors
Incident invasive breast cancer cases were identified through self-report on biennial questionnaires or through 24 state cancer registries in states in which >95% of BWHS participants live, and the diagnoses were confirmed by review of hospital and state cancer registry pathology records. All incident breast cancer cases were coded in accordance with the surveillance, epidemiology, and end results (SEER) coding guidelines. We excluded 376 women with breast cancer for whom we did not have data on stage at diagnosis and 61 who reported another cancer diagnosis before the breast cancer. The final analytic cohort comprised 1,621 study participants diagnosed with invasive breast cancer from 1995 to 2013.
Data on stage, tumor grade, estrogen receptor (ER) status, and progesterone receptor status were obtained from pathology reports and state cancer registry data. Data on initial treatment of the breast cancer, including surgery (mastectomy/lumpectomy/other), chemotherapy (yes/no), radiation therapy (yes/no), and endocrine therapy (yes/no), were obtained from medical records, state cancer registry records, and supplemental questionnaires completed by participants diagnosed with breast cancer.
Assessment of T2DM and covariates
On baseline and follow-up questionnaires, participants were asked whether they had ever been diagnosed with diabetes, age at first diagnosis, and use of injections or pills for diabetes. In a previous validation study, 217 of 229 (95%) of self-reports of diabetes were confirmed by physician checklists completed by the participants’ physicians [15]. Given the high accuracy of self-report, we accepted self-report to classify participants as having T2DM.
Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Because weight was updated by questionnaire every 2 years, we were able to calculate BMI for each 2-year period. BMI from the questionnaire immediately preceding the breast cancer diagnosis (thus <2 years before the breast cancer was diagnosed) was considered as a potential confounder. Data on other potential confounders were also taken from the questionnaire immediately preceding the breast cancer diagnosis (e.g., cigarette smoking, vigorous physical activity) or from the enrollment questionnaire (e.g., education level).
Assessment of study outcomes
Cause of death was determined from state-issued death certificates and the National Death Index. In each follow-up cycle, the National Death Index was searched for study participants who did not complete that questionnaire. Women were classified as having died from breast cancer if breast cancer was listed as an immediate or underlying cause of death.
Statistical analysis
Cox proportional hazard regression models with time since diagnosis (in months) as the time scale were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association of T2DM at time of breast cancer diagnosis with breast cancer-specific death and all-cause mortality. Participants were followed from the date of breast cancer diagnosis until death or end of follow-up, whichever came first.
All analyses were adjusted for age at breast cancer diagnosis and SEER stage at diagnosis. In addition, we evaluated the following factors as potential confounders: education level, pre-diagnosis BMI, hours per week of vigorous physical activity, menopausal status, use of hormone supplements, tumor grade, ER status, and breast cancer treatment (chemotherapy, endocrine therapy, radiation therapy, type of breast surgery). ER status, BMI (<20, 20–24, 25–29, 30–34, ≥35 kg/m2), and treatment variables were retained in the final multivariable model. Analyses for all-cause mortality also included terms for cigarette smoking, an established causal factor for cardiovascular mortality. Treatment data were missing for the following proportion of cases: surgery 7.7%; chemotherapy 25.8%; radiation therapy; 29.0%; endocrine therapy 39.7%. We used multiple imputation methods to impute missing data for treatment using a logistic model with the fully conditional specification. We imputed 40 data sets based on the following variables: diabetes status at baseline, follow-up time, age, educational level, SEER stage, ER status, and BMI. To assess the proportional hazards assumption, multiplicative interaction terms between T2DM and follow-up time were tested using the likelihood ratio test to compare models with and without interaction terms; no violations were observed. Analyses were conducted overall and within strata of ER status (ER+, ER−) and stage at diagnosis (SEER local, SEER regional, SEER distant). All statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute, Cary, NC, USA).
All, or nearly all, of the previous studies of T2DM in relation to breast cancer mortality have used T2DM status at the time of breast cancer diagnosis as the measure of exposure, without taking into account new diagnoses of T2DM during follow-up. So that results of the current study could be directly compared to the existing literature, we used the same approach. This approach also provides useful information for the clinician, who will know whether the new breast cancer patient has T2DM but will not know whether she will develop it a few years later. For completeness, we also carried out a secondary analysis in which we updated T2DM status (and BMI) every 2 years throughout follow-up of the breast cancer cohort.
Results
Among women with invasive breast cancer, 232 (14%) had T2DM at the time of breast cancer diagnosis. Characteristics of cases with and without T2DM are shown in Table 1. Women with T2DM were older (mean 59.6 years) than women without T2DM (mean 52.6 years). Women with T2DM were also less physically active and more likely to be obese. Cases with and without T2DM were similar with respect to SEER stage, tumor grade, ER status, and breast cancer treatments, with the exception of radiation therapy: A lower proportion of women with diabetes had undergone radiation treatment.
During the follow-up period, there were 273 deaths attributable to breast cancer. The overall HR for T2DM at the time of breast cancer diagnosis was 1.28 (95% CI 0.88–1.86) (Table 2). When diabetic women were grouped according to the number of years between first diagnosis of T2DM and diagnosis of breast cancer (<5, ≥5 years), a statistically significant positive association was observed for women diagnosed with diabetes at least 5 years before breast cancer, with a multivariable HR of 1.86 (95% CI 1.20–2.89). The association was present whether or not the diabetic women were taking medications for their T2DM at the time of breast cancer diagnosis: HR was 1.84 (95% CI 1.05–3.20) for women on diabetes medications and 1.91 (95% CI 1.01–3.62) for women who were not taking medications for their T2DM.
There was evidence of an association of T2DM with increased risk of both ER+ and ER− breast cancer (Table 3). The HR for long-term T2DM was higher for ER+ breast cancer, 2.86 (95% CI 1.43–5.72), than for ER− breast cancer, 1.67 (95% CI 0.85–3.28), but the two estimates were not statistically different (p-interaction 0.28). In analyses stratified on SEER stage (localized, regional, and distant), the strongest association of long-term T2DM with breast cancer mortality was observed among women with localized breast cancer at the time of breast cancer diagnosis, but there was not a significant interaction by SEER stage (p-interaction 0.35).
Because some women will have developed T2DM after the occurrence of breast cancer, we conducted a sensitivity analysis in which we updated T2DM status every 2 years as new questionnaire data were obtained. Only 7 of the 273 deaths due to breast cancer shifted from the “no diabetes” group to the “yes diabetes” group. The overall estimate for T2DM in relation to breast cancer mortality, with adjustment for age and SEER stage, was 1.07 (95% CI 0.76–1.50), and the estimate for T2DM duration ≥5 years was 1.32 (95% CI 0.91–1.92).
There were 368 all-cause deaths among the 1,621 women with breast cancer. As expected, T2DM was associated with increased all-cause mortality (Table 4). The overall HR was 1.54 (95% CI 1.15–2.07), and the HR for T2DM of at least 5-year duration was 2.26 (95% CI 1.62–3.15). Only 95 of the deaths were from causes other than breast cancer, with 33 from other cancers (including nine in diabetics), 28 from cardiovascular disease (including eight in diabetics), and 34 from other causes (including 10 in diabetics).
Discussion
We assessed whether Black women with a history of T2DM at the time of their breast cancer diagnosis experienced a worse prognosis compared to those who did not have T2DM. We found that women who had T2DM for at least 5 years before their breast cancer diagnosis had an 86% increased risk of dying from breast cancer as compared with those who did not have T2DM; fewer years of T2DM were not associated with increased risk. The positive association was observed for localized as well as regional breast cancer, and there was evidence of an association with both ER+ and ER− breast cancer. As expected, given that T2DM is associated with increased all-cause mortality in the general population, T2DM was found to be associated with all-cause mortality in this cohort of breast cancer survivors.
Most studies of diabetes and breast cancer-specific outcomes have focused on White women. Three studies suggested that diabetes increases the risk of breast cancer recurrence or breast cancer-specific death [6, 9, 12], and two studies showed no association [3, 5]. In a hospital-based cohort of breast cancer patients in Sweden, a 45% increased risk of breast cancer mortality was found among diabetics compared to non-diabetics (1.45, 95% CI 1.35–1.59) [12]. There was no adjustment for cancer stage, which may have resulted in overestimation of the association, as diabetics have been shown to have a more advanced stage of breast cancer or more aggressive tumor characteristics [3, 5, 8, 10]. In our study, adjusting for cancer stage slightly attenuated the effect of diabetes on breast cancer-specific death. The California Breast Cancer Survivorship Consortium (CBCSC), the only study to date to include a subgroup analysis of African-American women, found a 48% increased risk of breast cancer deaths among diabetics in all groups combined (1.48, 95% CI 1.18, 1.87), but there was not a significant association among African-Americans (1.17, 95% CI 0.72–1.90) [9]. That study followed 882 African-American women with breast cancer, of whom 101 had T2DM, whereas the present study followed 1,621 women, of whom 232 had T2DM. The CBCSC did not report HRs for varying durations of diabetes by race/ethnicity, but did find that across all groups, higher HRs were observed for a duration of 6 years or longer. Thus, the present results are not inconsistent with previous findings in Black women, but do present the first solid evidence of a positive association in this understudied group.
When new diagnoses of T2DM after the diagnosis of breast cancer were considered in a secondary analysis, the HR for ≥5-year duration of T2DM was reduced and no longer statistically significant. The weaker association observed in the time-dependent analysis may be due to the relatively shorter time of living with diabetes among women whose diabetes was diagnosed after breast cancer diagnosis as compared with those women who were diagnosed with diabetes at least 5 years before their breast cancer.
Associations of T2DM with increased all-cause mortality have been found in a number of studies, including some that found no association with breast cancer-specific mortality. The Women’s Health Initiative found no association between diabetes and breast cancer-specific deaths but observed an increase in all-cause mortality among diabetics, largely accounted for by cardiovascular disease [5]. In the Women’s Healthy Eating and Living cohort, all-cause mortality was increased among diabetic breast cancer patients compared to non-diabetic patients [3, 6]. A meta-analysis on T2DM and prognosis following breast cancer also found that diabetes was associated with increased non-cancer related mortality [7].
There is plausible evidence for biological mechanisms linking diabetes to breast cancer incidence. Insulin, insulin-like growth factor, and hyperinsulinemia, a characteristic of type 2 diabetes, have all been implicated in malignant transformation of normal breast epithelial cells [4, 17–19]. Unresolved systemic and local chronic inflammation is also characteristic of T2DM in adults and may alter the tumor microenvironment to promote cancer cell proliferation, survival, and metastasis. These biological mechanisms may be contingent upon a longer duration of diabetes in order to impact breast cancer-specific mortality [9]. Interestingly, the observed association did not differ according to whether or not the diabetic women were taking diabetes medication at the time of breast cancer diagnosis.
Our study is the largest to date to assess the relationship of diabetes with breast cancer-specific outcomes in Black women diagnosed with invasive breast cancer. Strengths include the relatively young age of the study population, the demonstrated validity of self-reported diabetes, and the ability to adjust for stage, treatment, ER status, and BMI. Despite the relatively large size, the study was underpowered to provide stable estimates of risk within specific ER subtypes.
In summary, T2DM diagnosed at least 5 years before breast cancer was associated with increased breast cancer mortality in this cohort of Black women. This association is consistent with associations observed in studies of White women. Because the prevalence of T2DM is markedly higher in Black women than White women, and the age at onset of T2DM is earlier in Black women, it is possible that T2DM contributes to the racial disparity in breast cancer mortality. Clinicians treating women newly diagnosed with breast cancer would be well advised to take into account their history of T2DM and management of the T2DM.
References
American Cancer Society (2013) Breast cancer facts and figures 2013–2014. American Cancer Society, Atlanta
Centers for Disease Control and Prevention (2014) National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. U.S. Department of Health and Human Services, Atlanta
Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD et al (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29:54–60
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y et al (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20:42–51
Luo J, Virnig B, Hendryx M, Wen S, Chelebowski R, Chen C et al (2014) Diabetes, diabetes treatment and breast cancer prognosis. Breast Cancer Res Treat 148:153–162
Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA et al (2010) Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat 122:859–865
Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL et al (2011) Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 29:40–46
Schrauder MG, Fasching PA, Haberle L, Lux MP, Rauh C, Hein A et al (2011) Diabetes and prognosis in a breast cancer cohort. J Cancer Res Clin Oncol 137:975–983
Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan TH et al (2015) Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomark Prev 24:361–368
Srokowski TP, Fang S, Hortobagyi GN, Giordano SH (2009) Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol 27:2170–2176
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159:1160–1167
Liu X, Ji J, Sundquist K, Sundquist J, Hemminki K (2012) The impact of type 2 diabetes mellitus on cancer-specific survival: a follow-up study in Sweden. Cancer 118:1353–1361
Krishnan S, Rosenberg L, Djousse L, Cupples LA, Palmer JR (2007) Overall and central obesity and risk of type 2 diabetes in U.S. black women. Obesity 15:1860–1866
Rosenberg L, Palmer JR, Wise LA, Adams-Campbell LL (2006) A prospective study of female hormone use and breast cancer among black women. Arch Intern Med 166:760–765
Wise LA, Rosenberg L, Radin RG, Mattox C, Yang EB, Palmer JR et al (2011) A prospective study of diabetes, lifestyle factors, and glaucoma among African-American women. Ann Epidemiol 21:430–439
Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L (2007) A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomark Prev 16:1795–1802
Papa V, Costantino A, Belfiore A (1997) Insulin receptor what role in breast cancer? Trends Endocrinol Metab 8:306–312
Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B (2005) Diabetes mellitus and breast cancer. Lancet Oncol 6:103–111
Xue F, Michels KB (2007) Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr 86:s823–s835
Acknowledgments
We acknowledge the continued commitment of the Black Women’s Health Study (BWHS) participants. We also acknowledge the dedication and support of the BWHS data collection staff. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Cancer Society, National Cancer Institute, or the National Institutes of Health. Data on breast cancer pathology were obtained from several state cancer registries (AZ, CA, CO, CT, DE, DC, FL, GA, IL, IN, KY, LA, MD, MA, MI, NJ, NY, NC, OK, PA, SC, TN, TX, VA), and results reported do not necessarily represent their views.
Financial support
This work was supported by the National Institutes of Health, National Cancer Institute grants R01 CA058420 (LR), UM1 CA164974 (LR, JRP), P01 CA151135 (JRP), and U01 CA182898 (GVD, JRP); the Boston Medical Center Carter Disparity Endowment (MC); and the American Cancer Society Institutional Research Grant IRG-72-001-36-IRG (MC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Charlot, M., Castro-Webb, N., Bethea, T.N. et al. Diabetes and breast cancer mortality in Black women. Cancer Causes Control 28, 61–67 (2017). https://doi.org/10.1007/s10552-016-0837-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-016-0837-z