Abstract
Purpose
Epidemiological studies indicated that type 2 diabetes mellitus may increase breast cancer risk and mortality. The aim of this retrospective cohort study was to examine the effect of diabetes on the clinical course and the prognosis of early stage breast cancer in relation to tumour and patient characteristics.
Methods
The cohort analyzed in this study consisted of 4,056 patients with invasive primary breast cancer. We compared overall survival, distant metastasis-free survival and local recurrence free survival between breast cancer patients with and without diabetes.
Results
In our cohort 276 breast cancer patients (6.8%) were affected by diabetes compared to 3,780 patients (93.2%) without diabetes. Women with diabetes were significantly older, had larger tumours, and a higher rate of lymph node involvement. After a follow-up period of 5 years, stratification for age and adjustment for other prognostic factors, overall mortality following breast cancer was significantly higher in diabetic breast cancer patients (hazard ratio, HR 1.92; 95% confidence interval, CI 1.49–2.48). We found no significant differences in distant metastasis-free survival and local recurrence free survival between the two groups, but we found a slightly significant higher rate of distant metastasis in the group of patients with diabetes and oestrogen receptor negative tumours (HR 2.28; CI 1.31–3.97).
Conclusion
In this study, patients with diabetes and oestrogen receptor negative breast cancer had a more than 2-fold higher risk for distant metastasis compared to patients without diabetes. Diabetes was also associated with an almost 2-fold increase in mortality within the 5 years follow-up period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast Cancer (BC) is one of the most common types of cancer diagnosed in women. It is a chronic disease associated with severe morbidity and mortality. There are many risk factors known to be associated with increased BC risk including age, family history, genetics, long menstrual history with early menarche and late menopause, nulliparity, obesity, and postmenopausal use of hormone therapy (Kelsey and Berkowitz 1988). Given a senescent population in the Western world the number of persons, who suffer from more than one chronic disease increases. Data from different cancer registries showed that about 60% of all new cancer patients older than 65 years suffer from at least one other serious disease. Most common among these comorbid conditions is diabetes mellitus (DM) (16%), which is also believed to be a possible risk factor for BC and a prognostic factor in BC patients (Wolf et al. 2005).
DM, especially type 2 diabetes mellitus (T2DM), which accounts for over 90% of all diagnosed DM cases, is a severe health problem of worldwide significance (Wild et al. 2004). The incidences of T2DM, as well as the prevalence of obesity and metabolic syndrome, have increased over the past decades just as the BC incidence. BC has been connected with T2DM, which is characterized by insulin resistance, hyperglycaemia and hyperinsulinemia, by the hypothesis that hyperinsulinemia may increase the risk of BC. But this relationship was unclear until experimental data and epidemiological studies demonstrated a significant association between T2DM and BC, as well as cancer of the colon, liver, endometrium, kidney and pancreas (Grote et al. 2010).
Obesity is related to T2DM, but it has also a complex relationship to BC risk that differs before and after the menopause. Surprisingly, adipose premenopausal women, particularly under the age of 35, are at lower risk for BC than lean women (Ursin et al. 1995). The mechanisms for this protective effect of adiposity in premenopausal women is not well understood, but the incidence of aggressive, oestrogen and progesterone receptor (ER/PR-) negative BC is much higher in this group of women and it has been suggested that this is due to a kind of selection of non-oestrogen-dependent tumour cells which are dependent on growth factors such as insulin, insulin-like growth factor-I (IGF-I) and adipokines like leptin (Vona-Davis et al. 2007; Rose and Vona-Davis 2010; Eng-Wong et al. 2009). The possible causative relationship between obesity, T2DM and BC has also been investigated in mouse models and it has been assumed that insulin resistance and hyperinsulinemia, as common features of obesity and T2DM, may be the major factors affecting cancer development and progression (LeRoith et al. 2008). Interestingly, there has been no associated risk of BC linked with type 1 DM (T1DM), suggesting that it is the hyperinsulinemia and insulin resistance of T2DM that lead to an increased risk of BC in postmenopausal women and increased mortality regardless of menopausal status (Rose and Vona-Davis 2009; Hjalgrim et al. 1997). Growing evidence in literature suggests an increased risk of BC in patients suffering from T2DM, which is independent of obesity (Grote et al. 2010; LeRoith et al. 2008; Larsson et al. 2007; Richardson and Pollack 2005). Data regarding the association of DM and BC recurrence with respect to obesity is limited.
In a large monocentric cohort of women with early stage primary BC from the University Breast Center for Franconia (Germany) we evaluated the influence of DM on survival, distant metastasis-free survival and local recurrence free survival in relation to common tumour and patient characteristics.
Patients and methods
Patients
Eligible patients were women with the diagnosis of early stage invasive BC who were treated at the University Breast Center for Franconia. Exclusion criteria were unknown ER or PR status, evidence of recurrent disease or distant metastasis, prior malignancy (except carcinoma-in situ of the cervix) and an incomplete medical record. Of the 5,420 patients treated between 1993 and 2008 at the University Breast Center for Franconia, 1,364 patients had to be excluded due to one or more of the aforementioned exclusion criteria and the final study population consisted of 4,056 patients. Among these women the majority (3,780 patients) had no history of DM, whereas 6.8% (276 patients) presented with DM in their medical history.
Pathology
Surgical pathologists specialized in breast pathology at the University Breast Center for Franconia examined the pathologic specimens of all patients in the course of routine patient care. The histological type, grade, resection status and TNM-stage were determined, and the expression of ER, PR, and Her2/neu was analyzed immunohistochemically according to the standard practice in certified breast centres in Europe (Blamey and Cataliotti 2006; Singletary et al. 2002).
Information concerning tumour characteristics was subsequently transferred from the histopathological reports into our clinical database. This database collects information about type and size of tumours, nodal status, grading, location of primary metastases and further information. The purpose of this database is to improve quality in the treatment and to provide the best possible care for BC patients.
Data collection and follow-up
The first presentation at our specialized BC unit with suspected or diagnosed breast cancer was defined as baseline visit. Height and weight were measured and the medical history was taken by the physician of the breast unit. Body mass index (BMI) was calculated and two cut-points (25 and 30 kg/m2) were used according to the literature to divide patients into three BMI groups. Information about DM was collected using the routine patients’ health records. Most patient characteristics were available in our clinical database, but no specific information on age at onset, severity or type of DM or any medication concerning DM.
All patients have been followed up for physical health issues and for the following parameters: all-cause and BC-specific mortality, occurrence of distant metastases and locoregional recurrence. Follow up time was limited to 5 years for each patient. During this period patients presented themselves at the study centre for regular check-ups following a general aftertreatment plan in accordance with German and international BC follow-up guidelines (Cardoso and Castiglione 2009). During those visits, information concerning the recent medical history, current medical problems and drug intake were retrieved, and re-staging procedures were disposed if indicated (e.g. abdominal sonogram, bone scan, CT or MRI to reveal distant metastases). An examination of the breast was part of each follow-up visit to detect possible local recurrence of the tumour.
Statistical analysis
Characteristics of patients with diabetes mellitus and patients without diabetes mellitus were compared with appropriate unpaired statistical tests. Student’s t-tests were used for continuous characteristics, chi-squared tests with continuity correction for categorical characteristics and the Armitage trend test for ordinal-categorical characteristics.
Overall survival, distant disease free survival and local recurrence free survival were the main outcomes of interest. Cox Proportional Hazard (PH) models were used to obtain hazard ratios (HR) for diabetes-patients versus nondiabetic patients. Each prognostic factor was modelled in a Cox PH model containing the prognostic factor, the diabetes status as factor and its interaction with the prognostic factor. In order to avoid confounding by unequal distributed cohorts all models were stratified by patient’s age at BC diagnosis. Therefore the patients were divided into four equal age classes. The no-interaction assumption of the strata was evaluated with the likelihood ratio test. Moreover, confounding was considered by adjusting the models by BMI and tumour stage (TNM-classification according to UICC and AJCC) (Singletary et al. 2002). The proportional hazard assumptions were checked by tests which correlate scaled Schoenfeld residuals with a suitable transformation of time (Grambsch and Therneau 1994). Furthermore, for each outcome an overall Cox PH model only with diabetes-status and the adjusting factors was modeled.
All of the tests were two-sided, and a P value <0.05 was regarded as statistically significant. All of the statistical analyses were carried out using the R system for statistical computing (version 2.10.1; R Development Core Team, Vienna, Austria, 2009).
Results
Study population
The study population consisted of 4,056 BC patients. Patients with DM were significantly older at diagnosis of BC and presented with a higher tumour stage (TNM-classification), see Table 1. The mean age of our cohort at baseline visit was 57.6 (12.9) years (standard deviation in parentheses), the mean BMI was 26.1 (4.8). The cohort included 2,596 postmenopausal and 1,069 premenopausal BC patients while in 391 patients the menopausal status was unclear or unknown.
The 276 patients with both DM and BC showed a mean BMI of 28.8 (5.6), whereas the mean BMI in the non-diabetic BC patient group was 25.9 (5.6). As shown in Table 1, more than half of all patients were at stage pT1 (57.2%) and 64.6% had no lymph node involvement, representing a cohort with an a priori low to average recurrence risk compared to similar studies (Coughlin et al. 2004; Hjalgrim et al. 1997).
Overall, 25% of all included patients presented with an ER-negative BC. 61.9% were moderately differentiated (G2) and 24.7% poorly differentiated (G3) carcinomas. The distribution of the Her2/neu-status was in accordance with the literature with 83.7% of the carcinomas showing no overexpression.
Data on tumour characteristics were comparable in both BC groups independent of DM, except that diabetic BC patients were older (mean: 67.4 vs. 57.6 years) and showed a significantly higher pathological tumour stage (pT), see Table 1.
Diabetes mellitus and overall survival
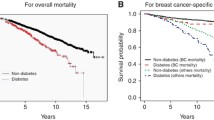
Until the end of data acquisition in 2009, there were 295 events of local recurrence, 417 events of distant metastasis and 576 deaths from all causes documented. After adjustment for age, BMI and tumour stage, mortality following BC was significantly higher among women with DM compared to BC patients without DM (HR 1.90; 95% CI 1.49–2.48). The increase in risk associated with DM showed no significant difference between pre- and postmenopausal BC patients (HR 2.35 vs. 1.91, P (interaction) = 0.56). Due to small patient numbers in the premenopausal subgroup with DM the 95% CI ranged from 0.73 to 7.53.
There were 576 deaths recorded in our cohort over the study period: 84 among women with DM (30.4%) and 492 among women without DM (13.0%). Significant predictors of increased risk of death following BC included higher age, higher tumour stage according to the TNM-classification, negative ER and PR status, positive Her2/neu status and higher grading. After adjustment for age, BMI and tumour stage, DM remained a significant independent predictor of death in the early stage BC cohort (HR 1.90; 95% CI 1.48–2.41, P < 0.00001) (Fig. 1).
Overall survival. Hazard ratio (HR) for diabetes mellitus yes versus no. Cox regression model for Diabetes and parameters (pT, pN, ER, PR, HER2, Grading, BMI), as well as “overall” Cox-model for Diabetes. All models are stratified by age and adjusted for BMI and tumour stage. The P value for differences in HR between the subgroups is given as P (int)
We categorized the BMI in groups (<25, 25–30, >30 kg/m2) according to body weight criteria (short or normal weight, overweight, and obese) described in the literature (Bray 1985). Surprisingly, DM seemed to be a stronger predictor for a worse outcome in the short/normal weight BC group compared to the overweight and obese group of patients. The hazard ratios for overall survival decreased with increasing BMI from 2.78 in the patients group with a BMI under 25, 1.97 in the group with BMI between 25 and 30, as well as 1.32 in the obese group with BMI over 30 (Fig. 1). The influence of DM on the overall survival was different according to the tumour stage. Comparing pT1 versus pT2-pT4 showed a different effect of DM which was stronger in the pT1 group of patients (HR = 2.71 vs. 1.61) and slightly significant [P(interaction) = 0.04].
Diabetes mellitus and distant metastasis-free survival
DM was not associated with distant metastasis-free survival in our study cohort (HR1.10; 95% CI 0.74–1.64) (Fig. 2). In view of the different subgroups we saw a trend towards a higher distant metastasis rate associated with DM in the subgroup with histological grade 3 tumours and PR-negative tumours (HR 1.66 and 1.50; P (interaction) = 0.09 and 0.11). Surprisingly, the subgroup of BC patients with ER-negative BC showed a significant association of DM with a higher rate of distant metastasis (HR 2.28; 95% CI 1.31–3.97, P (interaction) <0.01). The distant metastasis rate was 21% (15 out of 57) in the diabetic BC group with ER-negative tumours compared to 12.4% (123 out of 862) in the non diabetic ER-negative subgroup, as displayed in Fig. 2.
Distant metastasis-free survival. Hazard ratio (HR) for diabetes mellitus yes versus no. Cox regression model for Diabetes and parameters (pT, pN, ER, PR, HER2, Grading, BMI), as well as “overall” Cox-model for Diabetes. All models are stratified by age and adjusted for BMI and tumour stage. The P value for differences in HR between the subgroups is given as P (int)
Additionally, women with DM and a low BMI (<25) exhibited shorter distant metastasis-free survival by trend [P (interaction) = 0.09].
Diabetes mellitus and local recurrence-free survival
Similar to the aforementioned we found no association of DM with local recurrence-free survival (HR 0.82; 95% CI 0.45–1.48). The Cox-regression model for the event recurrence-free survival showed no significant association with DM in all examined subgroups after adjustment for age, BMI and tumour stage (Fig. 3).
Local recurrence-free survival. Hazard ratio (HR) for diabetes mellitus yes versus no. Cox regression model for Diabetes and parameters (pT, pN, ER, PR, HER2, Grading, BMI), as well as “overall” Cox-model for Diabetes. All models are stratified by age and adjusted for BMI and tumour stage. The P value for differences in HR between the subgroups is given as P (int)
Discussion
In this retrospective cohort study with 4056 BC patients we observed a significant higher risk for death in the group of BC patients with DM after adjustment for age, BMI and tumour stage. Moreover, DM showed no significant association with local recurrence-free survival and distant metastasis-free survival in the whole cohort. A significant association of DM with a higher distant metastasis rate during the follow-up period of 5 years was found in the ER-negative subgroup, only. Our findings confirm those from recent studies that have shown an association of DM with a worse prognosis of BC and reported HR between 1.4 and 1.61 (Verlato et al. 2003; Coughlin et al. 2004; Barone et al. 2008).
The HR of 1.90 in our study for mortality following BC among women with and without DM was similar to these previous published reports (Verlato et al. 2003; Coughlin et al. 2004; Barone et al. 2008). It has been suggested, that early survival following BC is reduced due to DM-related causes (Lipscombe et al. 2008). Our finding of a slightly significant higher risk of distant metastasis in the group of diabetic BC patients with ER-negative BC was unexpected and could possibly be due to chance. But it could also be the first clinical hint of a putative direct risk modifying effect of DM in BC beyond the DM-related mortality and morbidity.
In the hormone receptor negative subset of the Women’s Intervention Nutrition Study lower dietary fat intake and weight loss in the intervention group correlated with lower long-term mortality compared to the control group (7.5% vs. 18.1%). In this study no insulin level analysis was available, but the authors concluded, that the results are consistent with the notion that factors such as insulin or IGF may be important in the ER-negative subset of BC (Chlebowski et al. 2006; Chlebowski et al. 2008; Chlebowski et al. 1993). This theory could at least partly explain our finding of a greater impact of DM on distant disease free survival in patients with ER-negative BC.
Insulin and insulin like growth factors (IGF) are supposed to have a DM independent direct mitogenic and BC promoting effect. It has been shown in transgenic mice that premalignant and early malignant lesions of the mammary gland occur in mice that overexpress IGF-I or IGF-I receptor (IGF-IR) (Kleinberg et al. 2009). Insulin also inhibits the production of sex hormone-binding globulin (SHBG) and IGF binding protein-1, leading to an increase in free plasma oestrogen and free bioavailable IGF-1 (Michels et al. 2003). Moreover, higher levels of fasting insulin have been shown to be associated with the risk of BC development, as well as the disease outcome (distant recurrence and death) in DM free women with early BC (Goodwin et al. 2002; Del Giudice et al. 1998). The study by Goodwin et al. showed insulin levels to be significantly related to tumour stage, nodal stage and tumour grade. Insulin levels were not significantly related to nuclear grade, lymphatic invasion, ER, or PR (Goodwin et al. 2002).
The association of T2DM and postmenopausal BC risk is also well established and a meta-analysis of 5 case control studies and a meta-analysis of 15 cohort studies found a relative risk for BC of 1.18 and 1.20, respectively (95% CI, 1.05–1.32 and 1.11–1.30) (Larsson et al. 2007; Tsugane and Inoue 2010). Data from the Women’s Health Initiative Observational Study showed that fasting insulin levels were strongly positively associated with BC risk independently of obesity in postmenopausal women who did not have T2DM (Gunter et al. 2009). In this context, it is of interest that the positive relationship between T2DM and postmenopausal BC risk found in the Nurses’ Health Study was predominantly seen in patients with ER-positive tumours (HR 1.22 vs 1.13). However, the relevance of this finding is limited due to the small number of ER-negative diabetic BC patients. Moreover, information on prognosis is also missing in this study (Michels et al. 2003).
Being overweight has also been shown to affect BC risk as well as prognosis (Daling et al. 2001; Calle and Kaaks 2004). It has been suggested previously that the association of pre-existing obesity and postoperative weight gain with poor BC prognosis regardless of menopausal status is due to the association of adiposity with ER-negative, highly proliferative tumours with a propensity for distant metastasis (Rose and Vona-Davis 2009). Daling et al. found that women in the highest quartile of BMI were 2.5 times more likely to die of their disease within 5 years of BC diagnosis compared with women in the lowest quartile (Daling et al. 2001). They also reported that tumours of women in the highest quartile of BMI were more likely to be ER-negative and to have a high S-phase fraction, high histological grade, high mitotic cell count, and larger tumour size compared with women whose BMI was in the lowest quartile (Daling et al. 2001; Goodwin and Boyd 1990). In our study the reported effects of DM on mortality and distant recurrence were independent of BMI. We found less ER-negative tumours in the diabetic BC group (22.9% vs. 25.2%) and more histological grade 3 tumours in the non-diabetic BC group (25.0% vs. 21.5%) of our cohort. Keeping this in mind, one could argue that our finding of a higher rate of distant recurrence in the ER-negative subgroup of diabetic BC patients indicates an association of DM with a more aggressive ER-negative BC phenotype, but not with a higher rate of ER-negative BC, as shown for obesity (Vona-Davis et al. 2007; Stephenson and Rose 2003).
It is also very interesting that in the obese subgroup of BC patients DM does not seem to be as important for the overall survival as in the underweight/normal weight group of BC patients (HR 1.32 vs. 2.78, P (interaction) = 0.02). Moreover, DM seems to be more important in patients with a lower tumour stage (pT1) compared to patients with higher stages (pT2-pT4) (P (interaction) = 0.04). The explanation for this observation remains elusive. Possible reasons could be similar molecular mechanisms or changes in signalling pathways that determine the effect of DM and obesity on BC survival. Therefore, DM might have a worse effect on BC prognosis in lean patients where these tumour promoting pathways are not already activated by obesity. This DM effect on oncogenic pathways might be more relevant in patients with early stage tumours providing a possible explanation for the second finding.
DM is treated with a variety of medications, so there may also be a link between these medications and cancer. This link has already been suspected by several studies showing for example a higher pathologic complete response rate in BC patients receiving metformin during neoadjuvant chemotherapy, as well as suppressed cancer cell growth by metformin and rosiglitazone in vitro (Jiralerspong et al. 2009; Feng et al. 2010). Unfortunately, we did not have information on duration of DM, age at onset of DM and DM medication before or after BC diagnosis. Therefore, we cannot rule out a potential bias due to differences in DM treatment in our study group.
Another limitation of this study is that we were not able to distinguish between the different types of diabetes mellitus. T2DM is by far the most common type of DM, especially in the examined group of diabetic BC patients with a mean age of 67.4 years. Therefore, most cases of DM in this cohort are likely to be non-insulin-dependent. Nonetheless, taking into account the relative small numbers in some subgroups, the missing differentiation between T1DM and T2DM could be one confounding factor in this study.
Major strengths of the present study include the complete and highly reliable clinical information directly extracted from medical records by trained medical documentation assistants and the close follow-up of all patients presenting at our breast cancer unit in frequent intervals.
To our knowledge, only one prior study has examined associations between DM and BC recurrence in a large cohort. In this analysis, diabetic patients with early stage BC had over twofold the risk of additional BC events and mortality. The authors also examined univariate associations for variables like ER and PR status, but no results of subgroup analysis were published (Patterson et al. 2010).
The main findings of our study are an association between DM and mortality in early stage BC patients, together with the association of DM in ER-negative BC patients with worse distant metastasis-free survival. This is consistent with the idea of direct tumour promoting effects of DM in BC. The fact that DM is accountable for a worse BC prognosis in patients with ER-negative tumours is certainly not proven by our data, just as our finding of a weaker effect of DM on BC survival in obese patients, but these ideas warrant further investigation.
References
Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 300(23):2754–2764. doi:10.1001/jama.2008.824
Blamey RW, Cataliotti L (2006) Eusoma accreditation of breast units. Eur J Cancer 42(10):1331–1337. doi:10.1016/j.ejca.2006.04.003
Bray GA (1985) Obesity: definition, diagnosis and disadvantages. Med J Aust 142(7 Suppl):S2–S8
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4(8):579–591. doi:10.1038/nrc1408
Cardoso F, Castiglione M (2009) Locally recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(Suppl 4):15–18. doi:10.1093/annonc/mdp115
Chlebowski RT, Blackburn GL, Buzzard IM, Rose DP, Martino S, Khandekar JD, York RM, Jeffery RW, Elashoff RM, Wynder EL (1993) Adherence to a dietary fat intake reduction program in postmenopausal women receiving therapy for early breast cancer. The women’s intervention nutrition study. J Clin Oncol 11(11):2072–2080
Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM (2006) Dietary fat reduction and breast cancer outcome: Interim efficacy results from the women’s intervention nutrition study. J Natl Cancer Inst 98(24):1767–1776. doi:10.1093/jnci/djj494
Chlebowski RT, Anderson G, Pettinger M, Lane D, Langer RD, Gilligan MA, Walsh BW, Chen C, McTiernan A (2008) Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med 168(4):370–377. doi:10.1001/archinternmed.2007.123 (quiz 345)
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of us adults. Am J Epidemiol 159(12):1160–1167. doi:10.1093/aje/kwh161.159/12/1160
Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92(4):720–729. doi:10.1002/1097-0142(20010815)92:4<720::AID-CNCR1375>3.0.CO;2-T
Del Giudice ME, Fantus IG, Ezzat S, McKeown-Eyssen G, Page D, Goodwin PJ (1998) Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat 47(2):111–120
Eng-Wong J, Perkins SN, Bondy M, Li D, Eva Singletary S, Nunez N, Hursting S, Chang S (2009) Premenopausal breast cancer: estrogen receptor status and insulin-like growth factor-i (igf-i), insulin-like growth factor binding protein-3 (igfbp-3), and leptin. Breast J 15(4):426–428. doi:10.1111/j.1524-4741.2009.00753.x
Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC (2010) The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med. doi:10.1111/j.1582-4934.2010.01083.x
Goodwin PJ, Boyd NF (1990) Body size and breast cancer prognosis: a critical review of the evidence. Breast Cancer Res Treat 16(3):205–214
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N (2002) Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 20(1):42–51
Grambsch PM, Therneau TM (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81(3):515–526. doi:10.1093/biomet/81.3.515
Grote VA, Becker S, Kaaks R (2010) Diabetes mellitus type 2—an independent risk factor for cancer? Exp Clin Endocrinol Diabetes 118(1):4–8. doi:10.1055/s-0029-1243193
Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kaplan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD (2009) Insulin, insulin-like growth factor-i, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 101(1):48–60. doi:10.1093/jnci/djn415
Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A (1997) Cancer and diabetes—a follow-up study of two population-based cohorts of diabetic patients. J Intern Med 241(6):471–475
Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM (2009) Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27(20):3297–3302. doi:10.1200/JCO.2009.19.6410
Kelsey JL, Berkowitz GS (1988) Breast cancer epidemiology. Cancer Res 48(20):5615–5623
Kleinberg DL, Wood TL, Furth PA, Lee AV (2009) Growth hormone and insulin-like growth factor-i in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev 30(1):51–74. doi:10.1210/er.2008-0022
Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121(4):856–862. doi:10.1002/ijc.22717
LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S (2008) Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes 116(Suppl 1):S4–S6. doi:10.1055/s-2008-1081488
Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE (2008) The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat 109(2):389–395. doi:10.1007/s10549-007-9654-0
Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE (2003) Type 2 diabetes and subsequent incidence of breast cancer in the nurses’ health study. Diabetes Care 26(6):1752–1758
Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA, Laughlin GA, Erickson K, Thomson CA, Bardwell WA, Hajek RA, Pierce JP (2010) Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-010-0732-3
Richardson LC, Pollack LA (2005) Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2(1):48–53. doi:10.1038/ncponc0062
Rose DP, Vona-Davis L (2009) Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther 9(8):1091–1101. doi:10.1586/era.09.71
Rose DP, Vona-Davis L (2010) Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas 66(1):33–38. doi:10.1016/j.maturitas.2010.01.019
Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL (2002) Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J Clin Oncol 20(17):3628–3636
Stephenson GD, Rose DP (2003) Breast cancer and obesity: an update. Nutr Cancer 45(1):1–16
Tsugane S, Inoue M (2010) Insulin resistance and cancer: epidemiological evidence. Cancer Sci. doi:10.1111/j.1349-7006.2010.01521.x
Ursin G, Longnecker MP, Haile RW, Greenland S (1995) A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology 6(2):137–141
Verlato G, Zoppini G, Bonora E, Muggeo M (2003) Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care 26(4):1047–1051
Vona-Davis L, Howard-McNatt M, Rose DP (2007) Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev 8(5):395–408. doi:10.1111/j.1467-789X.2007.00396.x
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000, projections for 2030. Diabetes Care 27(5):1047–1053
Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B (2005) Diabetes mellitus and breast cancer. Lancet Oncol 6(2):103–111. doi:10.1016/S1470-2045(05)01736-5
Competing interests
The authors declare no competing interests. The authors have full control of all primary data and allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schrauder, M.G., Fasching, P.A., Häberle, L. et al. Diabetes and prognosis in a breast cancer cohort. J Cancer Res Clin Oncol 137, 975–983 (2011). https://doi.org/10.1007/s00432-010-0960-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0960-2