Abstract
The objectives of this study are to assess the impact of pre-existing diabetes and diabetes treatment on breast cancer prognosis. 8,108 women with centrally confirmed invasive breast cancer in the Women’s Health Initiative diagnosed between 1998 and 2013 were followed through the date of death or September 20, 2013. Information on diabetes and diabetes therapy were obtained via self-report and face-to-face review of current medication containers, respectively. Cox proportional hazard regression was used to estimate adjusted relative hazard ratios for overall mortality. The proportional subdistribution hazard model was used to estimate hazard ratios for breast cancer-specific mortality. Compared with women without diabetes, women with diabetes had significantly increased risk of overall mortality (HR 1.26 95 % CI 1.06–1.48), especially among those who took insulin or had longer duration of diabetes. However, diabetes was not associated with increased risk of breast cancer-specific mortality, regardless of type of treatment and duration of diabetes, despite the significant association of diabetes with unfavorable tumor characteristics. Our large prospective cohort study provides additional evidence that pre-existing diabetes increases risk of total mortality among women with breast cancer. The increased total mortality associated with diabetes was mainly driven by increased risk of dying from diseases other than breast cancer. Thus, the continuum of care for breast cancer patients with diabetes should include careful attention to CVD risk factors and other non-cancer conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Type 2 diabetes (hereafter referred to as diabetes) and breast cancer are common diseases in aging women. Recently, there has been great interest in investigating whether diabetes and its treatment increase breast cancer risk. A large body of epidemiologic evidence suggests that people with diabetes have a significantly higher risk of breast cancer incidence with a summary point relative risk of 1.20–1.27 based on three recent meta-analysis studies [6, 19, 21]. There is also convincing evidence that overall mortality following breast cancer is significantly higher in diabetic women with breast cancer than their non-diabetic counterparts [12, 18, 22, 34]. However, further interpretation of the observation for overall mortality is challenging, given the complex influences of diabetes on cancer prognosis.
First, diabetes may directly influence breast cancer progression and outcomes via the physiologic effects of hyperinsulinemia and/or hyperglycemia [28, 32]. However, diabetes may also increase the risk of overall mortality as a result of diabetes itself and its complications. Women with diabetes and breast cancer, for example, may be more likely to die from cardiovascular diseases. Only a few studies [14, 24, 37] have examined the impact of diabetes on breast cancer-specific mortality with mixed results. Thus, more definitive studies are needed to clarify whether the higher overall mortality seen in breast cancer patients with diabetes compared to those without diabetes is related to a poorer prognosis specific to breast cancer or due to risk of mortality from other causes (competing risk).
Second, breast cancer prognosis may be influenced by pharmacologic treatments for diabetes. Studies have shown that the effects of some glucose-lowering therapies are associated with breast cancer risk and that their effects vary by type of diabetes therapy. Metformin, an oral drug widely used as a first-line therapy for type 2 diabetes, has been associated with a lower risk of breast cancer incidence compared with other anti-diabetic therapies such as insulin and sulfonylureas [8, 10]. In contrast to incidence, however, few studies have examined treatment of diabetes in relation to breast cancer prognosis, with inconclusive results [11, 15].
In this study, we used the Women’s Health Initiative (WHI), a large prospective cohort study, to address the following questions, with the goal of advancing understanding of how pre-existing diabetes influences breast cancer prognosis: (1) is there a total mortality difference between diabetic and non-diabetic women with breast cancer? (2) Is there a breast cancer-specific mortality difference between diabetic and non-diabetic women with breast cancer? (3) Is diabetes associated with unfavorable tumor characteristics? 4) Do different diabetes pharmacologic treatments have different impacts on breast cancer prognosis.
Methods
Women’s Health Initiative (WHI)
The WHI is an ongoing, ethnically and geographically diverse, multi-center clinical trial (CT) and observational study (OS) designed to address major causes of morbidity and mortality in postmenopausal women. In brief, between September 1993 and December 1998, the WHI enrolled 161,808 postmenopausal women aged 50–79 years at 40 clinical centers throughout the United States. The WHI CT included four overlapping components: two Hormone Therapy Trials (27,347 women), a Dietary Modification Trial (48,835 women), and a Calcium/Vitamin D Supplementation Trial (36,282 women). CT follow-up ended March 31, 2005 and observational follow-up immediately commenced among participants consenting to the WHI Extension 1 (2005–2010) and subsequently to WHI Extension 2 (2010–2015). Participants in the OS included 93,676 women who were screened for the clinical trials but proved to be ineligible or unwilling to participate, or were recruited through a direct invitation for the observational study. In 2005, OS follow-up continued for participants who consented to WHI Extension 1 and subsequently to WHI Extension 2. Details of the scientific rationale, eligibility requirements, and baseline characteristics of the participants in the WHI have been published elsewhere [1]. Consent rates to the WHI Extensions are posted to the WHI website [2]. The study was overseen by IRBs at all 40 clinical centers and at the coordinating center, as well as by a data safety and monitoring board. All participants in the WHI gave informed consent. Both the CT and OS are used for this study.
Follow-up and ascertainment of breast cancer cases
Incident breast cancer cases were identified by self-administered questionnaires (administered every 6 months in the CT through 2005, and annually in the CT after 2005 and in OS), with all cases confirmed by medical record review. All primary breast cancer cases were then coded centrally in accordance with the Surveillance Epidemiology and End Results (SEER) coding guidelines. The breast cancer stage was categorized as: in situ, localized (confined to primary site); regional (spread to regional lymph nodes); distant (cancer has metastasized); or unknown (unstaged). Other tumor characteristics that are available in the WHI database include tumor size, positive lymph nodes, tumor grade, histology, estrogen receptor status, progesterone receptor status, and HER2 status.
Study population
As of September 20, 2013, there were a total of 10,911 breast cancer cases. After excluding cases of breast cancer in situ (1,970 women), breast cancers with missing information on stage (107 women), 713 women with prevalent cancer at baseline (other than non-melanoma skin cancer), and 13 type 1 diabetes cases (identified by self-report of being told of a diabetes diagnosis at 21 years of age or younger), a total of 8,108 cases were available for final analysis. The study population included 6,115 localized, 1,879 regional, and 114 distant breast cancer cases that occurred during the WHI follow-up 1994–2013.
Measurements
Outcomes
Our primary outcome was breast cancer-specific mortality. However, we also examined overall mortality as an outcome for comparison with the results of previous studies.
Diabetes status, diabetes duration
In the WHI, diabetes at enrollment (prevalent diabetes) was defined as a positive answer to the question “did a doctor ever say that you had sugar diabetes or high blood sugar when you were not pregnant”. Medically treated diabetes at enrollment was defined as whether or not the participant reported ever having been treated for diabetes with pills or insulin shots. Incidence of medically treated diabetes was also determined during WHI follow-up. The definition of incident diabetes was a positive response to the question on either the semiannual or annual follow-up questionnaires: “Since the date given on this form has a doctor prescribed for the first time any of the following pills or treatments?”, and subsequent selection of any of the following responses: pills for diabetes, insulin shots for diabetes. Self-reported diabetes in the WHI has been found to be reliable based on medication inventories, fasting glucose levels, and medical record review [17, 26]. We determined diabetes status up to the date of breast cancer diagnosis. The duration of diabetes for prevalent diabetes (diabetes at enrollment) was estimated as the difference between age of the participant when first diagnosed with diabetes and age at breast cancer diagnosis. The duration of diabetes for incident diabetes (diagnosed during WHI follow-up) was estimated from the date of the report of diabetes to the date of breast cancer diagnosis.
Diabetes therapies
Information on diabetes therapies was extracted from WHI medication inventory data. The WHI Clinical Centers collected current medication on CT participants at baseline and years 1, 3, 6, and 9. For OS participants, medication inventories took place at baseline and year 3. During each visit, participants were asked to bring all current prescription medications. Information on medications included the product name or generic name of the medication, the prescribed dose and frequency, the actual dose and frequency, and the duration. Information on different types of anti-diabetic drugs was extracted from the WHI medication inventory collected at baseline and follow-ups. Exposure to various anti-diabetic medications was categorized into insulin (ever used alone or with other oral medications), metformin (ever used alone or with other oral medications), or only used other anti-diabetic medications. If treated diabetes was reported but medication data were not available at a particular medication inventory, the exposure was coded as “unknown treatment”. Because the WHI only requested participants to report newly diagnosed treated diabetes during follow-up, in this analysis, we defined incident diabetes as treated diabetes before breast cancer diagnosis. Diabetes therapies were also defined up to breast cancer diagnosis.
Comorbidity
Information on comorbidity was based on eight diseases most prevalent at baseline in the study population. These eight included hypertension, cardiovascular problems (including nonfatal MI, CHD death, coronary artery bypass graft (CABG) or percutaneous transluminal coronary angioplasty (PTCA), stroke, and heart failure), depression, osteoporosis, osteoarthritis or arthritis, hyperlipidemia, asthma, and cancer. Other than asthma (only prevalent cases collected at baseline), all other comorbid conditions were updated through the date of breast cancer diagnosis or September 30, 2010. Women were categorized by the number of comorbid conditions (0—no comorbidity before their breast cancer diagnosis, 1—one comorbid condition, 2—two conditions, 3—three comorbid conditions, and 4—four or more comorbid conditions).
Demographics, breast cancer risk factors and other covariates
Other covariates included age at diagnosis, race/ethnicity, education level, body mass index (BMI), physical activity, alcohol intake, family history of cancer among females, total daily energy intake, percent of daily dietary calories from fat, history of hormone therapy use, and participation in study cohorts (participation in OS or CTs, and different treatment assignments for all three clinical trials). Other than age at breast cancer diagnosis, and different treatment assignments, all other information was ascertained at baseline. Measurement of these variables is described more completely in the Tables.
Statistical analysis
Distribution of women’s characteristics was compared between women with and without diabetes. Chi-square tests were used to evaluate differences for categorical covariates, and t-tests were used for continuous variables. Overall mortality and breast cancer-specific mortality stratified by diabetes status was estimated with the Kaplan–Meier method first. Survival time was measured as the days from date of breast cancer diagnosis until death or September 20, 2013, whichever came first. For overall mortality analyses, we treated data of persons alive at the end of follow-up as censored observations.
Multivariable Cox proportional hazards regressions were then used to estimate adjusted relative hazard ratios for overall mortality in relation to diabetes status after adjusting for potential confounders. The proportional subdistribution hazard model proposed by Fine and Gray [13] was used to estimate hazard ratios for breast cancer-specific mortality associated with diabetes status by accounting for non-breast cancer mortality as competing risk. For the purpose of comparison with previous studies, we also used conventional epidemiology methods (Cox proportional hazards) to analyze breast cancer-specific mortality by considering the dates of death from causes other than breast cancer as censored observations. In all multivariate models, potential confounders included age at breast cancer diagnosis; cancer stage (localized or regional); race/ethnicity, education level, obesity, physical activity, alcohol intake, family history of cancer among female relatives, total daily energy intake, percent of daily dietary calories from fat, history of hormone therapy use, and comorbidity. We fitted models with and without adjustment for stage along with other covariates, to see how much the stage adjustment attenuated the mortality risk.
Results
Of a total of 8,108 women with invasive breast cancer, 727 (9.0 %) had pre-existing treated diabetes before breast cancer diagnosis (referred to hereafter as diabetes). Baseline patient characteristics by diabetes status are shown in Table 1. Compared with women without diabetes, women with diabetes were significantly more likely to be older, be members of non-White race/ethnicity groups, be heavier, be physically inactive, have no current alcohol use, be less educated, and not use hormones or to use estrogen alone, and have greater number of comorbid conditions. However, there was no substantial difference between the diabetes group and no diabetes group in family history of cancer among female relatives (Table 1).
There were 1498 (20.3 %) deaths in the no diabetes group and 181 (24.9 %) deaths in the diabetes group. In the no diabetes group, 36.7 % were from breast cancer, 17.8 % from other cancers, 18.4 % from cardiovascular disease, 19.6 % from other known causes, and 7.5 % from unknown causes. In the diabetes group, 26.0 % were from breast cancer, 12.7 % from other cancers, 23.2 % from cardiovascular disease, 29.8 % from other known causes, and 8.3 % from unknown causes.
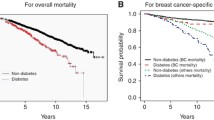
Figure 1 shows the Kaplan–Meier survival curves stratified by diabetes status for overall mortality (Fig. 1a) and the survival curves stratified by diabetes status for breast cancer-specific mortality using competing mortality model (Fig. 1b). There was a significant difference between women without and with diabetes for overall mortality (p < 0.0001) but not for breast cancer-specific mortality (p = 0.9) by the log-rank test.
Table 2 shows hazard ratios for overall mortality in relation to diabetes characteristics. Compared with women without diabetes, women with diabetes had significantly increased risk of overall mortality (HR 1.26 95 % CI 1.06–1.48). Examining type of treatment and diabetes duration, women who received insulin or other non-metformin oral drugs, or had longer duration of diabetes prior to breast cancer diagnosis had significantly higher risk of overall mortality. Comparing models with and without adjusting for cancer stage, the hazard ratios for overall mortality were slightly attenuated, but were not substantially changed by stage adjustment.
Table 2 also shows hazard ratios for breast cancer-specific mortality in relation to diabetes using the competing risk method. There was no increased risk for breast cancer-specific mortality associated with diabetes regardless of type of treatment or duration of diabetes. Results were not substantially different with or without adjustment for stage. Results for breast cancer-specific mortality using conventional epidemiology methods (censoring women experiencing competing events at the time of these events) also demonstrated no significant association with diabetes, although the point estimates were slightly overestimated (e.g., HR 0.81, 95 % CI 0.58–1.12 without competing risk model vs HR 0.74, 95 % CI 0.53–1.02 with competing risk model.)
Among the 8,108 women with breast cancer, compared to women without diabetes, women with diabetes were more likely to have large tumor size, advanced tumor stage, moderately and poorly differentiated grade tumors, ductal histology, and a longer interval from the last mammogram to breast cancer diagnosis. There were no statistically significant differences in positive lymph nodes, estrogen, progesterone, and HER2 receptor status (Table 3), although women with diabetes were less likely to have missing tumor markers for HER2.
Discussion
Our large prospective study showed that pre-existing diabetes increased adjusted risk of overall mortality among women with breast cancer, especially among women with diabetes who required insulin therapy or had longer duration of diabetes. However, pre-existing diabetes was not associated with increased risk of breast cancer-specific mortality regardless of type of treatment and duration of diabetes, despite the significant association of diabetes with unfavorable tumor characteristics. Compared with women without pre-existing diabetes, cause of death among women with pre-existing diabetes was more likely to be attributed to cardiovascular disease and other known causes of death than to breast cancer or other cancer types.
Our observed increased risk of overall mortality associated with pre-existing diabetes is in line with a recent published meta-analysis where pre-existing diabetes was significantly associated with overall mortality in six of seven studies with a significant pooled HR of 1.49 (95 % CI 1.35–1.65) [29]. A few studies [7, 9, 14, 24, 37] have examined diabetes in relation to breast cancer-specific mortality with mixed results. Two studies [7, 24] reported an increased risk for breast cancer-specific mortality in women with diabetes, while two other studies [9, 14] reported no increase in risk. Of the two studies [7, 24] that reported an increased risk, one study [24] was based on Swedish registry data where diabetes was defined as hospitalized for diabetes, which may represent more severe cases than diabetes in the general population. The other study [7] was conducted in Taiwan and was unable to adjust for some important confounders, such as physical activity and BMI. Srokowski et al. [37] used the Surveillance, Epidemiology, and End Results—Medicare database and observed an elevated breast cancer-specific mortality in women with diabetes who received chemotherapy compared with their non-diabetic counterparts, but not in women who had not received chemotherapy. In addition, among all previous studies examining breast cancer-specific mortality, none of them considered competing risk. Rather, they censored women experiencing competing events at the time of these events, which may overestimate the absolute risk of the event of interest [31, 38]. We overcame this limitation through applying the proportional subdistribution hazard model proposed by Fine and Gray [13] to estimate breast cancer-specific mortality by accounting for non-breast cancer mortality outcomes as competing risk. Although in this case, the results were essentially the same in both methods, other studies have demonstrated that competing risk analysis yields less upward bias in risk estimates [25].
Experimental studies have shown that diabetes may increase breast cancer-specific mortality via direct physiologic effects of hyperinsulinemia [33, 39]. However, our data did not support the initial hypothesis that diabetes may be associated with adverse prognosis specific to breast cancer. If there is an unfavorable effect of diabetes on breast cancer-specific mortality this may be counterbalanced by beneficial effects of positive lifestyle changes or some diabetes therapies among diabetic women.
Pre-clinical studies suggest that metformin, an oral drug widely used as a first-line therapy for type 2 diabetes, may decrease breast cancer progression [5]. A few epidemiological studies have examined the effect of metformin on breast cancer prognosis with inconclusive results [4, 11, 16, 20, 30, 40]. Three studies [16, 30, 40] found a significant inverse association between metformin therapy and all-cause mortality, while three studies [4, 11, 20] did not. Two other studies [20, 30] reported null associations between metformin and breast cancer-specific mortality. Our data did not support the hypothesis that metformin improves overall survival and breast cancer-specific mortality in women with breast cancer. However, study power for breast cancer-specific mortality may be limited because of the relatively small sample size. Thus, larger studies are needed to assess the prognostic value of metformin for cancer patients.
Our finding that breast cancer-specific mortality was not significantly higher among women with diabetes could have occurred for at least two reasons. First, effective treatments for breast cancer, such as adding trastuzumab to adjuvant chemotherapy or chemotherapy with anthracycline is associated with increased risk of cardiomyopathy and congestive heart failure [35], which may increase the risk of dying from cardiovascular disease, especially in patients with diabetes [36]. Radiation therapy may also increase cardiovascular disease [3]. However, the WHI does not include information on cancer treatment so we are not able to support or refute this using the current data. A second explanation could lie in misclassification when assigning cause of death. Patients with diabetes may be more likely to be assigned cardiovascular diseases as a cause of death, even when they have advanced cancer, because of the well-known association of diabetes with cardiovascular complications and mortality. However, in our study women with diabetes were also more likely to have their deaths attributed to non-cancer, non-CVD causes.
Our data revealed that diabetes was associated with less favorable tumor characteristics including larger tumor size, more poorly differentiated grade, and more advanced tumor stage. This may be because women with diabetes had less frequent breast cancer screening compared with those without diabetes; our data show that women with diabetes were more likely to have had their most recent mammogram more than 1 year before diagnosis. Other studies also documented underuse of breast cancer screening among women with diabetes compared to those without diabetes [23, 27], which may lead to delayed diagnosis. However, our data do not suggest that diagnostic delay or unfavorable tumor characteristics in women with diabetes resulted in increased breast cancer mortality even after considering competing non-cancer mortality.
Although the WHI includes detailed and comprehensive data on demographic and breast cancer risk factors, tumor characteristics and physician-adjudicated causes of death, there is no cancer treatment information available in the WHI data, thus, we could not account for cancer treatment in our data analysis. Second, diabetes diagnosis was based on self-report. This may have resulted in some degree of misclassification of the exposure. This misclassification may have biased our effect estimates toward the null. However, a validation study in the WHI has shown a high concordance of self-report with a gold standard based on medical record review and with medication inventories [17, 26]. In addition, the statistical power for breast cancer-specific mortality analysis may have been limited due to the few breast cancer deaths. Other limitations include lack of information regarding glucose control and diabetes progression.
In conclusion, our large prospective cohort study provides additional evidence that pre-existing diabetes increases risk of total mortality among women with breast cancer. The increased total mortality associated with diabetes was mainly driven by increased risk of dying from diseases other than breast cancer. The continuum of care for breast cancer patients with diabetes should include careful attention to CVD risk factors and other non-cancer conditions, since these caused the majority of deaths among women in our study. Further study is needed to understand how treatment choices for cancer and non-cancer conditions may differ among breast cancer patients with and without diabetes.
References
The Women’s Health Initiative Study Group (1998) Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 19:61–109
Women’s Health Initiative. https://www.whi.org/about/SitePages/About%20WHI.aspx
Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, Constine LS (2003) Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat oncol 13:346–356. doi:10.1016/S1053-4296(03)00026-2
Bayraktar S, Hernadez-Aya LF, Lei X, Meric-Bernstam F, Litton JK, Hsu L, Hortobagyi GN, Gonzalez-Angulo AM (2012) Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 118:1202–1211. doi:10.1002/cncr.26439
Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F (2011) Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71:4366–4372. doi:10.1158/0008-5472.CAN-10-1769
Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, Fairley LL, Boniol M, Zheng T, Zhang Y, Pasterk M, Smans M, Curado MP, Mullie P, Gandini S, Bota M, Bolli GB, Rosenstock J, Autier P (2012) Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. doi:10.1038/bjc.2012.414
Chen WW, Shao YY, Shau WY, Lin ZZ, Lu YS, Chen HM, Kuo RN, Cheng AL, Lai MS (2012) The impact of diabetes mellitus on prognosis of early breast cancer in Asia. Oncologist 17:485–491. doi:10.1634/theoncologist.2011-0412
Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JE, Aragaki AK, Rohan T, Ipp E, Kaklamani VG, Vitolins M, Wallace R, Gunter M, Phillips LS, Strickler H, Margolis K, Euhus DM (2012) Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol 30:2844–2852. doi:10.1200/JCO.2011.39.7505
Cleveland RJ, North KE, Stevens J, Teitelbaum SL, Neugut AI, Gammon MD (2012) The association of diabetes with breast cancer incidence and mortality in the Long Island breast cancer study project. Cancer Causes Control 23:1193–1203. doi:10.1007/s10552-012-9989-7
Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT (2012) Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Tr 135:639–646. doi:10.1007/s10549-012-2170-x
Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL (2012) Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 35:299–304. doi:10.2337/dc11-1313
Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, Saquib N, Rock CL, Pierce JP (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29:54–60. doi:10.1200/JCO.2010.29.3183
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Fleming ST, Rastogi A, Dmitrienko A, Johnson KD (1999) A comprehensive prognostic index to predict survival based on multiple comorbidities: a focus on breast cancer. Med Care 37(601–614):18
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685. doi:10.2337/dc10-0666
Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J (2013) Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Tr 137:807–816. doi:10.1007/s10549-012-2404-y
Jackson JM, Defor TA, Crain AL, Kerby T, Strayer L, Lewis CE, Whitlock E, Williams S, Bonds DE, Vitolins MZ, Rodabough RJ, Margolis KL (2013) Self-reported diabetes is a valid outcome in pragmatic clinical trials and observational studies. J Clin Epidemiol 66:349–350. doi:10.1016/j.jclinepi.2012.01.013
Kaplan MA, Pekkolay Z, Kucukoner M, Inal A, Urakci Z, Ertugrul H, Akdogan R, Firat U, Yildiz I, Isikdogan A (2012) Type 2 diabetes mellitus and prognosis in early stage breast cancer women. Med Oncol 29:1576–1580. doi:10.1007/s12032-011-0109-4
Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121:856–862. doi:10.1002/ijc.22717
Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL (2013) Association Between Metformin Therapy and Mortality After Breast Cancer A population-based study. Diabetes Care 36:3018–3026. doi:10.2337/Dc12-2535
Liao S, Li J, Wei W, Wang L, Zhang Y, Li J, Wang C, Sun S (2011) Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pacific J Cancer Prevention 12:1061–1065
Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE (2008) The impact of diabetes on survival following breast cancer. Breast Cancer Res Tr 109:389–395. doi:10.1007/s10549-007-9654-0
Lipscombe LL, Hux JE, Booth GL (2005) Reduced screening mammography among women with diabetes. Arch Intern Med 165:2090–2095
Liu XD, Ji JG, Sundquist K, Sundquist J, Hemminki K (2012) The impact of type 2 diabetes mellitus on cancer-specific survival a follow-up study in Sweden. Cancer 118:1353–1361. doi:10.1002/Cncr.26420
Luo J, Lin HC, He K, Hendryx M (2014) Diabetes and prognosis in older persons with colorectal cancer. Br J Cancer 110:1847–1854. doi:10.1038/bjc.2014.68
Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, Phillips LS (2008) Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 5:240–247. doi:10.1177/1740774508091749
McBean AM, Yu XH (2007) The underuse of screening services among elderly women with diabetes. Diabetes Care 30:1466–1472. doi:10.2337/Dc06-2233
Morss AS, Edelman ER (2007) Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem 282:14635–14644. doi:10.1074/jbc.M608565200
Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC (2011) Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 29:40–46. doi:10.1200/JCO.2009.27.3011
Peeters PJ, Bazelier MT, Vestergaard P, Leufkens HG, Schmidt MK, de Vries F, De Bruin ML (2013) Use of metformin and survival of diabetic women with breast cancer. Current drug safety 8:357–363
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430. doi:10.1002/sim.2712
Richardson LC, Pollack LA (2005) Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2:48–53. doi:10.1038/ncponc0062
Sachdev D, Yee D (2001) The IGF system and breast cancer. Endocr Relat Cancer 8:197–209
Schrauder MG, Fasching PA, Haberle L, Lux MP, Rauh C, Hein A, Bayer CM, Heusinger K, Hartmann A, Strehl JD, Wachter DL, Schulz-Wendtland R, Adamietz B, Beckmann MW, Loehberg CR (2010) Diabetes and prognosis in a breast cancer cohort. J Cancer Res Clin Oncol. doi:10.1007/s00432-010-0960-2
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20:1215–1221
Serrano C, Cortes J, De Mattos-Arruda L, Bellet M, Gomez P, Saura C, Perez J, Vidal M, Munoz-Couselo E, Carreras MJ, Sanchez-Olle G, Tabernero J, Baselga J, Di Cosimo S (2012) Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol 23:897–902. doi:10.1093/annonc/mdr348
Srokowski TP, Fang S, Hortobagyi GN, Giordano SH (2009) Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol 27:2170–2176. doi:10.1200/JCO.2008.17.5935
Wolbers M, Koller MT, Witteman JC, Steyerberg EW (2009) Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 20:555–561. doi:10.1097/EDE.0b013e3181a39056
Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B (2005) Diabetes mellitus and breast cancer. Lancet Oncol 6:103–111. doi:10.1016/S1470-2045(05)01736-5
Xiao Y, Zhang S, Hou G, Zhang X, Hao X, Zhang J (2013) Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer. Tumour Biol. doi:10.1007/s13277-013-1270-5
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R15CA179463. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.” The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.” We also request that you consult www.whi.org for exact wording if you include a description of the WHI trials in your manuscript. A short list of WHI investigators appears in the appendix.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Appendix: short list of whi investigators
Appendix: short list of whi investigators
-
Program Office (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
-
Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
-
Investigators and Academic Centers (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
-
Women’s Health Initiative Memory Study (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Rights and permissions
About this article
Cite this article
Luo, J., Virnig, B., Hendryx, M. et al. Diabetes, diabetes treatment and breast cancer prognosis. Breast Cancer Res Treat 148, 153–162 (2014). https://doi.org/10.1007/s10549-014-3146-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3146-9