Abstract
The aim of this study was to evaluate the influence of body mass index (BMI), weight change on triple-negative breast cancer (TNBC) prognosis in a population-based prospective cohort study. The current analysis included 518 participants diagnosed with TNBC in Shanghai Breast Cancer Survival Study. Weight at 1 year prior to cancer diagnosis, at diagnosis, and at 6, 18 and 36 months after cancer diagnosis and height at 6 months after cancer diagnosis were assessed. Disease-free survival (DFS) and overall survival (OS) were evaluated in relation to BMI and weight change using Cox proportional hazard models. Obesity (BMI ≥ 28.0 kg/m2) at 1-year pre-diagnosis was associated with higher risk of total mortality and recurrence/disease-specific mortality, with multivariate hazard ratios (HRs) of 1.79 (95 % CI 1.06–3.03) and 1.83 (95 % CI 1.05–3.21), respectively. The associations between BMI and TNBC prognosis attenuated over time from pre-diagnosis to post-diagnosis. Compared with stable weight (change within 5 %), weight loss ≥5 % at 18- or 36-month post-diagnosis was related with higher risk of total mortality and recurrence/disease-specific mortality. Respective multivariate HRs were 2.08 (95 % CI 1.25–3.46) and 1.42 (95 % CI 0.77–2.63) for OS, and 2.50 (95 % CI 1.45–4.30) and 2.17 (95 % CI 1.14–4.12) for DFS. However, the association of weight loss and OS/DFS attenuated after excluding patients whose weight was measured after recurrence. Weight gain ≥5 % at 18- or 36-month post-diagnosis was associated with a non-significant increased risk of death. The results showed that obesity pre-diagnosis and weight loss post-diagnosis was inversely associated with TNBC prognosis. Emphasis on maintaining stable weight after cancer diagnosis for TNBC patients may be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies have suggested that obesity at the time of cancer diagnosis or pre-diagnosis is associated with poor prognosis for breast cancer all types combined [1–6]. Triple-negative breast cancer (TNBC), namely negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2), accounts for 10–20 % of breast cancers and it is characterized by aggressive clinical behavior and lack of effective targeted therapies and worse prognosis [7]. Limited research has evaluated the associations of obesity at diagnosis or pre-diagnosis on TNBC prognosis, and the findings are mixed [8–14].
Weight gain is a common and persistent problem in breast cancer patients, especially those who are younger, closer to ideal body weight and who have been treated with chemotherapy [15]. Several studies [16–18] have demonstrated that weight gain post-diagnosis may increase the risk of all-cause mortality and recurrence, including a report from our study [19], whereas other studies find no relation [20–22]. Negative effects of weight loss on breast cancer prognosis have been reported in majority of previous studies [16–20, 22]. To our knowledge, no research has examined whether changes in weight after cancer diagnosis influence outcomes of TNBC. Given the limited treatment options and the poor survival of TNBC, the prognostic effects of weight change may be of particular relevance for its outcome and the clarification may provide a chance to improve the survival rate through weight control.
In an effort to address this gap, we conducted a comprehensive study to elucidate the relationship between BMI, weight change post-diagnosis, and TNBC prognosis, using data from a population-based prospective cohort study Shanghai Breast Cancer Survival Study (SBCSS).
Materials and methods
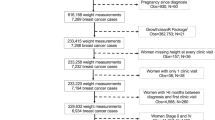
Study participants were women who were diagnosed with a primary breast cancer and enrolled in SBCSS, a population-based cohort study in Shanghai, China. Details of the study design and method have been reported elsewhere [23, 24]. Briefly, through the population-based Shanghai Cancer Registry, 6,299 eligible cases were identified approximately 6 months after cancer diagnosis, and 5,042 were enrolled (participation rate, 80 %) and completed baseline interviews between March 2002 and April 2006. Of these participants, 532 patients were TNBC based on information abstracted from the medical charts and receptor assays done in the Vanderbilt Molecular Epidemiology Lab [25]. For this analysis, we excluded patients with noninvasive breast cancer (TNM stage 0, n = 13) and stage IV breast cancer (n = 1). The final cohort consisted of 518 patients.
In-person interviews by trained interviewers who were mostly retired medical professionals were performed approximately 6 months by using a standardized questionnaire. The baseline survey covered demographic characteristics, cancer diagnosis and treatment, reproductive factors, selected life style factors (e.g., exercise participation, tea consumption, cigarette smoking), dietary intake, use of complementary and alternative medicine, cancer therapies, and quality of life. Medical record charts were reviewed to verify clinical information and treatment. Information on validity of physical activity questionnaire has previously been published [26]. Briefly, all participants were asked to report whether they participated in regular exercise and information on frequency and duration for up to five types of the most common exercises. A Charlson comorbidity index was created for each woman based on a validated comorbidity scoring system [27]. Anthropometric measurements (height, weight, waist and hip circumference) were measured by trained interviewers according to a standard protocol at the baseline survey [19]. In short, weight was measured to the nearest 0.1 kg using a digital weight scale. Height and circumferences were measured at 2.5 cm above the umbilicus and hip circumference at the level of maximum width of the buttocks with subject in a standing position. Participants were also asked to report their pre-diagnosis weight around 1 year before diagnosis and weight at diagnosis.
The cohort has been being followed up by in-person interviews at 18, 36, 60, and 120 months after cancer diagnosis to update exposure information and collect data on disease progression and survival status. The follow-up rate for the 18-, 36-, and 60-month post-diagnosis interview was 91, 84, and 77 %, respectively. The 10-year post-diagnosis survey has been completed for 55 % survivors, and the follow-up survey for the remaining survivors is ongoing. Information on survival status was also collected and ascertained by periodical linkage with the Shanghai Vital Statistics database.
Weight at approximate 18, 36 and 60 months after diagnosis was measured by trained interviewers using the same standard protocol. For subjects with missing data at a specific survey, we substituted the information with a mean value or the value assessed at adjacent surveys. BWI was calculated by dividing weight by height squared (kg/m2).
Change in weight from pre- to post-diagnosis was calculated by subtracting the weight measurement taken at 1-year pre-diagnosis from that taken at each follow-up survey. The relative percent of weight change between pre-diagnosis and each follow-up survey post-diagnosis was evaluated [(weight at follow-up − weight pre-diagnosis)/weight pre-diagnosis × 100]. This current study was focused on the weight change between 1-year pre-diagnosis and 18- or 36-month post-diagnosis to allow sufficient time for patients to recover from the treatment related immediate weight change. A positive and negative value was indicative of weight gain and loss, respectively.
The SBCSS was approved by the institutional review boards of all institutions involved in this study and the participants provided written informed consent prior to interview.
Statistical analysis
BMI was categorized according to Chinese standard: underweight, <18.5 kg/m2; normal weight, 18.5–23.9 kg/m2; overweight, 24.0–27. 9 kg/m2, and obesity ≥28.0 kg/m2. A weight change of <5 % of the pre-diagnosis weight was considered as weight stable (reference); weight gain and loss were defined as a ≥5 % change.
Differences in patient characteristics across baseline BMI categories were evaluated using analysis of variance (ANOVA) for continuous variables and χ 2 test for categorical variables. The endpoints for the analysis were any death for overall survival (OS) and cancer recurrence/metastasis or death related to breast cancer for disease-free survival (DFS). Survival status was censored at the date of last in-person contact or 31 December 2013 (the most recent date for linkage to the Vital Statistics database). For disease-free analysis, censoring occurred at date of last in-person contact or date of death for non-breast cancer death. Multivariable Cox proportional hazard models were used to estimate the adjusted hazard ratios (HRs) and 95 % confidence intervals (CIs) in association with BMI and weight change using age as time scale [28]. Entry time was defined as age at diagnosis and exit time was defined as age at the event or censoring. The following covariates were adjusted for in the multivariate models: age at diagnosis, education level, menopausal status, Charlson comorbidity index, exercise participation, TNM stage, type of surgery received, chemotherapy, and radiotherapy. Smoking rate was very low in our study population, with only 2 current smokers and 6 former smokers seen in our study participants. Thus, we were not able to adjust for smoking in the analysis. Stratified analyses were conducted to explore whether the associations of obesity or weight change were modified by TNM stage, menopausal status, comorbidity, exercise participation, or pre-diagnostic obesity.
Tests for trend were performed by entering the category variable as continuous parameter in the models. Multiplicative interactions were tested for using −2 log likelihood ratio test statistics, which compared models with and without the interaction terms. All analyses were performed using SAS version 9.3. Tests of statistical significance were based on two-sided probability, and p values <0.05 were considered statistically significant.
Results
During the median follow-up of 9.1 years (range 0.6–11.8 years) after cancer diagnosis, 128 deaths and 112 recurrences or breast cancer deaths were documented among 518 TNBC patients. The mean age at cancer diagnosis was 53.4 years (standard deviation, SD 10.6). Overall, 30.9 % of women were overweight (BMI 24.0–27.9 kg/m2) and 12.0 % were obese (BMI ≥ 28.0 kg/m2) at 1 year prior to cancer diagnosis. The corresponding prevalence rates of overweight and obesity were 29.3 and 11.0 % at diagnosis, 35.3 and 12.6 % at 6-month post-diagnosis, and 39.0 and 14.4 % at 18-month post-diagnosis, respectively. The mean weight at 1-year pre-diagnosis, diagnosis, 6-month post-diagnosis, 18-month post-diagnosis, 36-month post-diagnosis, and 60-month post-diagnosis was 59.2 kg (SD 9.4), 58.8 kg (SD 9.3), 60.0 kg (SD 9.2), 60.7 kg (SD 9.4), 60.3 kg (SD 9.2), and 60.1 kg (SD 9.5), respectively. The mean weight changes and SDs from pre-diagnosis to diagnosis, 6-, 18-, and 36-month post-diagnosis were −0.4 ± 2.1 kg (median 0 kg), 0.8 ± 4.0 kg (median 1.0 kg), 1.5 ± 4.6 kg (median 1.0 kg), and 1.5 ± 4.8 kg (median 1.0 kg), respectively.
Table 1 showed the distribution of patient characteristics by baseline BMI. Obese patients tended to be older, post-menopausal, and to have higher WHR. There was no significant difference among BMI groups with regard to time interval from diagnosis to study enrollment, level of education, marriage status, Charlson comorbidity index, exercise participation, tea consumption, chemotherapy, radiotherapy, immunotherapy, tamoxifen use, or tumor stage.
BMI pre-diagnosis and TNBC prognosis
As shown in Table 2, women who were obese (BMI ≥ 28.0 kg/m2) at 1-year pre-diagnosis had higher mortality than normal weight women. The adjusted HRs were 1.79 (95 % CI 1.06–3.03) for total mortality and 1.83 (95 % CI 1.05–3.21) for recurrence/disease-specific mortality. No such association was found for women who were obese at diagnosis or 6-month post-diagnosis. WHR at diagnosis was not significantly associated with mortality, with and without adjustment for BMI at 6-month post-diagnosis.
In stratification analysis, while the negative influence of overweight on TNBC prognosis was limited among pre-menopausal women, no modified effects were observed (data not shown). The association of BMI at pre-diagnosis and mortality also did not varied by TNM stage, comorbidity, or exercise participation (data not shown).
Weight change post-diagnosis and TNBC prognosis
Tables 3 and 4 presented associations of weigh change at 18- and 36-month post-diagnosis with total mortality and recurrence/disease-specific mortality of TNBC, respectively. Compared with women who maintained the weight (change within 5 %), those who lost weight from pre-diagnosis to 18-month post-diagnosis (≥5 %) had HRs of 2.08 (95 % CI 1.25–3.46) for total mortality and 2.50 (95 % CI 1.45–4.30) for recurrence/disease-specific mortality. Similarly, weight loss at 36-month post-diagnosis was associated with TNBC survival with HRs of 1.42 (95 % CI 0.77–2.63) for total mortality and 2.17 (95 % CI 1.14–4.12) for recurrence/disease-specific mortality. Weight gain ≥5 % at 18-month or at 36-month post-diagnosis was not significantly increased the risk of mortality of TNBC.
We further investigated whether the association of weight change with mortality was modified by pre-diagnostic BMI, menopausal status, TNM stage, comorbidity, and exercise participation. While the association of weight loss with mortality appeared to be pronounced among women who were pre-menopausal, or women who were overweight initially, no significant multiplicative interaction was displayed (Tables 3, 4). Exercise participation, TNM stage, and comorbidity did not affect the relationship between weight change and TNBC prognosis (data not shown).
As a sensitivity analysis, all models were run excluding the 8 women who died within the first year of diagnosis, and results were not substantially altered. Similarly, sensitivity analysis done using only data for subjects with weight measurements at all time points (458 cases at 18-month survey and 400 cases at 36-month survey) also showed comparable results. Additionally, results from lag analysis excluding those whose weights were assessed close to the time of death (within 6 months, 14 cases at 18-month survey and 4 cases at 36-month survey) were unchanged. Furthermore, there were about 20 % of women taking tamoxifen in the current study. After excluding subjects who had used tamoxifen, the association pattern for weight loss at 18- or 36-month survey was not substantially changed. However, the association of weight loss and OS/DFS attenuated after excluding these patients whose weight measurement at 18- or 36-month survey was taken after cancer recurrence (data not shown).
Discussion
In this population-based prospective cohort study of women diagnosed with TNBC, we found that obesity prior to diagnosis and weight loss post-diagnosis was associated with worse outcomes. Furthermore, the negative effects of weight loss on prognosis appeared more evident for those who overweight initially or pre-menopausal. It should be noted that the association of weight loss and TNBC prognosis attenuated after excluding patients whose weight was measured after recurrence.
Lots of observational studies have shown that obesity before or after diagnosis is associated with poorer survival for all types of breast cancer combined [1–6]. A systemic review that included 82 follow-up breast cancer studies showed that compared with normal weight women, obesity was associated with poorer overall and breast cancer survival in pre- and post-menopausal breast cancer, regardless of when BMI was ascertained [6]. The limited studies that evaluated influence of BMI on TNBC survival have yielded mixed findings [8–12]. The largest retrospective study published so far, including 2,311 women with stage I–III TNBC tumors, found no difference in DFS or OS across BMI groups at diagnosis [12]. Similar null association between BMI at diagnosis and TNBC survival was also observed in two retrospective studies of TNBC patients [8, 11]. However, a large pooled analysis including 5,683 operable breast cancer patients [13] indicated that the magnitude of the negative effect of severe obesity at diagnosis on survival outcomes was similar across the three breast cancer subtypes (ER/PR-positive/HER-2-negative, HER-2- positive, TNBC). Our study showed that obesity pre-diagnosis was associated with higher risk of TNBC mortality and this association appeared to be stronger among women who were pre-menopausal, consistent with Turkoz’s report [14]. The difference in the study design, the characteristics of study population, or time of BMI assessment may be partly contributed to the discrepancy.
Several plausible biological mechanisms, including changes in glucose metabolism, high circulating levels of estrogen, increased insulin and insulin-like growth factors [5, 29, 30], have been proposed to support the effects of obesity in breast cancer patients, mainly for hormone receptor positive breast cancer. The mechanism underlying the association between obesity and TNBC prognosis is unclear. It may be partly explained by other medical conditions such as diabetes and heart disease [15], and obesity-related regulatory protein (such as leptin and adiponectin) secreting by adipose tissue which plays a key role in proliferation, apoptosis, and/or migration of breast tumor cells [31, 32]. In our study, the association was limited to BMI pre-diagnosis and the association attenuated over time from pre-diagnosis to post-diagnosis. BMI at diagnosis and taken soon after diagnosis may be affected by cancer stage/treatment and disease progression, which may attenuate the association between obesity and breast cancer prognosis. Of note, chance finding, misclassification, and confounding effect cannot be completely ruled out in our study. However, misclassification on weight due to self-report was likely to be non-differential which would bias the results toward null. Triple-negative breast cancers comprise a very heterogeneous group of cancers, and obesity may influence molecular subgroup distribution. Much remains to be learned about the role of obesity in TNBC survival, and further studies are needed to confirm the association and clarify the possible mechanisms.
The association of weight gain with higher mortality risk of breast cancer was previously reported [16–18], including the SBCSS study [19], although the evidence was not entirely consistent [20–22]. Many literatures demonstrated that weight loss was associated with worse prognosis [16–20, 22]. A pooling project [20] observed that weight loss ≥10 % was related to a 40 % increased risk of death (HR 1.41; 95 % CI 1.14–1.75) among American women with breast cancer and over three times the risk of death (HR 3.25; 95 % CI 2.24–4.73) in Chinese women with breast cancer. To our knowledge, no study has specifically evaluated the association of change in weight with TNBC outcomes. Our study showed that weight loss was associated with higher risk of morality and the negative effects of weight loss on prognosis seemed to be greater for those who were overweight initially or who were pre-menopausal.
For our observed higher risk of mortality among TNBC survivors who lost weight, one of potential explanation was that it may be an early marker of caner cachexia or pre-cachexia, which results in not only weight loss but substantial loss of lean body mass [20, 33]. It is hypothesized that exaggerated loss of lean body mass in cancer survivors may be related to chronic inflammation, insulin resistance, and reduction in physical activity [33]. In addition, weight loss may be an early marker of comorbid overweight women, which appeared to be at risk for weight loss, and comorbidity may also be related to receiving less chemotherapy, experiencing more toxicity of treatment, and thus higher risk of mortality [20, 34]. Unfortunately, we were unable to carry out more in depth stratified analyses such as by comorbidity and treatment cycle due to the limited sample size or lack of data. When the association was evaluated based on BMI initially, data were significant only among those who were overweight initially, suggesting that early in the disease process, weight loss in this most aggressive form of breast cancer may be a marker of a worse prognosis, especially among women who were initially overweight or obese. Therefore, it raised a question about the safety of intentional weight loss in the first few years post-diagnosis among TNBC survivors, even though they were overweight. Of not, some of the 18- or the 36-month weight assessment was taken after cancer recurrence and the sensitivity analysis showed that the association of weight loss and TNBC prognosis attenuated after excluding these patients from the analysis, suggesting that weight loss could be a result of disease progression and reverse causation may be as the reason for such association. More research with larger sample size is warranted to examine the influence of weight loss on TNBC prognosis and clarify the related biologic mechanisms.
This study was a population-based prospective cohort study with up to 10 years of follow-up. Sequential information of weight taken at different time point allowed us to describe the changing patterns of weight/BMI overtime and analyze the effects of such change on survival of TNBC. It should be noted that our study has some limitations. Although weight information at each follow-up survey was collected, we had limited power to comprehensively estimate the influence of long-term weight change on TNBC survival. Another shortfall of this study was the potential for reverse confounding to influence the results (i.e., those who were with cancer recurrence or who are near death were more likely to lose weight). Additionally, statistical power was not sufficient to explore interactions by BMI and other factors, and this could be one potential explanation for lack of significance.
In summary, this population-based prospective cohort study found that obesity at 1-year pre-diagnosis and weight loss during first several years post-diagnosis were associated with worse outcomes of TNBC patients. It should be noted that the associations of weight loss and TNBC prognosis attenuated after excluding patients whose weight was measured after recurrence. Our findings would suggest that TNBC patients may require closer surveillance and proper intervention to maintain stable weight.
References
Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN (2011) Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat 129:565–574
Protani M, Coory M, Martin JH (2010) Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 123:627–635
Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH (2013) Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol 24:2506–2514
Kwan ML, Chen WY, Kroenke CH et al (2012) Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat 132:729–739
Azrad M, Demark-Wahnefried W (2014) The association between adiposity and breast cancer recurrence and survival: a review of the recent literature. Curr Nutr Rep 3:9–15
Chan DS, Vieira AR, Aune D et al (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25:1901–1914
Boyle P (2012) Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 23(Suppl 6):vi7–vi12
Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB (2011) Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer 117:4132–4140
Sparano JA, Wang M, Zhao F et al (2012) Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer 118:5937–5946
Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH (2013) Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res 184:253–259
Tait S, Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX (2014) Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat 146:189–197
Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM (2012) Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer 12:364–372
Pajares B, Pollan M, Martin M et al (2013) Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast Cancer Res 15:R105
Turkoz FP, Solak M, Petekkaya I et al (2013) The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON 18:335–341
Makari-Judson G, Braun B, Jerry DJ, Mertens WC (2014) Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol 5:272–282
Nichols HB, Trentham-Dietz A, Egan KM et al (2009) Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev 18:1403–1409
Thivat E, Therondel S, Lapirot O et al (2010) Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer 10:648
Bradshaw PT, Ibrahim JG, Stevens J et al (2012) Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology 23:320–327
Chen X, Lu W, Zheng W et al (2010) Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat 122:823–833
Caan BJ, Kwan ML, Shu XO et al (2012) Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 21:1260–1271
Jeon YW, Lim ST, Choi HJ, Suh YJ (2014) Weight change and its impact on prognosis after adjuvant TAC (docetaxel–doxorubicin–cyclophosphamide) chemotherapy in Korean women with node-positive breast cancer. Med Oncol 31:849
Caan BJ, Kwan ML, Hartzell G et al (2008) Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control 19:1319–1328
Shu XO, Zheng Y, Cai H et al (2009) Soy food intake and breast cancer survival. JAMA 302:2437–2443
Epplein M, Zheng Y, Zheng W et al (2011) Quality of life after breast cancer diagnosis and survival. J Clin Oncol 29:406–412
Su Y, Zheng Y, Zheng W et al (2011) Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer 11:292
Matthews CE, Shu XO, Yang G et al (2003) Reproducibility and validity of the Shanghai Women’s Health Study physical activity questionnaire. Am J Epidemiol 158:1114–1122
Grunau GL, Sheps S, Goldner EM, Ratner PA (2006) Specific comorbidity risk adjustment was a better predictor of 5-year acute myocardial infarction mortality than general methods. J Clin Epidemiol 59:274–280
Korn EL, Graubard BI, Midthune D (1997) Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 145:72–80
Rock CL, Demark-Wahnefried W (2002) Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol 20:3302–3316
Demark-Wahnefried W, Campbell KL, Hayes SC (2012) Weight management and its role in breast cancer rehabilitation. Cancer 118:2277–2287
Cole SW (2009) Chronic inflammation and breast cancer recurrence. J Clin Oncol 27:3418–3419
Oh SW, Park CY, Lee ES et al (2011) Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: a cohort study. Breast Cancer Res 13:R34
Dodson S, Baracos VE, Jatoi A et al (2011) Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62:265–279
Zauderer M, Patil S, Hurria A (2009) Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat 117:205–210
Acknowledgments
The authors thank Dr. Wei Lu and Dr. Fan Jin for her support in study implements and the participants and staff members of the SBCSS for making this study possible. The SBCSS was supported by Grants from the Department of Defense Breast Cancer Research Program (DAMD 17-02-1-0607 to X.-O. Shu) and the National Cancer Institute (R01 CA118229 to X.-O. Shu), and grants from the Shanghai Municipal Commission of Health and Family Planning (Grant No. 20134070 to P. P. Bao), as well as from the National Natural Science Foundation of China (Grant No. 81402734 to P. P. Bao).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bao, PP., Cai, H., Peng, P. et al. Body mass index and weight change in relation to triple-negative breast cancer survival. Cancer Causes Control 27, 229–236 (2016). https://doi.org/10.1007/s10552-015-0700-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0700-7