Abstract
Purpose
Studies of vitamin D-pathway genetic variants in relation to cancer risk have been inconsistent. We examined the associations between vitamin D-related genetic polymorphisms, plasma 25-hydroxyvitamin D [25(OH)D], and breast cancer risk.
Methods
In a population-based case–control study of 967 incident breast cancer cases and 993 controls, we genotyped 25 polymorphisms encoding the vitamin D receptor (VDR) gene, 1α-hydroxylase (CYP27B1), 24-hydroxylase (CYP24A1), and vitamin D-binding protein (GC) and measured plasma 25(OH)D. We used multivariable logistic regression to estimate adjusted odds ratios (ORs) and 95 % confidence intervals (CIs).
Results
Among CYP24A1 polymorphisms, rs6068816 was associated with a 72 % reduction in breast cancer risk (TT vs. CC, OR 0.28, 95 % CI 0.10–0.76; p trend = 0.01), but for rs13038432, the 46 % decrease included the null value (GG vs. AA, OR 0.54, 95 % CI 0.17–1.67; p trend = 0.03). Increased risk that included the null value was noted for CYP24A1 rs3787557 (CC vs. TT, OR 1.34, 95 % CI 0.92–1.89). The VDR polymorphism, TaqI (rs731236), was associated with a 26 % risk reduction (TT vs. CC, OR 0.74, 95 % CI 0.56–0.98; p trend = 0.01). For other polymorphisms, ORs were weak and included the null value. The inverse association for plasma 25(OH)D with breast cancer was more pronounced (OR 0.43, 95 % CI 0.27–0.68) among women with the common allele for CYP24A1, rs927650 (p for interaction on a multiplicative scale = 0.01).
Conclusion
Breast cancer risk may be associated with specific vitamin D-related polymorphisms, particularly CYP24A1. Genetic variation in the vitamin D pathway should be considered when designing potential intervention strategies with vitamin D supplementation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D in the body comes from two main sources: endogenous production from sun exposure (accounting for up to 90 %) or ingestion of food or supplements [1]. Epidemiologic studies have consistently reported reduced breast cancer incidence and mortality associated with greater exposure to sunlight and ultraviolet B (UVB) irradiation [2–11]. However, results for studies evaluating dietary and supplemental intake of vitamin D and breast cancer risk are mixed [12–18]. Circulating 25-hydroxyvitamin D [25(OH)D] is an objective measure of vitamin D status from sunlight exposure, dietary, or supplement intake. Two recent meta-analysis of prospective studies showed that overall 25(OH)D blood levels are associated with reduced breast cancer risk [19, 20]. However, three recent prospective studies observed no association between 25(OH)D levels and breast cancer risk [21–23], and only one recent prospective study found an inverse association among whites, but not other ethnic groups [24].
Several enzymatic steps are involved in vitamin D metabolism. Genetic variants involved in vitamin D metabolism potentially modify cancer risk [25]. UVB exposure converts 7-dehydrocholesterol into vitamin D3 (cholecalciferol). Metabolism is initiated when vitamin D3 is hydroxylated in the liver to 25(OH)D through a reaction catalyzed by 25-hydroxylase enzyme. If calcium levels drop, parathyroid hormone (PTH) is released and activates 1α-hydroxylase (encoded by CYP27B1) that hydroxylates 25(OH)D to the active metabolite, 1α, 25-dihydroxyvitamin D [1,25(OH)2D]. 1,25(OH)2D binds to the vitamin D receptor (VDR), a ligand-dependent transcription factor, that regulates transcription of a number of genes involved in cell proliferation, differentiation, apoptosis, growth factor signaling, and immunomodulation [25, 26]. Both 25(OH)D and 1,25(OH)2D can also be degraded into less-active forms by 24-hydroxylase (encoded by CYP24A1). The group-specific component (GC) gene encodes the vitamin D-binding protein (DBP), which facilitates the transport of vitamin D metabolites.
Vitamin D-pathway genetic polymorphisms may influence breast cancer risk. Most well studied are vitamin D receptor (VDR) polymorphisms. A comprehensive review found no evidence of a consistent association between VDR polymorphisms and breast cancer risk [27]. Studies of single nucleotide polymorphisms (SNPs) in GC found no significant association with breast cancer risk [25, 28, 29]. CYP27B1 and CYP24A1 are involved in the activation and degradation of 25(OH)D and 1,25(OH)2D. Only five studies examined the association between SNPs on these genes and breast cancer risk [25, 28–31]. A review suggests that some SNPs on these genes may be associated with breast cancer risk, but results are inconclusive [32].
Variations in these genes may influence vitamin D synthesis and levels of circulating vitamin D. Potential interactions between genotypes and vitamin D levels have not been adequately addressed in epidemiologic studies. Only three previous studies examined interactions between circulating 25(OH)D and VDR gene polymorphisms, specifically those detected by digestion with BsmI (rs1544410) and FokI (rs10735810) [33–35]. Effect modification of GC polymorphisms, CYP27B1 and CYP24A1, may also be important to breast cancer development. Less is known about these vitamin D-related genes and their association with breast cancer risk and interaction with circulating 25(OH)D.
Among participants in a population-based case–control study, the Long Island Breast Cancer Study Project (LIBCSP), we previously observed an inverse association between circulating 25(OH)D and breast cancer risk [36]. Our objective here was to examine whether polymorphisms in genes involved in the vitamin D pathway may modify the association between 25(OH)D and breast cancer in an effort to identify susceptible subgroups of the population who may be at highest risk or who may benefit most from vitamin D exposure.
Materials and methods
This study utilizes the LIBCSP, a population-based case–control study conducted on Long Island, New York [37]. Full details have been reported previously [37]. Institutional Review Board approval was obtained from all participating institutions.
Study population
Breast cancer cases were women 20 years of age or older, residents of Nassau or Suffolk County, English speaking, and newly diagnosed with in situ or invasive breast cancer between 1 August 1996 and 31 July 1997. Eligible cases were identified through daily or weekly contact with the 28 hospitals in these two counties, and three hospitals in New York City that treat Long Island residents diagnosed with breast cancer. Controls were women without breast cancer identified by random digit dialing for women under 65 years of age and through Health Care Finance Administration (now the Center for Medicare and Medicaid Services) rosters for women 65 years or older. Controls were frequency-matched to the expected age distribution of the cases by 5-year age groups.
Trained interviewers administered the structured 2-h case–control questionnaire where respondents were asked about breast cancer risk factors and demographic characteristics [37]. In-person interviews were completed by 82.1 % (n = 1,508) of the eligible cases and 62.7 % (1,556) of the eligible controls. Respondents ranged in age from 20 to 98 years, were primarily postmenopausal (67 %), and 93 % self-reported as white, 5 % black, and 2 % other, which is consistent with the underlying racial distribution of the study area at the time of data collection [37].
Medical records of cases were abstracted to obtain information on tumor characteristics of the first primary breast cancer. Non-fasting blood samples were obtained at the time of the interview from 73.1 % of the case and 73.3 % of the control respondents (n = 1,102 and 1,141, respectively). Samples were collected prior to chemotherapy for 77.2 % (851/1,102) of the case respondents [37]. Plasma 25(OH)D measurements are absent in 6.9 % of cases and 5.8 % of controls, due to insufficient sample to complete the assay [36].
We limited the study reported here to white women due to population stratification concerns, and thus, our final sample size was 967 breast cancer cases and 993 controls. LIBCSP case and control participants who reported their race as white and with both DNA and serum available for this study had a mean age of 58.6 and 56.5 years, respectively [36]. Cases more often reported nulliparity, a first-degree family history of breast cancer and history of benign breast disease. Season of blood draw was also slightly different between cases and controls. Cases had higher percentage of women with blood drawn in October to December as compared to controls (31.5 vs. 27.8 %, respectively). However, controls had higher percentage of blood drawn in January to March as compared to cases (24.5 vs. 18.2 %, respectively). For the remaining months, April to September the frequency of blood draws was similar between cases and controls.
Measurement of plasma 25(OH)D
Quantification of 25(OH)D in plasma was done via Diasorin radioimmunoassay (RIA) method. Prior to measurement, plasma samples were stored at −80 °C. Samples were analyzed in batches between September and December 2007 using eight lots of the assay, as described previously [36]. Quality controls were utilized to assess inter-assay accuracy and precision. During each run, quality control (QC) samples (n = 5) were run together with the study samples. The QC samples came from the following sources, provided by Diasorin (n = 2; 17.3 and 50.4 ng/mL), pooled plasma sample (n = 1; 23.6 ng/mL), and commercially available external QC samples (n = 2; 63.9 and 107.9 ng/mL). The inter-assay precision determined for each QC from n = 56 runs was 14.2, 15.7, 16.4, 14.2, and 5.7 %, respectively. In addition, the laboratory successfully ran external proficiency samples from the UK-based vitamin D proficiency program DEQAS. Measurement of plasma 25(OH)D were performed in the laboratory of Dr. Serge Cremers at Columbia University Medical Center (CUMC).
Genotyping assays
We selected 35 SNPs for genotyping with known or suspected impact on the vitamin D pathway or that had been associated with breast cancer in previous studies [27, 32]. They included 20 SNPs in VDR: rs6823, BsmI (rs1544410), rs2071358, rs2107301, rs2239181, rs2239182, rs2408876, rs2544038, rs3782905, rs4073729, rs4760674, rs7299460, TaqI (rs731236), rs739837, rs7974708, ApaI (rs7975232), FokI (rs10735810), rs10875694, rs11168287, and rs11168314; 12 SNPs from 24-hydroxylase (CYP24A1): rs927650, rs2181874, rs2296241, rs2244719, rs2245153, rs2585428, rs2762939, rs3787557, rs4809960, rs6022999, rs6068816, and rs13038432; two from the vitamin D-binding protein (GC): rs4588 and rs7041; and one from 1α-hydroxylase (CYP27B1): rs4646537.
As previously described, genomic DNA was extracted from mononuclear cells in whole blood separated by Ficoll (Sigma Chemical Co, St. Louis, MO, USA) and washed twice with phosphate-buffered saline [37]. Pelleted cells were frozen at −80 °C until DNA isolation by standard phenol and chloroform/isoamyl alcohol extraction. Master DNA 96-well plates containing 10 ng/μL were used to make replica plates. Genotyping of the SNPs was performed by the fluorogenic 5′-nuclease or TaqMan assay, using the TaqMan Core Reagent Kit (Applied Biosystems, Foster City, CA, USA). Polymerase chain reactions were carried out by using standard conditions recommended by the manufacturer. The fluorescence profile of each well was measured in an ABI 7500HT Sequence Detection System, and the results were analyzed with Sequence Detection Software (Applied Biosystems). Controls for genotype at each locus and two no DNA controls were included on each plate. Any samples that were outside the parameters defined by the controls were identified as non-informative and were retested. Three SNPs VDR (rs2239182, rs2239181) and CYP24A1 (rs6068816) had concordance >98 %. Most other SNPs fell between 95 and 98 %, except four SNPs with concordance below 95 % (rs2107301 and rs6823 had 94 %, rs4760674 91 % and rs4073729 85 % concordance) [38]. All SNPS had a call rate of 95 % or better, except four: VDR (rs1544410: 89.1 %; rs3782905 93.0 %), CYP24A1 (rs2762939: 93.3 %), and GC (rs7041: 94.6 %). Laboratory personnel were blinded to case–control status. Genotyping assays were performed in the laboratory of Dr. Regina Santella at CUMC.
Statistical methods
For each of the 35 polymorphisms assayed, white subjects were divided into three groups based on the genotype. We tested for deviation from Hardy–Weinberg equilibrium (HWE) among controls for each polymorphism using observed genotype frequencies and a χ2 test with one degree of freedom [39]. VDR (FokI, rs10735810) had significant departure from HWE, and CYP27B1 (rs464537) had a minor allele frequency (MAF) of <5 %; thus, both SNPs were excluded. We also excluded the four SNPs with concordance below 95 %. The following ten SNPs were in linkage disequilibrium: VDR ApaI (rs7975232) and VDR rs739837; VDR BsmI (rs1544410) and VDR TaqI (rs731236); VDR rs3782905 and VDR rs7974708; CYP24A1 rs2585428 and CYP24A1 rs2296241; CYP24A1 rs4809960 and CYP24A1 rs2245153. Given the relative importance of BsmI and TaqI in other published literature, we elected to include these SNPs. We used ApaI instead of rs739837 as previous studies have suggested an association between ApaI and breast cancer, whereas rs739837 has only been associated with fair skin and melanoma risk [40, 41]. We selected rs3782905 and rs4809960 instead of rs7974708 and rs2245153, respectively; previous studies suggest an association with prostate cancer prognosis [42], whereas to our knowledge rs7974708 has not been investigated in relation to cancer. For the CYP24A1 SNPs, we selected rs2585428, instead of rs2296241, because a prior study found no association between breast cancer risk and rs2296241 [43]. Thus, the final number of SNPS included in our statistical analyses was 25.
Quantile regression was used to compare plasma 25(OH)D concentrations across all three genotypes and comparing a dominant model among controls [44]. We used log-transformed plasma 25(OH)D concentrations to normalize the distribution of 25(OH)D. To obtain plasma 25(OH)D concentrations that are adjusted for seasonal trend, we estimated the trend using a sine function among the controls, and then, we subtracted the estimated trend from measured plasma 25(OH)D. We used these adjusted values for all analyses that incorporated 25(OH)D.
We examined the association between genotype and breast cancer risk by unconditional logistic regression to estimate odds ratios (ORs) and 95 % confidence intervals (CIs) [45]. The genotype that was homozygous for the common allele was used as the referent category.
We conducted polytomous logistic regression for the association between genotype and subgroups of breast cancer defined by tumor characteristics [45]. ORs were estimated with cases classified by stage of disease (in situ vs. invasive) and hormone receptor status [estrogen receptor (ER)+ or progesterone receptor (PR)+ vs. ER−/PR− or ER+/PR+ vs. ER−/PR−]. The ratio of the ORs (RORs) was used as an indicator of etiological heterogeneity across disease stage and hormone receptor subtype [46].
Effect modification of plasma 25(OH)D across level of genotype was evaluated on a multiplicative scale comparing the likelihood ratio tests of logistic regression models with and without interaction terms [45]. Plasma 25(OH)D was divided into two categories (<19.1 and ≥19.1 ng/mL), based on the lowest quartile of 25(OH)D versus all above. Multiplicative interactions were assessed using indicator variables, where low plasma 25(OH)D (<19.1 ng/mL) was the referent category in a dominant genetic model, stratified by homozygous common allele and heterozygous or homozygous minor allele.
We identified potential confounders using a directed acyclic graph (DAG): first-degree family history of breast cancer, body mass index (BMI), oral contraceptive use, alcohol consumption, smoking, hormone replacement use, breast-feeding, and mammogram use. Potential confounders were included in the final models as a confounder if their inclusion significantly changed the log-likelihood of the model. Only two of these variables (family history of breast cancer and mammogram use) confounded the models. Therefore, all final statistical models include adjustment for age, first-degree family history of breast cancer, and mammogram use.
To aid in the interpretation of our results, we accounted for multiple comparisons using the Bonferroni correction [47]. Given we examined 25 SNPs, the corrected p value denoting a significant association was p < 0.002. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Among white control women with DNA and serum samples available for this study, we found a difference in median plasma 25(OH)D concentrations across genotypes for several polymorphisms, as shown in Table 1. For almost half of control participants (44.9 %), regardless of genotype, the geometric mean of plasma 25(OH)D was <30 ng/mL. For two VDR SNPs (rs2071358, rs2408876), we observed different geometric mean 25(OH)D levels across genotype. For the CYP27A1 (rs13038432), it appears that geometric mean plasma 25(OH)D levels are lower among those with the GG genotype than those with AA genotype. For both GC polymorphisms (rs4588, rs7041), the lowest median plasma 25(OH)D was among those with homozygous minor alleles (p = 0.001 and p = 0.001, respectively).
As shown in Table 2, CYP24A1 rs6068816 was associated with a 72 % reduction in breast cancer risk (TT vs. CC, OR 0.28, 95 % CI 0.10–0.76, p trend = 0.01). Increased breast cancer risk was noted for CYP24A1 rs6022999, rs2181874, and rs3787557; however, the confidence intervals included the null value (GG vs. AA, OR 1.35 95 % CI 0.95–1.90, p trend = 0.11; AA vs. GG, OR 1.37, 95 % CI 0.96–1.95, p trend = 0.11; CC vs. TT, OR 1.34, 95 % CI 0.92–1.89, p trend = 0.49, respectively). For VDR polymorphisms, TaqI (rs731236), BsmI (rs1544410), and rs2544038 showed a decrease in odds of breast cancer (TT vs. CC, OR 0.74, 95 % CI 0.56–0.98, p trend = 0.01; GG vs. AA, OR 0.74, 95 % CI 0.55–1.00, p trend = 0.03; TT vs. CC, OR 0.74, 95 % CI 0.57–0.97, p trend = 0.03, respectively). For the remaining polymorphisms, associations with breast cancer were weak and confidence intervals included the null value. Once we adjusted for multiple comparisons, none of the SNP-breast cancer risk p values were <0.002, the threshold determined using the Bonferroni correction.
As presented in Table 3, we observed little or no heterogeneity in the association between vitamin D-related SNPs and breast cancer across tumor characteristics of the first primary breast cancer, with a few exceptions. For VDR rs2408876, there was a 42 % decreased breast cancer risk among patients either ER+ or PR+ tumors as compared to women with ER−/PR− tumors (ROR 0.59, 95 % CI 0.36–0.98). We also examined heterogeneity between ER+/PR− and ER−/PR− tumors and found similar variations in the RORs, with attenuation of most of the ORs (Supplemental Table 1). Other SNPs showed apparent variability across tumor subtypes, but the confidence intervals for the measure of heterogeneity included the null value.
We noted effect modification on a multiplicative scale (p ≤ 0.05) for CYP24A1 polymorphism rs927650. Women homozygous for the common allele of CYP24A1 rs927650 who had plasma 25(OH)D of ≥19.1 ng/mL had reduced breast cancer risk compared to women with plasma 25(OH)D <19.1 ng/mL (OR 0.43, 95 % CI 0.27–0.68; Supplemental Table 2). With adjustment for multiple comparisons, none of the interaction p values were below the Bonferroni-determined threshold. Our findings, however, were based on small numbers of women and therefore should be interpreted with caution. In analyses restricted to postmenopausal women, the interaction for CYP24A1 (rs927650) was no longer significant (Supplemental Table 3).
Discussion
In this population-based case–control study, we observed reduced risks for breast cancer in association with selecting biologically plausible vitamin D-related gene polymorphisms, particularly CYP24A1. Specifically, we observed potential 72 and 46 % reductions in breast cancer risk in association with the homozygous minor allele genotype for CYP24A1 polymorphism rs6068816 and rs13038432. After accounting for multiple comparisons, however, we found no interactions between CYP24A1 and GC polymorphisms and plasma 25(OH)D. To our knowledge, this is the first study to examine the effect modification of breast cancer risk by plasma 25(OH)D among vitamin D-related gene polymorphisms other than VDR.
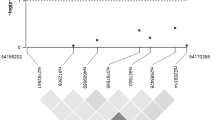
CYP24A1 is located on chromosome 20 (Fig. 1b) and plays an important role in vitamin D metabolism, specifically regulating the level of active vitamin D [27]. CYP24A1 is amplified in breast tumors, which may nullify growth control [48]. Two previous studies found no association between CYP24A1 polymorphisms (rs2296241, rs2181874, rs4809958 and rs601305) and breast cancer risk [25, 28], and another study found an increased risk with one polymorphism (rs6091822) and a decreased risk with two other CYP24A1 polymorphisms (rs8124792 and rs6097809) [29]. We found decreased breast cancer risk of two CYP24A1 polymorphisms that were not examined in these previous studies, rs13038432 and rs6068816.
In our study, there was a potential interaction between CYP24A1 polymorphism rs927650 and plasma 25(OH)D; breast cancer risk was reduced among women with the homozygous common allele with plasma 25(OH)D ≥19.1 ng/mL compared to those with 25(OH)D <19.1 ng/mL. A recent genome-wide association study (GWAS) demonstrated that variation in CYP24A1 was related to circulating levels of 25(OH)D [49]. CYP24A1 encodes 24-hydroxylase, which degrades 1,25(OH)2D, reducing the growth control of 1,25(OH)2D and potentially increasing breast cancer risk among women with certain CYP24A1 polymorphisms [27]. We did not test for rs6013897, which has been highlighted in a recent GWAS study [49] as associated with vitamin D insufficiency. To our knowledge, no previous publication has examined linkage disequilibrium between rs6013897 and any CYP24A1 polymorphisms. Our findings appear to be compatible with the known function of CYP24A1, which suggests that the association with breast cancer may be modified through 25(OH)D.
We also found potential breast cancer risk reductions for a number of VDR polymorphisms, including BsmI (rs1544410), TaqI (rs731236), and rs2544038. It is interesting to note that all the VDR polymorphisms associated with decreased breast cancer risk or plasma 25(OH)D in our study were closer to the 3′ end of the promoter region and part of block B (Fig. 1a) [50]. The functionality of these VDR polymorphisms is not completely understood [51]. The TaqI (rs731236) polymorphism is on block B and part of the ligand-binding domain [27]. Our findings of a reduced risk comparing CC versus TT in TaqI are consistent with the magnitude of effect observed in previous studies [35, 52]. However, a few other studies have found an increased risk or no association, but these studies were composed of slightly different populations, either premenopausal women only or women in other countries with differing sun exposure [53, 54]. Our findings of a reduced risk with BsmI are consistent with one previous study among white women [17], and two other studies [55, 56] conducted within populations of different racial backgrounds. However, other studies conducted within white populations showed an increased breast cancer risk with BsmI [33, 34, 57–59]. We know of only one study that also assessed rs2544038, which found a slightly increased breast cancer risk with the CC versus TT genotype [60].
Among VDR polymorphisms associated with increased risk, one SNP has not been previously published in relation to breast cancer, rs2239182. Our study showed ApaI was associated with an increased breast cancer risk, which is consistent with three previous studies [53, 61, 62] and inconsistent with three others [25, 52, 56]. It is unclear whether ApaI is associated with increased breast cancer risk or whether these are chance findings.
Within the vitamin D-binding protein encoded by GC, we examined two relatively common SNPs, rs7041 and rs4588 (Fig. 1c). Previous breast cancer studies have found varied results [25, 28]. In our population, we observed weak increases in breast cancer risk, with confidence intervals that included the null value, for both of these polymorphisms. Our breast cancer risk estimates were similar for rs7041 to two recent studies [25, 28] and similar in rs4588 to one of these studies [25]. However, two other studies found a decreased breast cancer risk of rs7041 [63, 64], only one examined rs4588 and also found an inverse association [63]. For both rs7041 and rs4588, we observed some variation in plasma 25(OH)D levels across genotype. Overall, our findings are in agreement with a recent study that showed GC variation is associated with 25(OH)D concentrations [65]. However, a GWAS study showed that only GC rs2282679 was associated with vitamin D insufficiency, and thus, variation in 25(OH)D across genotype may be influenced by other mechanisms [49].
These results support the concept that breast carcinogenesis may be influenced by the vitamin D axis, including the interaction between the different components of vitamin D, which includes circulating vitamin D, the VDR, and the vitamin D-binding protein [66]. Few previous studies have assessed interactions between circulating 25(OH)D and vitamin D polymorphisms on breast cancer risk [33–35, 63].
We acknowledge the following limitations of our study. First, we used a biologically based approach for SNP selection [67–69]; however, with adjustments for multiple comparisons, none of the associations we observed met the conservative Bonferroni threshold for significance. Thus, our results could be due to chance. Second, given that blood was collected near diagnosis and that 25(OH)D has a relatively short half-life of approximately 2–3 weeks [70], circulating vitamin D levels at the time of diagnosis may not reflect the etiologically relevant window time frame. Third, we limited our analyses to white women, given genotypes in VDR have been shown to vary by race and ethnicity [71]. This may limit generalizability of our findings; however, our homogenous population is also a study strength, because there is less genetic variability. Fourth, our results are based on a small number of case subjects with the homozygous minor allele. It is unclear whether our findings would be replicated in a larger study with more women with the homozygous minor alleles in CYP24A1 gene.
Our study improves upon previous studies in a number of different ways. First, we examined a number of biologically plausible polymorphisms, not just those on VDR. Our results show that other vitamin D-related genes—particularly CYP24A1—may also be important in understanding the relationship between vitamin D and breast cancer risk. Second, our study is based on incident breast cancer cases. Vitamin D levels can also be affected by treatment [72–74] and changes in lifestyle behaviors after diagnosis with breast cancer. Blood samples in our population were collected prior to the treatment with chemotherapy in 70 % of the cases. Third, our study was population based, reducing the likelihood of unquantified selection biases that are inherent in using select populations. As previously reported [37], LIBCSP participants for whom blood samples were available were more likely to be younger, report their race as white, to ever use alcohol, ever used hormone replacement, breast-fed for more than 6 months, ever had a mammogram, and less likely to be past smokers. However, all statistical analyses included the frequency matching factor age, were limited to whites only, and adjusted for ever having a mammogram, which may have helped to limit some of the potential selection bias associated with these differences. In addition, alcohol use, hormone replacement use, breast-feeding, and smoking were not confounders in our analyses. Further, our incidence density sampling approach improves our ability for estimating rate ratios, which enhances interpretation of our findings.
In conclusion, in our population-based study, breast cancer risk was associated with specific vitamin D-related SNPs, supporting the biologic plausibility of a relationship between vitamin D and breast cancer risk. Prospective studies evaluating 25(OH)D and breast cancer risk have had mixed results; some studies found 25(OH)D decreases breast cancer risk [19, 20, 24], whereas others reported no association [21–23]. We observed that the inverse association with vitamin D may be stronger among women with polymorphisms within CYP24A1. Genetic variation in the vitamin D pathway, specifically in CYP24A1, is potentially important to breast cancer risk, which should be considered when designing potential intervention strategies with vitamin D supplementation.
References
Holick MF (2006) Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol 92(1):49–59. doi:10.1016/j.pbiomolbio.2006.02.014
Gorham ED, Garland FC, Garland CF (1990) Sunlight and breast cancer incidence in the USSR. Int J Epidemiol 19(4):820–824
Blot WJ, Fraumeni JF Jr, Stone BJ (1977) Geographic patterns of breast cancer in the United States. J Natl Cancer Inst 59(5):1407–1411
Kuper H, Yang L, Sandin S, Lof M, Adami HO, Weiderpass E (2009) Prospective study of solar exposure, dietary vitamin D intake, and risk of breast cancer among middle-aged women. Cancer Epidemiol Biomark Prev 18(9):2558–2561. doi:10.1158/1055-9965.EPI-09-0449
Millen AE, Pettinger M, Freudenheim JL, Langer RD, Rosenberg CA, Mossavar-Rahmani Y, Duffy CM, Lane DS, McTiernan A, Kuller LH, Lopez AM, Wactawski-Wende J (2009) Incident invasive breast cancer, geographic location of residence, and reported average time spent outside. Cancer Epidemiol Biomark Prev 18(2):495–507. doi:10.1158/1055-9965.EPI-08-0652
Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F (2011) Joint effects of dietary vitamin D and sun exposure on breast cancer risk: results from the French E3N cohort. Cancer Epidemiol Biomark Prev 20(1):187–198. doi:10.1158/1055-9965.EPI-10-1039
Yang L, Veierod MB, Lof M, Sandin S, Adami HO, Weiderpass E (2011) Prospective study of UV exposure and cancer incidence among Swedish women. Cancer Epidemiol Biomark Prev 20(7):1358–1367. doi:10.1158/1055-9965.EPI-11-0071
Anderson LN, Cotterchio M, Kirsh VA, Knight JA (2011) Ultraviolet sunlight exposure during adolescence and adulthood and breast cancer risk: a population-based case–control study among Ontario women. Am J Epidemiol 174(3):293–304. doi:10.1093/aje/kwr091
Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC (2008) Relationship between low ultraviolet B irradiance and higher breast cancer risk in 107 countries. Breast J 14(3):255–260. doi:10.1111/j.1524-4741.2008.00571.x
Grant WB (2010) An ecological study of cancer incidence and mortality rates in France with respect to latitude, an index for vitamin D production. Dermatoendocrinol 2(2):62–67. doi:10.4161/derm.2.2.13624
Garland FC, Garland CF, Gorham ED, Young JF (1990) Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 19(6):614–622
John EM, Schwartz GG, Dreon DM, Koo J (1999) Vitamin D and breast cancer risk: the NHANES I epidemiologic follow-up study, 1971-1975 to 1992. National health and nutrition examination survey. Cancer Epidemiol Biomark Prev 8(5):399–406
Rossi M, McLaughlin JK, Lagiou P, Bosetti C, Talamini R, Lipworth L, Giacosa A, Montella M, Franceschi S, Negri E, La Vecchia C (2009) Vitamin D intake and breast cancer risk: a case–control study in Italy. Ann Oncol 20(2):374–378. doi:10.1093/annonc/mdn550
Frazier AL, Li L, Cho E, Willett WC, Colditz GA (2004) Adolescent diet and risk of breast cancer. Cancer Causes Control 15(1):73–82. doi:10.1023/B:CACO.0000016617.57120.df
Levi F, Pasche C, Lucchini F, La Vecchia C (2001) Dietary intake of selected micronutrients and breast-cancer risk. Int J Cancer 91(2):260–263
Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM (2007) Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med 167(10):1050–1059. doi:10.1001/archinte.167.10.1050
McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE (2005) Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomark Prev 14(12):2898–2904. doi:10.1158/1055-9965.EPI-05-0611
Robien K, Cutler GJ, Lazovich D (2007) Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women’s Health Study. Cancer Causes Control 18(7):775–782. doi:10.1007/s10552-007-9020-x
Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW (2013) Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumour Biol 34(6):3509–3517. doi:10.1007/s13277-013-0929-2
Kim Y, Je Y (2014) Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer 110(11):2772–2784. doi:10.1038/bjc.2014.175
Wang J, Eliassen AH, Spiegelman D, Willett WC, Hankinson SE (2014) Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses’ Health Study II. Cancer Causes Control 25(7):819–827. doi:10.1007/s10552-014-0383-5
Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, Arslan AA, Chen Y, Hallmans G, Lundin E, Rinaldi S, Toniolo P, Shore RE, Zeleniuch-Jacquotte A (2013) Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case–control study. Breast Cancer Res 15(1):R15. doi:10.1186/bcr3390
Kuhn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, Kvaskoff M, Dossus L, Tjonneland A, Olsen A, Overvad K, Chang-Claude J, Lukanova A, Buijsse B, Boeing H, Trichopoulou A, Lagiou P, Bamia C, Masala G, Krogh V, Sacerdote C, Tumino R, Mattiello A, Buckland G, Sanchez MJ, Menendez V, Chirlaque MD, Barricarte A, Bueno-de-Mesquita HB, van Duijnhoven FJ, van Gils CH, Bakker MF, Weiderpass E, Skeie G, Brustad M, Andersson A, Sund M, Wareham N, Khaw KT, Travis RC, Schmidt JA, Rinaldi S, Romieu I, Gallo V, Murphy N, Riboli E, Linseisen J (2013) Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: a nested case–control study. Int J Cancer 133(7):1689–1700. doi:10.1002/ijc.28172
Kim Y, Franke AA, Shvetsov YB, Wilkens LR, Cooney RV, Lurie G, Maskarinec G, Hernandez BY, Le Marchand L, Henderson BE, Kolonel LN, Goodman MT (2014) Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case–control study in the multiethnic cohort study. BMC Cancer 14:29. doi:10.1186/1471-2407-14-29
McCullough ML, Stevens VL, Diver WR, Feigelson HS, Rodriguez C, Bostick RM, Thun MJ, Calle EE (2007) Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case–control study. Breast Cancer Res 9(1):R9. doi:10.1186/bcr1642
Welsh J, Wietzke JA, Zinser GM, Smyczek S, Romu S, Tribble E, Welsh JC, Byrne B, Narvaez CJ (2002) Impact of the Vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. J Steroid Biochem Mol Biol 83(1–5):85–92
McCullough ML, Bostick RM, Mayo TL (2009) Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr 29:111–132. doi:10.1146/annurev-nutr-080508-141248
Anderson LN, Cotterchio M, Cole DE, Knight JA (2011) Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomark Prev 20(8):1708–1717. doi:10.1158/1055-9965.EPI-11-0300
Dorjgochoo T, Shi J, Gao YT, Long J, Delahanty R, Xiang YB, Cai Q, Shu XO (2012) Genetic variants in vitamin D metabolism-related genes and body mass index: analysis of genome-wide scan data of approximately 7000 Chinese women. Int J Obes 36(9):1252–1255. doi:10.1038/ijo.2011.246
Fuhrman BJ, Freedman DM, Bhatti P, Doody MM, Fu YP, Chang SC, Linet MS, Sigurdson AJ (2013) Sunlight, polymorphisms of vitamin D-related genes and risk of breast cancer. Anticancer Res 33(2):543–551
Yao S, Zirpoli G, Bovbjerg DH, Jandorf L, Hong CC, Zhao H, Sucheston LE, Tang L, Roberts M, Ciupak G, Davis W, Hwang H, Johnson CS, Trump DL, McCann SE, Ademuyiwa F, Pawlish KS, Bandera EV, Ambrosone CB (2012) Variants in the vitamin D pathway, serum levels of vitamin D, and estrogen receptor negative breast cancer among African-American women: a case–control study. Breast Cancer Res 14(2):R58. doi:10.1186/bcr3162
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ (2014) The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 14(5):342–357. doi:10.1038/nrc3691
Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW (2005) Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer 41(8):1164–1169. doi:10.1016/j.ejca.2005.01.017
Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE (2005) Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomark Prev 14(10):2335–2339. doi:10.1158/1055-9965.EPI-05-0283
Abbas S, Nieters A, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J (2008) Vitamin D receptor gene polymorphisms and haplotypes and postmenopausal breast cancer risk. Breast Cancer Res 10(2):R31. doi:10.1186/bcr1994
Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, Shane E, Terry MB, Desai M, Teitelbaum SL, Neugut AI, Santella RM (2009) Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila Pa) 2(6):598–604. doi:10.1158/1940-6207.CAPR-08-0138
Gammon MD, Neugut AI, Santella RM, Teitelbaum SL, Britton JA, Terry MB, Eng SM, Wolff MS, Stellman SD, Kabat GC, Levin B, Bradlow HL, Hatch M, Beyea J, Camann D, Trent M, Senie RT, Garbowski GC, Maffeo C, Montalvan P, Berkowitz GS, Kemeny M, Citron M, Schnabe F, Schuss A, Hajdu S, Vincguerra V, Collman GW, Obrams GI (2002) The Long Island Breast Cancer Study Project: description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat 74(3):235–254
Fleiss JL, Levin B, Paik MC (2003) Statistical methods for rates and proportions. Wiley series in probability and statistics, 3rd edn. Wiley-Interscience, Hoboken
Ziegler A, König IR (2010) A statistical approach to genetic epidemiology: [concepts and applications], 2nd edn. Wiley-VCH, Weinheim
Barroso E, Fernandez LP, Milne RL, Pita G, Sendagorta E, Floristan U, Feito M, Aviles JA, Martin-Gonzalez M, Arias JI, Zamora P, Blanco M, Lazaro P, Benitez J, Ribas G (2008) Genetic analysis of the vitamin D receptor gene in two epithelial cancers: melanoma and breast cancer case–control studies. BMC Cancer 8:385. doi:10.1186/1471-2407-8-385
John EM, Schwartz GG, Koo J, Wang W, Ingles SA (2007) Sun exposure, vitamin D receptor gene polymorphisms, and breast cancer risk in a multiethnic population. Am J Epidemiol 166(12):1409–1419. doi:10.1093/aje/kwm259
Holt SK, Kwon EM, Koopmeiners JS, Lin DW, Feng Z, Ostrander EA, Peters U, Stanford JL (2010) Vitamin D pathway gene variants and prostate cancer prognosis. Prostate 70(13):1448–1460. doi:10.1002/pros.21180
Dorjgochoo T, Delahanty R, Lu W, Long J, Cai Q, Zheng Y, Gu K, Gao YT, Zheng W, Shu XO (2011) Common genetic variants in the vitamin D pathway including genome-wide associated variants are not associated with breast cancer risk among Chinese women. Cancer Epidemiol Biomark Prev 20(10):2313–2316. doi:10.1158/1055-9965.EPI-11-0704
Koenker R, Bassett G Jr (1978) Regression quantiles. Econometrica 46(1):33–50. doi:10.2307/1913643
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley series in probability and statistics texts and references section, 2nd edn. Wiley, New York
Begg CB, Zhang ZF (1994) Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomark Prev 3(2):173–175
Rothman KJ, Greenland S, Lash TL (2008) Modern epidemiology. 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D (2000) Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet 25(2):144–146. doi:10.1038/75985
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD (2010) Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 376(9736):180–188. doi:10.1016/S0140-6736(10)60588-0
Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E, Tuomilehto J, Newport MJ, Clayton DG, Todd JA (2004) Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet 13(15):1633–1639. doi:10.1093/hmg/ddh169
Rukin NJ, Strange RC (2007) What are the frequency, distribution, and functional effects of vitamin D receptor polymorphisms as related to cancer risk? Nutr Rev 65(8 Pt 2):S96–S101
Sillanpaa P, Hirvonen A, Kataja V, Eskelinen M, Kosma VM, Uusitupa M, Vainio H, Mitrunen K (2004) Vitamin D receptor gene polymorphism as an important modifier of positive family history related breast cancer risk. Pharmacogenetics 14(4):239–245
Curran JE, Vaughan T, Lea RA, Weinstein SR, Morrison NA, Griffiths LR (1999) Association of A vitamin D receptor polymorphism with sporadic breast cancer development. Int J Cancer 83(6):723–726
Buyru N, Tezol A, Yosunkaya-Fenerci E, Dalay N (2003) Vitamin D receptor gene polymorphisms in breast cancer. Exp Mol Med 35(6):550–555
Ingles SA, Garcia DG, Wang W, Nieters A, Henderson BE, Kolonel LN, Haile RW, Coetzee GA (2000) Vitamin D receptor genotype and breast cancer in Latinas (United States). Cancer Causes Control 11(1):25–30. doi:10.1023/A:1008979417618
Hou MF, Tien YC, Lin GT, Chen CJ, Liu CS, Lin SY, Huang TJ (2002) Association of vitamin D receptor gene polymorphism with sporadic breast cancer in Taiwanese patients. Breast Cancer Res Treat 74(1):1–7
Bretherton-Watt D, Given-Wilson R, Mansi JL, Thomas V, Carter N, Colston KW (2001) Vitamin D receptor gene polymorphisms are associated with breast cancer risk in a UK Caucasian population. Br J Cancer 85(2):171–175. doi:10.1054/bjoc.2001.1864
Guy M, Lowe LC, Bretherton-Watt D, Mansi JL, Peckitt C, Bliss J, Wilson RG, Thomas V, Colston KW (2004) Vitamin D receptor gene polymorphisms and breast cancer risk. Clin Cancer Res 10(16):5472–5481. doi:10.1158/1078-0432.CCR-04-0206
McKay JD, McCullough ML, Ziegler RG, Kraft P, Saltzman BS, Riboli E, Barricarte A, Berg CD, Bergland G, Bingham S, Brustad M, Bueno-de-Mesquita HB, Burdette L, Buring J, Calle EE, Chanock SJ, Clavel-Chapelon F, Cox DG, Dossus L, Feigelson HS, Haiman CA, Hankinson SE, Hoover RN, Hunter DJ, Husing A, Kaaks R, Kolonel LN, Le Marchand L, Linseisen J, McCarty CA, Overvad K, Panico S, Purdue MP, Stram DO, Stevens VL, Trichopoulos D, Willett WC, Yuenger J, Thun MJ (2009) Vitamin D receptor polymorphisms and breast cancer risk: results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomark Prev 18(1):297–305. doi:10.1158/1055-9965.EPI-08-0539
Engel LS, Orlow I, Sima CS, Satagopan J, Mujumdar U, Roy P, Yoo S, Sandler DP, Alavanja MC (2012) Vitamin D receptor gene haplotypes and polymorphisms and risk of breast cancer: a nested case–control study. Cancer Epidemiol Biomark Prev 21(10):1856–1867. doi:10.1158/1055-9965.EPI-12-0551
Chakraborty A, Mishra AK, Soni A, Regina T, Mohil R, Bhatnagar D, Bhatnagar A, Chintamani C, Sharma PC, Saxena S (2009) Vitamin D receptor gene polymorphism(s) and breast cancer risk in north Indians. Cancer Detect Prev 32(5–6):386–394
Dalessandri KM, Miike R, Wiencke JK, Farren G, Pugh TW, Manjeshwar S, DeFreese DC, Jupe ER (2012) Vitamin D receptor polymorphisms and breast cancer risk in a high-incidence population: a pilot study. J Am Coll Surg 215(5):652–657. doi:10.1016/j.jamcollsurg.2012.06.413
Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J (2008) The Gc2 allele of the vitamin D binding protein is associated with a decreased postmenopausal breast cancer risk, independent of the vitamin D status. Cancer Epidemiol Biomark Prev 17(6):1339–1343. doi:10.1158/1055-9965.EPI-08-0162
Wang W, Ingles SA, Torres-Mejia G, Stern MC, Stanczyk FZ, Schwartz GG, Nelson DO, Fejerman L, Wolff RK, Slattery ML, John EM (2014) Genetic variants and non-genetic factors predict circulating vitamin D levels in hispanic and non-hispanic white women: the breast cancer health disparities study. Int J Mol Epidemiol Genet 5(1):31–46
Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, Nexo E (2005) Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int 77(1):15–22. doi:10.1007/s00223-004-0227-5
Thyer L, Ward E, Smith R, Fiore MG, Magherini S, Branca JJ, Morucci G, Gulisano M, Ruggiero M, Pacini S (2013) A novel role for a major component of the vitamin D axis: vitamin D binding protein-derived macrophage activating factor induces human breast cancer cell apoptosis through stimulation of macrophages. Nutrients 5(7):2577–2589. doi:10.3390/nu5072577
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1(1):43–46
Thomas DC, Clayton DG (2004) Betting odds and genetic associations. J Natl Cancer Inst 96(6):421–423
Savitz DA, Olshan AF (1995) Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol 142(9):904–908
Holick MF (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19(2):73–78. doi:10.1016/j.annepidem.2007.12.001
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338(2):143–156. doi:10.1016/j.gene.2004.05.014
Jacot W, Pouderoux S, Thezenas S, Chapelle A, Bleuse JP, Romieu G, Lamy PJ (2012) Increased prevalence of vitamin D insufficiency in patients with breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 134(2):709–717. doi:10.1007/s10549-012-2084-7
Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, Sunga AY (2009) Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis 24(2):219–224. doi:10.1007/s00384-008-0593-y
Nogues X, Servitja S, Pena MJ, Prieto-Alhambra D, Nadal R, Mellibovsky L, Albanell J, Diez-Perez A, Tusquets I (2010) Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas 66(3):291–297. doi:10.1016/j.maturitas.2010.03.012
Acknowledgments
This study is funded in part by Grants Nos. U01 CA/ES66572, P30ES009089, P30ES10126, from the National Cancer Institute and the National Institute of Environmental Health Sciences, an award from the Breast Cancer Research Foundation and the Jean Sindab Foundation. Laura Reimers was supported by a training grant from the National Cancer Institute (T32 CA009529). This manuscript was also made possible by Grant Number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Conflict of interest
The authors report no financial conflicts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reimers, L.L., Crew, K.D., Bradshaw, P.T. et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control 26, 187–203 (2015). https://doi.org/10.1007/s10552-014-0497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0497-9