Abstract
There were some case–control studies, nested case–control studies, and cohort studies with controversial results on the association between serum 25-hydroxyvitamin D [25(OH)D] and breast cancer risk. Case–control studies are prone to selection bias, which limit the strength and quality of the evidence. To overcome the shortcoming of the case–control studies, the meta-analysis of prospective studies including nested case–control studies and cohort studies was conducted. PubMed, Embase, and Web of Science databases were searched, and the last retrieval date was March 24, 2013. For the highest versus the lowest level of serum 25(OH)D, the relative risks (RRs) and its 95 % confidence intervals (CIs) from each study were used to estimate summary RR and its 95 % CI. Subgroup analyses by geographic region, menopausal status, and adjusted status of RR were also performed, respectively. A dose–response association between serum 25(OH)D concentration and breast cancer risk was assessed. Fourteen articles with 9,110 breast cancer cases and 16,244 controls were included in the meta-analysis. Overall, serum 25(OH)D levels were inversely significantly associated with breast cancer risk (RR = 0.845, 95 % CI = 0.750–0.951). Inversely statistically significant associations were observed in North American studies, postmenopausal women, and studies with adjusted and unadjusted RR, respectively. No statistically significant associations were observed in European studies and premenopausal women, respectively. Dose–response analysis showed that every 10 ng/mL increment in serum 25(OH)D concentration was associated with a significant 3.2 % reduction in breast cancer risk. This meta-analysis provides evidence of a significantly inverse association between serum 25(OH)D concentration and breast cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D in humans is mainly derived from both sun exposure and diet. 25-hydroxyvitamin D [25(OH)D] is the main blood circulation form of vitamin D. At physiological concentrations, 25(OH)D does not seem to have a function of its own, but it is the precursor of the active hormone, 1,25-dihydroxyvitamin D [1,25(OH)2D] [1]. 1,25(OH)2D induces growth arrest, triggers cell death, and promotes differentiation of cancer cells including breast cancer cells [2]. Therefore, vitamin D may play a protective role in breast cancer risk through the above potential mechanism. 1,25(OH)2D is the most active vitamin D metabolite, although its concentration in serum is one thousandth that of 25(OH)D [3], and its assay should never be used for detecting vitamin D deficiency because levels will be normal or even elevated as a result of secondary hyperparathyroidism [4]. Thus, monitoring vitamin D status by serum 25(OH)D analysis is the most accurate way to determine the appropriate level and the route of supplementation [2]. Therefore, serum 25(OH)D rather than vitamin D ingestion or serum 1,25(OH)2D was chosen as an index to clarify the association between vitamin D and breast cancer risk in this meta-analysis.
There were some case–control studies, nested case–control studies, and cohort studies with controversial results on the association between serum 25(OH)D and breast cancer risk. Of note, case–control studies are prone to selection bias because in case–control studies, 25(OH)D is assessed after a cancer diagnosis and it is unclear how changes in health behaviors after a cancer diagnosis influence 25(OH)D levels. The prospective studies including nested case–control studies and cohort studies overcome the above shortcoming and allow for measurement of exposures before the outcome occurs, an appropriate time sequence for a cause–effect relationship [5]. In addition, it is still not clear whether geographic region, menopausal status, and adjusted status of relative risk (RR) would affect the association between serum 25(OH)D and breast cancer risk. A dose–response association between serum 25(OH)D concentration and breast cancer risk was also assessed. Thus, the meta-analysis of prospective studies was conducted.
Materials and methods
Search strategy
A literature search was performed up to March 24, 2013 in PubMed, Embase, and Web of Science databases without restrictions using the following search strategy: (“25-OH-D” or “25(OH)D” or “cholecalciferol” or “calcidiol” or “25-hydroxyvitamin D” or “hydroxycholecalciferol*” or “25-hydroxyvitamin D3 1-alpha-hydroxylase” or “vitamin D”) and (“breast” or “mammary glands” or “mamma”) and (“cancer” or “tumor” or “tumour” or “neoplasm” or “carcinoma”) and (“serum” or “plasma”). Moreover, references cited in the studies were reviewed to identify any additional articles that were not indexed by the electronic databases.
Eligibility criteria
Studies were included in the meta-analysis if they met the following criteria: (1) had a prospective nested case–control or cohort study design; (2) the exposure was plasma or serum 25(OH)D; (3) the outcome was breast cancer risk; and (4) RRs or odd ratios (ORs) with its 95 % confidence intervals (CIs) (or data to calculate these) were reported. If included population were duplicated in more than one study, only the most complete and the largest study was included in the total analysis. If the duplicated study with the smaller sample size could meet one of the standards in the subgroup analyses but the largest study did not give the data in the subgroup analyses, it could also be included in the subgroup analyses.
Data extraction
The following information were extracted from included articles: first author, year of publication, study design, country, geographic region, ethnicity, menopausal status, number of cases and controls, adjustment factors of RR or OR, time from 25(OH)D measurement to breast cancer development, median (or the cut-points to calculate the median) of serum 25(OH)D concentration in each quantile, and RRs or ORs with its 95 % CIs in each quantile of serum 25(OH)D levels. As cancer is a relatively rare disease, the distinction between the various estimates of relative risk (i.e., odds ratio, rate ratio, risk ratio) was ignored and all measures were interpreted as relative risk. To ensure the accuracy of extracted information, two investigators extracted information independently and differences were settled by reaching an agreement among all investigators.
Statistical analysis
Statistical analysis was performed using the software program STATA (version 12.0). Heterogeneity among studies was determined using a χ 2-based Q statistic and I 2 statistic [6]. For the highest versus the lowest quantile of serum 25(OH)D levels, the RRs and its 95 % CIs from each study were used to estimate summary RR and its 95 % confidence interval (CI). When there was some evidence of heterogeneity in the analysis (P Q statistic < 0.10 or I 2 statistic >50 %), summary RR was determined using a random-effects model [7] in which the contribution of each study is weighted by the inverse of the sum of the inter- and intra-study variance, otherwise using fixed-effects model [8] in which the weight of each study is estimated by the intra-study variance. Sensitivity analysis was also conducted by omitting one study in each turn and recalculating the combined RR for the remaining studies. Subgroup analyses by geographic region, menopausal status, and adjusted status of RR were also performed, respectively. Publication bias was assessed by funnel plot and Egger’s regression test [9]. All the P values were two sided.
A dose–response analysis was conducted based on data for the median value of serum 25(OH)D concentration in each quantile, number of cases and participants, and adjusted logarithm of the RR with its SE [10]. Studies were not eligible if the necessary data were not reported or could not be estimated. Data of the median 25(OH)D concentration were measured in nanograms per milliliter (ng/mL), where 1 ng/mL = 2.5 nmol/L (nmol/L: nanomoles per liter) [11].
Results
Literature search and study characteristics
Through literature search and selection based on the inclusion criteria, 14 articles [12–25] with a total of 9,110 breast cancer cases and 16,244 controls were finally included in the meta-analysis (Fig. 1). These 14 articles were all prospective studies (13 nested case–control studies and 1 cohort study) published from 2005 to 2013. The characteristics of the articles in the meta-analysis are presented in Table 1. One article [20] reported the data in 1990 and 2000, respectively. So, this article was looked as two individual studies (no. 9 and no. 10) in the meta-analysis. Thus, this meta-analysis included 15 individual prospective studies. Among these articles, the article [25] (no. 15 study) was left out of the total analysis because it was a subset of the larger study [12] (no. 1 study), but it contributed to subgroup analysis of postmenopausal women.
High versus low quantile of 25(OH)D levels
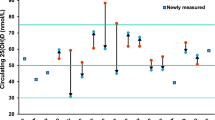
The RRs for each study and combination of all studies for the highest versus the lowest quantile of serum 25(OH)D levels are shown in Fig. 2. Results from the studies on serum 25(OH)D levels in relation to breast cancer risk were inconsistent. Because there was a statistically significant heterogeneity among the studies (P = 0.076, I 2 = 37.7 %), the random-effects model was used to pool the results. Overall, serum 25(OH)D concentration was inversely significantly associated with breast cancer risk (RRrandom effects = 0.845, 95 % CI = 0.750–0.951; Table 2 and Fig. 2).
The sensitivity analyses omitting one study at a time and calculating the combined RR for the remaining studies showed that the combined risk estimate was not substantially affected by any single study. Of note, the summary RRs were all statistically significant and similar among each other, with a narrow range from 0.818 (95 % CI = 0.732–0.914), when the study no. 8 [19] was excluded, to 0.857 (95 % CI = 0.755–0.973), when the study no. 7 [18] was excluded.
The results of subgroup analyses are presented in Table 2. In subgroup analyses by geographic region, an inversely statistically significant association was observed in North American studies (RRfixed effects = 0.851, 95 % CI = 0.765–0.946), but not in European studies (RRrandom effects = 0.838, 95 % CI = 0.605–1.160). In subgroup analyses by menopausal status, an inversely statistically significant association was observed in postmenopausal women (RRfixed effects = 0.846, 95 % CI = 0.750–0.955), but not in premenopausal women (RRrandom effects = 0.839, 95 % CI = 0.523–1.346). In subgroup analyses by adjusted status of RR, inversely statistically significant associations were observed both in studies with adjusted RR (RRfixed effects = 0.885, 95 % CI = 0.788–0.993) and unadjusted RR (RRfixed effects = 0.785, 95 % CI = 0.677–0.911).
Dose–response meta-analysis
A significant dose–response association between serum 25(OH)D concentration and risk of breast cancer incidence using data from 11 studies [12–15, 17–19, 21–24] was identified. The risk of breast cancer incidence decreased, on average, by 3.2 % for every 10 ng/mL increment of serum 25(OH)D concentration (RRfixed effects = 0.968, 95 % CI = 0.943–0.994, P for trend = 0.016, P heterogeneity = 0.130).
Publication bias
Funnel plot and Egger’s regression test were performed to assess the publication bias of the literatures. All funnel plots did not reveal obvious evidence of asymmetry (e.g., Fig. 3), and all the P Egger tests were greater than 0.05 (Table 2). These results did not suggest obvious publication biases in all comparisons.
Discussion
Vitamin D and its metabolites reduce the incidence of many types of cancer by inhibiting tumor angiogenesis, stimulating mutual adherence of cells, and enhancing intercellular communication through gap junctions, thereby strengthening the inhibition of proliferation that results from tight physical contact with adjacent cells within a tissue (contact inhibition) [3]. 1,25(OH)2D can inhibit mitosis of breast epithelial cells. Pulsatile release of ionized calcium from intracellular stores, including the endoplasmic reticulum, induces terminal differentiation and apoptosis, and 1,25(OH)2D enhances this release [3]. Those above are the molecular biological mechanism of vitamin D effects on breast cancer risk. In addition, an animal experiment also showed that the rats treated with lα(OH)D3 (a synthetic analog of vitamin D) at 0.5 μg/kg dose significantly inhibited tumor progression when compared with the control group which treated with steroid suspension medium alone (P = 0.03) [26]. In this meta-analysis, individuals in the highest compared with the lowest quantile of serum 25(OH)D levels showed an overall reduction in the risk of breast cancer by approximately 15.5 % (RRrandom effects = 0.845, 95 % CI = 0.750–0.951; Table 2 and Fig. 2). In the dose–response association, the incidence of breast cancer decreased by 3.2 % for every 10 ng/mL increment in serum 25(OH)D concentration (RRfixed effects = 0.968, 95 % CI = 0.943–0.994). Findings from this meta-analysis indicate that serum 25(OH)D concentration is inversely associated with breast cancer risk from an epidemiological aspect. Therefore, molecular biological mechanism, animal experiment, and epidemiological evidence all confirm the protective effects of vitamin D on breast cancer risk.
In this meta-analysis of the total studies, only three epidemiological studies found significantly inverse associations between levels of serum 25(OH)D and breast cancer risk (no. 5, no. 7, and no. 9), while 11 failed to detect significant associations (nos. 1–4, no. 6, no. 8, and nos. 10–14) (Table 1 and Fig. 2). The time from 25(OH)D measurement to breast cancer development in each of the studies was more than 3 months, except the study no. 5 which did not mention about the time (Table 1). Serum 25(OH)D represents recent (within the past 3 months) vitamin D exposure and may not be reflective of lifetime exposure. The null findings of most of the prospective studies of 25(OH)D–breast cancer association may be due to longer interval between measurement of 25(OH)D and breast cancer diagnosis. Sensitivity analyses omitting one study at a time and calculating the combined RR for the remaining studies showed that the combined significantly inverse association was not substantially affected by any single study, including omitting the study (no. 5) which did not mention about the time from 25(OH)D measurement to breast cancer diagnosis. These results showed that our findings were robust. Because the combined result was based on a larger sample size and had a more sufficient power, the conclusion of the combined significantly inverse association is more reliable than a single study.
In subgroup analyses by geographic region, an inversely statistically significant association was observed in North American studies, but not in European studies. Interestingly, ethnic distributions were consistent with geographic regions in the original studies, which were mixed population (Caucasians in major) all in North America and Caucasians all in Europe. The different results between the two geographic regions indicate that besides a possible role of ethnic differences in genetic profiles, other factors such as people’s living environment and dietary habits contribute as well.
In subgroup analyses by menopausal status, an inversely statistically significant association was observed in postmenopausal women, but not in premenopausal women. These results indicate that menopausal status, as an effect modifier, may play an important role in the association between serum 25(OH)D concentration and breast cancer risk. The reason may be as follows: Almost all postmenopausal women are aged people. The likelihood of vitamin D deficiency increases with age, as the cutaneous production of vitamin D decreases, and with estrogen deficiency, which seems to reduce the activation of vitamin D and expression of the VDR [12]. Consequently, postmenopausal women with lower serum 25(OH)D concentration may be at increased risk of breast cancer. As to premenopausal women, with stronger physical status, the effect of lower serum 25(OH)D may turn not to be obvious on breast cancer risk.
In the subgroup analyses by geographic region and menopausal status, no statistically significant associations between 25(OH)D and breast cancer risk were observed in the European studies and among premenopausal women, respectively. However, the risk estimates are comparable to what was seen in the North American studies and among postmenopausal women, respectively. Besides the possible reasons for the different results that we discussed above, perhaps, the lack of statistical significance is due to smaller numbers and the lack of sufficient power to detect a difference.
In the studies with adjusted RR, there were some adjustments for age, season of serum collection, BMI, etc. which are known to influence 25(OH)D levels (Table 1). These studies with adjusted RR more accurately reflected the association between 25(OH)D and breast cancer risk than those with unadjusted RR. However, in subgroup analyses by adjusted status of RR, inversely statistically significant associations were observed both in studies with adjusted RR and unadjusted RR. These similar results suggest that the adjusted status of RR affect little on the association in this meta-analysis.
Some limitations of this study should be considered. First, there were variant adjusted factors of RR, which were different from each other (Table 1). Therefore, a more precise analysis should be conducted if individual data are available, which could permit the same adjusted factors. Second, only European studies and North American studies were included in this meta-analysis. More original studies are needed to explore the associations in different geographic regions such as Africa, Asia, and Latin America.
In spite of those limitations, this meta-analysis also had some important advantages. First, the previous meta-analyses [27–31] on the association all collected both retrospective and prospective studies. In this meta-analysis, the original studies all used a prospective study design, which greatly reduced the likelihood of selection bias. Second, we performed subgroup analyses by geographic region, menopausal status, and adjusted status of RR, which all the previous meta-analyses [27–31] were not performed. Third, we detected no publication biases in all the comparisons, indicating that the pooled results may be unbiased.
In conclusion, this meta-analysis suggests that serum 25(OH)D concentration is inversely associated with breast cancer risk. Inversely statistically significant associations are presented in North American studies, postmenopausal women, and both of the adjusted status of RR, respectively. No statistically significant associations are presented in European studies and premenopausal women, respectively. More large well-designed prospective studies in different geographic regions are needed to further investigate the association.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- 1,25(OH)2D:
-
1,25-Dihydroxyvitamin D
- lα(OH)D3 :
-
1-α-Hydroxycholecalciferol
- RR:
-
Relative risk
- CI:
-
Confidence interval
- SE:
-
Standard error
- VDR:
-
Vitamin D receptor
References
Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–55.
Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys. 2012;523:107–14.
Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Essebag V, Genest Jr J, Suissa S, Pilote L. The nested case–control study in cardiology. Am Heart J. 2003;146:581–90.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9.
Gilbert R, Martin RM, Beynon R, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose–response meta-analysis. Cancer Causes Control. 2011;22:319–40.
Bertone-Johnson ER, Chen WY, Holick MF, et al. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–7.
Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–91.
Freedman DM, Chang SC, Falk RT, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17:889–94.
McCullough ML, Stevens VL, Patel R, et al. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11:R64.
Rejnmark L, Tietze A, Vestergaard P, et al. Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: a nested case–control study. Cancer Epidemiol Biomarkers Prev. 2009;18:2655–60.
Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk—a prospective nested case–control study. Int J Cancer. 2010;127:2159–68.
Engel P, Fagherazzi G, Boutten A, et al. Serum 25(OH) vitamin D and risk of breast cancer: a nested case–control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:2341–50.
Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast Cancer Res. 2011;13:R50.
Eliassen AH, Tworoger SS, Bertone-Johnson E, Rosner B, Willett WC, Hankinson SE. Circulating vitamin D and breast cancer risk: repeated exposure assessment and 18 years of follow-up in the Nurses’ Health Study. Cancer Res. 2011;71:4633.
Amir E, Cecchini RS, Ganz PA, et al. 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res Treat. 2012;133:1077–88.
Neuhouser ML, Manson JE, Millen A, et al. The influence of health and lifestyle characteristics on the relation of serum 25-hydroxyvitamin D with risk of colorectal and breast cancer in postmenopausal women. Am J Epidemiol. 2012;175:673–84.
Mohr SB, Gorham ED, Alcaraz JE, et al. Serum 25-hydroxyvitamin D and breast cancer in the military: a case–control study utilizing pre-diagnostic serum. Cancer Causes Control. 2013;24:495–504.
Ordonez-Mena JM, Schottker B, Haug U, et al. Serum 25-hydroxyvitamin D and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2013;22:905–16.
Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127:667–74.
Colston KW, Chander SK, Mackay AG, Coombes RC. Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochem Pharmacol. 1992;44:693–702.
Chen P, Li M, Gu X, et al. Higher blood 25(OH)D level may reduce the breast cancer risk: evidence from a Chinese population based case–control study and meta-analysis of the observational studies. PLoS One. 2013;8:e49312.
Mohr SB, Gorham ED, Alcaraz JE, et al. Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res. 2011;31:2939–48.
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–205.
Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–24.
Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–77.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (no. 30972516), Natural Science Foundation of Hebei Province (no. C2010000481), and Hebei Province Health Bureau Foundation (no. 20090004).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, D., Vélez de-la-Paz, O.I., Zhai, JX. et al. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumor Biol. 34, 3509–3517 (2013). https://doi.org/10.1007/s13277-013-0929-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0929-2