Abstract
Objective
To examine the association between occupational exposure to silica and lung cancer from a systematic review (and meta-analysis) of the epidemiologic literature, with special reference to the methodological quality of observational studies.
Methods
We searched Medline, Toxline, BIOSIS, and Embase (1966–December 2007) for original articles published in any language. Observational studies (cohort and case–control studies) were selected if they reported the result of dose–response analyses relating lung cancer to occupational exposure to silica after appropriate adjustment for smoking.
Results
Ten studies (4 cohort studies and 6 case–control studies) met the inclusion criteria of the meta-analysis, nine of which contributing to the main analysis (dose–response analysis, no lag time). We found increasing risk of lung cancer with increasing cumulative exposure to silica, with heterogeneity across studies however. Posthoc analyses identified a set of seven more homogeneous studies. Their meta-analysis resulted in a dose–response curve that was not different from that obtained in the main analysis.
Conclusion
Silica is a lung carcinogen. This increased risk is particularly apparent when the cumulative exposure to silica is well beyond that resulting from exposure to the recommended limit concentration for a prolonged period of time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From a recent meta-analysis, we estimated that, overall and after controlling for smoking, silicosis is associated with a 60% increase in risk of lung cancer [1]. Whether the association between silicosis and lung cancer is due to the effect of the fibrotic process or to the effect of respirable silica itself is unclear [2], since lung fibrosis (as it is seen in idiopathic pulmonary fibrosis and asbestosis) increases the risk of lung cancer [3, 4].
Although the International Agency for Research on Cancer (IARC) classified silica as a human lung carcinogen [5], the opinion that exposure to crystalline silica in itself (i.e., in the absence of silicosis) induces lung cancer is still challenged [6]. Our objective was to reexamine the epidemiologic evidence regarding the association between occupational exposure to silica and lung cancer through a systematic review (and meta-analysis) of the epidemiologic literature. The methods that we used are in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group’s recommendations [7].
Materials and methods
Literature search
We searched Toxline, BIOSIS, Embase, and Medline [8] (1966–December 2007) for original articles published in any language using the following strategy: ([silicon dioxide, MeSH Major Topic] OR [silicosis, MeSH Major Topic]) AND [lung neoplasms, MeSH Major Topic]. All terms were exploded. We also searched for additional articles from the reference list of relevant articles obtained from the electronic search.
Study selection
Criteria for inferring causation from epidemiologic investigations have been proposed [9, 10]. These criteria include, among others, the strength of association, the consistency of results across studies, the demonstration of a biologic gradient, plausibility from mechanistic investigation, and supportive evidence from experimentation. From these criteria, the finding that an increasing cumulative exposure to respirable silica is consistently associated, with an increasing risk of cancer, would represent a strong argument supporting the IARC’s conclusion that silica is carcinogenic. We therefore included published and peer-reviewed observational studies (cohort or case–control studies) that reported on dose–response analyses relating occupational exposure to silica to risk of lung cancer. In cohort studies with internal comparison, dose–response analyses are particularly appealing since workers with high exposure are compared to workers with low exposure, both groups presumably sharing similar smoking habits and clinical characteristics otherwise [11]. Cohort studies with external comparison groups and case–control studies were also considered if risks were adjusted for smoking. We included only studies in which cumulative exposure to silica was quantified in terms of mg/m3 per years. To avoid confounding bias, studies reporting on concomitant exposures to silica and other lung carcinogens (e.g., asbestos, arsenic, uranium, radon [12]) were excluded, unless the measure of association had been adjusted for confounders. To limit selection bias, autopsy studies were also excluded [13]. In addition, narrative reviews, letters to the editor, clinical commentaries, case series, and case reports were disregarded.
Three reviewers (YL, SM, and DG) successively applied these criteria to the titles and abstracts of all citations obtained. If the title of an article or, when available, its abstract suggested any possibility that it might be relevant, the article was retrieved and independently assessed by the same reviewers for a final decision about its inclusion into the meta-analysis. Throughout this process, the reviewers were blinded to authors’ names, journal, and year of publication of the articles. Those published in languages other than English and French were translated in French. Any disagreement was resolved by consensus. When we identified studies that had been reported in multiple articles, we limited our analysis to the most recent report, unless the necessary data had appeared only in an earlier article. Agreement between coders was measured using quadratic weighted Kappa statistics [14]. We kept a log of reasons for rejection of citations identified from the searches.
Information extraction
Two reviewers (YL and SM) abstracted information from all articles selected for inclusion in the meta-analysis. The abstracted information included: (1) the study design; (2) the industry and country where the occupational exposure to silica occurred; (3) the methods of exposure quantification; (4) whether patients with silicosis were excluded from the study; and (5) the risks [relative risks (RR) or odds ratios (OR), with their 95% confidence intervals (CI) or variance estimates] of lung cancer at various thresholds of exposure to silica.
Statistical analysis
Given the low event rate in the control groups, we assimilated OR to RR in case–control studies and used RR throughout the analyses. For the studies providing only RR, we transformed the confidence intervals to the log scale to estimate the variances of log (RR). The standard error was estimated by dividing the transformed interval length by 3.92 and the corresponding variance then equalled to the square of the standard error [15]. Each study reported the risk of several ranges of exposure to silica with respect to a reference range. This reference range differed across studies and corresponded to the “non-exposed” (i.e., control) category. Within each category, we assigned as exposure value the median point when available or the mid-point of the corresponding range. When the highest category was open-ended, we assigned the lower point of the interval plus 20% as the exposure level [16].
Standard dose–response meta-analyses are usually conducted by performing separate analyses of each individual study first and then combining results by weighted averages. Such approach requires at least four exposure levels within each individual study when a nonlinear trend is suspected. Since several studies considered in our analysis reported less than four exposure levels, we could not be apply this approach. We rather considered an alternative approach (the “prepool” method) consisting of pooling data available from all studies into a joint analysis. No assumption was made about the relationship between log (RR) and the exposure level. Following Bagnardi et al., we considered splines regression models [17]. Heterogeneity between different studies was modeled by an additional random component of variance [18]. Models with and without the latter additional component are known as mixed-effects and fixed-effects models, respectively. We used the difference of the maximum log likelihood of these models to test the heterogeneity between studies. Both models parameters were estimated by the algorithm proposed by Stram [19]. Technical and computational details are given in Appendix 1.
A similar exposure–response meta-analysis was conducted with cumulative exposures to silica lagged by 10–20 years. In this method, for each period of follow-up, employment history in the most recent 10–20 years is ignored in order to take into account disease latency.
Sensitivity analyses
We decided a priori to conduct subgroup analyses to identify sources of heterogeneity, if any, according to the following hypotheses: (1) cohort and case–control studies would yield different results; (2) dose–response relationships would vary according to the methods of measurement of respirable silica (i.e., job-exposure matrices versus longstanding personal shift measurements). In addition, we planned a posteriori analyses to assess the impact of each individual study on heterogeneity and to examine the stability of the results. The sensitivity investigation consisted of iterative “leave-on-out” procedures. In this method, the influence of individual studies is estimated from the average dose–response curve by considering all studies except one [20].
Results
Literature search/agreement studies
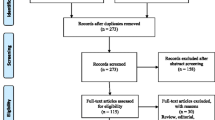
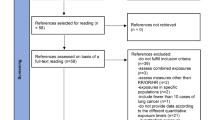
A total of 1,284 separate publications were retrieved. We reduced these to a list of 449 potentially eligible articles, of which 400 were excluded for the following reasons: silicosis without specific reference to silica exposure (n = 120), exposure other than silica (n = 104), commentaries or reviews (n = 85), no report of measure of association (n = 36), autopsy studies (n = 10), outcome other than lung cancer (n = 22), animal studies (n = 13), other reasons (n = 10). Forty-nine publications from 23 studies reported on risk of lung cancer with quantitative assessment of exposure. Thirteen of these studies were excluded because of differences in quantitative assessment of exposure (Appendix 2, [21–33]). The primary reviewers finally agreed to include 12 articles [34–45] reporting on 10 studies (4 cohort studies [34–37] and 6 case–control studies [38, 39, 42–45]). Only two studies excluded patients with silicosis [38, 40]. In the remaining eight studies, the confounding effect of silicosis on the association between silica exposure and lung cancer was not assessed. Agreement among the reviewers was good (Kappa: 0.53). Disagreement was always resolved by consensus. Table 1 summarizes the 10 studies that met the inclusion criteria of the meta-analysis.
Dose–response meta-analysis
Figure 1 illustrates the contribution of the nine studies that provided data on the dose–response relationship between respirable silica exposure and lung cancer with no lag time [34–40, 42, 43]. From these data, we estimated dose–response relationship between exposure to silica and RR of lung cancer along with its confidence limit (Fig. 2). We found increasing risk of lung cancer with increasing cumulative exposure to silica. For instance, considering two levels of exposure x 1 = 1.0 mg/m3 per year and x 2 = 6 mg/m3 per year, the corresponding RRs were 1.22 (CI: 1.01–1.47) and 1.84 (CI: 1.48–2.28), respectively. The difference of these risks is significant. In fact, the risk associated with any exposure level superior to 1.84 mg/m3 per year has a significant difference with that associated with x = 0. Figure 2 also suggested that the risk of lung cancer plateaus at a level exposure is greater than 6 mg/m3 per year. The log likelihood test detected a strong heterogeneity between studies (χ2 = 20.19 on 1 degree of freedom, p < 0.001). The exposure–response meta-analysis of six studies with cumulative exposures to silica lagged by 10–20 years yielded similar results.
Sensitivity analyses
The separate analyses of cohort studies and case–control studies yielded similar results and also revealed heterogeneity. We also considered that all the methods of measurement of exposure to silica used to derive the cumulative exposure provided only rough estimates (Table 1). Therefore, we did not proceed with further subgroup analyses based on the methods of measurement of respirable silica. Finally, the results of the a posteriori analysis are presented in Fig. 3. Two studies generated heterogeneity in dose–response relationship estimation [37, 41]. The design and measurement methods of these two studies were different. In one cohort study, estimates of exposure to silica were based on occupational categories defined in a national census [37]. In the other (a nested case–control study conducted in industrial sand workers), exposure to silica was derived from visits to the plants, records of past changes in process and dust control, and interviews with long-term employees [41, 43]. The remaining seven studies were more homogeneous, although some heterogeneity remained (χ2 = 4.45 on 1 degree of freedom, p = 0.035). Overall, their meta-analysis yielded the same results as those obtained in the main analysis.
Discussion
From a systematic review of the literature and a meta-analysis, we found that an increasing cumulative exposure to respirable silica is associated, across high-quality epidemiological studies, with an increasing risk of lung cancer. The interpretation of our meta-analysis is restricted by our finding of significant heterogeneity across studies.
Heterogeneity is however inherent to the purpose and methods of meta-analysis. Primary studies may differ considerably in their designs, data collection processes, and definitions of exposure and confounders [46]. Important sources of heterogeneity include variability in exposure characterization, study design and quality, and control for confounding [47]. Although heterogeneity is often seen as a limitation to meta-analysis, its exploration may improve understanding of the degree of comparability among studies, identify stratifying variables to remove heterogeneity, and generate hypotheses for further research [47, 48]. None of our a priori hypotheses satisfactorily explain heterogeneity. Subgroup analyses were however limited by the small number of studies included.
Another source of heterogeneity is exposure measurement error [49]. Unfortunately, exposure-measurement error is frequently ignored in epidemiologic studies [50]. In addition, different methods to measure silica exposure exist [51] and variation in cutpoints across studies are also problematic in meta-analysis [49]. Cumulative exposure to respirable silica was most often estimated from job descriptions and historical data to create job-exposure matrices (Table 1). In the main analysis (i.e., dose–response analysis, no lag time), only three studies [34, 40, 42] included contemporary—although limited—air sampling from which cumulative estimates of exposure were based. The effect of measurement error on the study results was formally tested in only one study [42]. In all studies, cumulative exposure quantification could therefore only represent rough estimates that could certainly account, in part, for heterogeneity. Also, measurement error probably accounted for some of the uncertainty regarding the carcinogenicity of silica following small cumulative exposure, where the relative risk of lung cancer is around 1.0. It is nevertheless reassuring to observe at least a trend in the dose–response relationship between cumulative silica exposure and the risk of lung cancer in most individual studies (Fig. 1).
Another limitation of the studies that met the inclusion criteria of the meta-analysis is that, with two exceptions [38, 40], patients with silicosis were not excluded. The meta-analysis of these two case–control studies lacked statistical power and could not demonstrate any dose–response relationship between silica exposure and lung cancer (data not shown). Since silicosis is a risk factor for lung cancer [1], this situation may overestimate the association between silica exposure and lung cancer. However, the magnitude of this error is unknown because the proportion of silicotics among patients exposed to silica was not specified in any of the studies. The statistical model describing the association between silica exposure and lung cancer with silicosis as an intermediate outcome is a typical illness-death model [52]. Advanced statistical methods are employed to investigate such models, and these methods have not been adapted to meta-analysis.
In a pooled exposure–response analysis of 10 silica-exposed cohorts to investigate the association with lung cancer, Steenland et al. also concluded that silica is carcinogenic [11]. Although pooled and meta-analyses both aim at reconciling previously conducted studies that yielded inconsistent results [46], fundamental differences exist between the two types of reviews. A pooled analysis results from the collaboration of investigators who share individual data from related studies. Exposure measures and other covariates can be applied uniformly across the studies combined [53]. A meta-analysis combines aggregated data from studies retrieved from a systematic search of the literature. Pooled and meta-analyses on the same topic may therefore be conducted on quite different sets of studies. For instance, only two published studies [34, 35] were common to Steenland’s pooled analysis and our meta-analysis. In addition, 6 of the 10 studies that met the inclusion criteria of our review became available after Steenland’s publication. “Participation bias” in pooled analysis has been assimilated to “publication bias” in meta-analysis [53]. Few head-to-head comparison between pooled and meta-analyses has been published. A rare instance is the study by Gordon et al. of sinonasal cancer among wood workers [53]. Overall, both analyses yielded similar results, as did Steenland’s and ours.
Our results and those of Steenland et al. bring only partial guidance to compensatory boards evaluating patients with lung cancer and past exposure to respirable silica. It is now well established that silicosis is a risk factor for lung cancer [1]. Whether the association between silicosis and lung cancer is due to the effect of the fibrotic process or to the effect of quartz dust itself is unclear [2]. Although there is a dose–response relationship between cumulative exposure to respirable silica and lung cancer, the increased risk is especially apparent when the cumulative exposure to silica is extremely high and well beyond that resulting from exposure to the limit concentration recommended by the National Institute of Occupational Safety and Health (0.05 mg/m3 as a time-weighted average for up to 10-h workday during 40-h workweek [54]) for a prolonged period of time (>30 years). Such cumulative exposures are unlikely in developed countries where strict occupational safety and health regulations exist. The risk of lung cancer at lower cumulative exposure remains uncertain.
Conclusion
From our results and those of Steenland et al. [11], we would concur with the IARC that silica is a lung carcinogen. Our results indicate that there is an exposure–response relationship between silica and lung cancer above a threshold level estimated to be >1.84 mg/m3 per year. The interpretation of our findings by compensatory boards is however limited by the wide range of exposure to respirable silica reported in the original studies, the heterogeneity across studies, and the confounding effect of silicosis that cannot be fully assessed.
References
Lacasse Y, Martin S, Simard S, Desmeules M (2005) Meta-analysis of silicosis and lung cancer. Scand J Work Environ Health 31:450–458
Koskela RS, Klockars M, Jarvinen E, Rossi A, Kolari PJ (1990) Cancer mortality of granite workers 1940–1985. IARC Sci Publ (97):43–53
Hughes JM, Weill H (1991) Asbestosis as a precursor of asbestos related lung cancer: results of a prospective mortality study. Br J Ind Med 48:229–233
Hubbard R, Venn A, Lewis S, Britton J (2000) Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 161:5–8
International Agency for Research on Cancer (IARC) (1997) IARC monographs on the evaluation of carcinogenic risks to humans—silica, some silicates, coal dust and para-aramid fibrils. World Health Organization
Weill H, McDonald JC (1996) Exposure to crystalline silica and risk of lung cancer: the epidemiological evidence. Thorax 51:97–102. doi:10.1136/thx.51.1.97
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012. doi:10.1001/jama.283.15.2008
Gehanno JF, Paris C, Thirion B, Caillard JF (1998) Assessment of bibliographic databases performance in information retrieval for occupational and environmental toxicology. Occup Environ Med 55:562–566. doi:10.1136/oem.55.8.562
Hill AB (1965) The environment and disease: association or causation? Proc R Soc Med 58:295–300
Lagiou P, Adami HO, Trichopoulos D (2005) Causality in cancer epidemiology. Eur J Epidemiol 20:565–574. doi:10.1007/s10654-005-7968-y
Steenland K, Mannetje A, Boffetta P et al (2001) Pooled exposure-response analyses and risk assessment for lung cancer in 10 cohorts of silica-exposed workers: An IARC multicentre study. Cancer Causes Control 12:773–784. doi:10.1023/A:1012214102061
Steenland K, Loomis D, Shy C, Simonsen N (1996) Review of occupational lung carcinogens. Am J Ind Med 29:474–490. doi:10.1002/(SICI)1097-0274(199605)29:5<474::AID-AJIM6>3.0.CO;2-M
Puntoni R, Goldsmith DF, Valerio F et al (1988) A cohort study of workers employed in a refractory brick plant. Tumori 74:27–33
Kramer MS, Feinstein AR (1981) Clinical biostatistics. LIV. The biostatistics of concordance. Clin Pharmacol Ther 29:111–123
Rothman KJ, Greenland S (1998) Modern epidemiology. Lippincott-Raven Publishers, Philadelphia
Il’yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C (2005) Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control 16:383–388. doi:10.1007/s10552-004-5025-x
Bagnardi V, Zambon A, Quatto P, Corrao G (2004) Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol 159:1077–1086. doi:10.1093/aje/kwh142
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. doi:10.1016/0197-2456(86)90046-2
Stram DO (1996) Meta-analysis of published data using a linear mixed-effects model. Biometrics 52:536–544. doi:10.2307/2532893
Bonovas S, Filioussi K, Tsavaris N, Sitaras NM (2006) Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol 24:4808–4817. doi:10.1200/JCO.2006.06.3560
Hnizdo E, Murray J, Klempman S (1997) Lung cancer in relation to exposure to silica dust, silicosis and uranium production in South African gold miners. Thorax 52:271–275
Checkoway H, Hughes JM, Weill H, Seixas NS, Demers PA (1999) Crystalline silica exposure, radiological silicosis, and lung cancer mortality in diatomaceous earth industry workers. Thorax 54:56–59
Chen W, Chen J (2002) Nested case-control study of lung cancer in four Chinese tin mines. Occup Environ Med 59:113–118. doi:10.1136/oem.59.2.113
Calvert GM, Rice FL, Boiano JM, Sheehy JW, Sanderson WT (2003) Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med 60:122–129. doi:10.1136/oem.60.2.122
Kauppinen T, Heikkila P, Partanen T et al (2003) Mortality and cancer incidence of workers in Finnish road paving companies. Am J Ind Med 43:49–57. doi:10.1002/ajim.10161
Menvielle G, Luce D, Fevotte J et al (2003) Occupational exposures and lung cancer in New Caledonia. Occup Environ Med 60:584–589. doi:10.1136/oem.60.8.584
Attfield MD, Costello J (2004) Quantitative exposure-response for silica dust and lung cancer in Vermont granite workers. Am J Ind Med 45:129–138. doi:10.1002/ajim.10348
Smailyte G, Kurtinaitis J, Andersen A (2004) Mortality and cancer incidence among Lithuanian cement producing workers. Occup Environ Med 61:529–534. doi:10.1136/oem.2003.009936
L’Abbate N, Di Pierri C, Nuzzaco A, Caputo F, Carino M (2005) Radiological and functional progression in silicosis. Med Lav 96:212–221
Baccarelli A, Khmelnitskii O, Tretiakova M et al (2006) Risk of lung cancer from exposure to dusts and fibers in Leningrad Province, Russia. Am J Ind Med 49:460–467. doi:10.1002/ajim.20316
Chen W, Yang J, Chen J, Bruch J (2006) Exposures to silica mixed dust and cohort mortality study in tin mines: exposure-response analysis and risk assessment of lung cancer. Am J Ind Med 49:67–76. doi:10.1002/ajim.20248
Zeka A, Mannetje A, Zaridze D et al (2006) Lung cancer and occupation in nonsmokers: a multicenter case-control study in Europe. Epidemiology 17:615–623. doi:10.1097/01.ede.0000239582.92495.b5
Yu ITS, Tse LA, Leung CC, Wong TW, Tam CM, Chan ACK (2007) Lung cancer mortality among silicotic workers in Hong Kong—no evidence for a link. Ann Oncol 18:1056–1063. doi:10.1093/annonc/mdm089
Checkoway H, Heyer NJ, Seixas NS et al (1997) Dose-response associations of silica with nonmalignant respiratory disease and lung cancer mortality in the diatomaceous earth industry. Am J Epidemiol 145:680–688
Steenland K, Sanderson W (2001) Lung cancer among industrial sand workers exposed to crystalline silica. Am J Epidemiol 153:695–703. doi:10.1093/aje/153.7.695
Brown TP, Rushton L (2005) Mortality in the UK industrial silica sand industry: 2. A retrospective cohort study. Occup Environ Med 62:446–452. doi:10.1136/oem.2004.017731
Pukkala E, Guo J, Kyyronen P, Lindbohm ML, Sallmen M, Kauppinen T (2005) National job-exposure matrix in analyses of census-based estimates of occupational cancer risk. Scand J Work Environ Health 31:97–107
Ulm K, Waschulzik B, Ehnes H et al (1999) Silica dust and lung cancer in the German stone, quarrying, and ceramics industries: results of a case-control study. Thorax 54:347–351
Bruske-Hohlfeld I, Mohner M, Pohlabeln H et al (2000) Occupational lung cancer risk for men in Germany: results from a pooled case-control study. Am J Epidemiol 151:384–395
Cocco P, Rice CH, Chen JQ, McCawley MA, McLaughlin JK, Dosemeci M (2001) Lung cancer risk, silica exposure, and silicosis in Chinese mines and pottery factories: the modifying role of other workplace lung carcinogens. Am J Ind Med 40:674–682. doi:10.1002/ajim.10022
Hughes JM, Weill H, Rando RJ, Shi R, McDonald AD, McDonald JC (2001) Cohort mortality study of North American industrial sand workers. II. Case-referent analysis of lung cancer and silicosis deaths. Ann Occup Hyg 45:201–207
Westberg HB, Bellander T (2003) Epidemiological adaptation of quartz exposure modeling in Swedish aluminum foundries: nested case-control study on lung cancer. Appl Occup Environ Hyg 18:1006–1013. doi:10.1080/10473220390244676
McDonald JC, McDonald AD, Hughes JM, Rando RJ, Weill H (2005) Mortality from lung and kidney disease in a cohort of North American industrial sand workers: an update. Ann Occup Hyg 49:367–373. doi:10.1093/annhyg/mei001
Cassidy A, Mannetje A, Van Tongeren M et al (2007) Occupational exposure to crystalline silica and risk of lung cancer: a multicenter case-control study in Europe. Epidemiology 18:36–43. doi:10.1097/01.ede.0000248515.28903.3c
Chen W, Bochmann F, Sun Y (2007) Effects of work related confounders on the association between silica exposure and lung cancer: a nested case–control study among Chinese miners and pottery workers. Int Arch Occup Environ Health 80:320–326. doi:10.1007/s00420-006-0137-0
Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C (1999) Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol 28:1–9. doi:10.1093/ije/28.1.1
Blair A, Burg J, Foran J et al (1995) Guidelines for application of meta-analysis in environmental epidemiology. ISLI Risk Science Institute. Regul Toxicol Pharmacol 22:189–197. doi:10.1006/rtph.1995.1084
Berlin JA (1995) Invited commentary: benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol 142:383–387
Colditz GA, Burdick E, Mosteller F (1995) Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol 142:371–382
Jurek AM, Maldonado G, Greenland S, Church TR (2006) Exposure-measurement error is frequently ignored when interpreting epidemiologic study results. Eur J Epidemiol 21:871–876. doi:10.1007/s10654-006-9083-0
Dahmann D, Taeger D, Kappler M et al (2007) Assessment of exposure in epidemiological studies: the example of silica dust. J Expo Sci Environ Epidemiol 18:452–461. doi:10.1038/sj.jes.7500636
Wang W (2004) Nonparametric estimation of the Sojourn time distributions for a multi-path model. J R Stat Soc Ser B 65:921–936
Gordon I, Boffetta P, Demers PA (1998) A case study comparing a meta-analysis and a pooled analysis of studies of sinonasal cancer among wood workers. Epidemiology 9:518–524. doi:10.1097/00001648-199809000-00008
Department of Health and Human Services, Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health (2002) NIOSH hazard review: HEALTH effects of occupational exposure to respirable crystalline silica. http://www.cdc.gov/niosh/02-129pd.html
Acknowledgments
We thank Dr. Marc Baril from the Quebec Research Institute for Occupational Health and Safety (Institut de recherche Robert-Sauvé en santé et en sécurité du travail—IRSST) for his support during all steps of this project. We also acknowledge the contributions of Hélène Girard and Jocelyne Bellemare, respectively, technician in documentation and librarian at Laval Hospital.
Source of funding:
Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST), Grant 99-163.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
Appendix 1: statistical methods for meta-analysis
Let I = 1,…, I indexes independent studies and J = 1,…, n i indexes exposure levels within studies. Let Y ij = log(RR ij ) be the estimated log risk ratio corresponding to exposure level x ij . The fixed-effects model is then written as
where f is a smooth continuous function, describing the relationship between the exposure level and the log relative risk response. ε ij → N(0, s 2 ij ) are the sampling errors in Y ij since the latter are estimated rather than observed. Estimated s 2 ij are given by individual studies. Finally, e ij → N(0, σ2) are the overall error terms. It is assumed that ε ij and e ij are independent.
For the mixed-effects model, a random effect term γ i common to points of the same study is added. This yield
where γ i → N(0,τ2) and are independent from ε ij and e ij . Testing heterogeneity corresponds them to performing the following:
The null hypothesis H0 is rejected at level α if
where χ 21,α satisfies \( P\left( {\chi_{1}^{2} > \chi_{1,\alpha }^{2} } \right) = \alpha. \) In both models, f is left completely unspecified providing flexibility. It is nonparametically estimated by a spline of order 3 [17]:
where (x − k i )+ = max(x − k i ,0) is the positive part and \( \left\{ {k_{i} ,\;i = 1, \ldots ,C - 1} \right\} \) are knots. We set C = 3. The models parameters \( \left\{ {\beta_{0 1} ,\beta_{0 2} ,\beta_{0 3} ,\beta_{ 1 3} ,\beta_{ 2 3,} \tau^{ 2} ,\sigma^{ 2} } \right\} \) are estimated by the algorithm given by Stram [19].
Rights and permissions
About this article
Cite this article
Lacasse, Y., Martin, S., Gagné, D. et al. Dose–response meta-analysis of silica and lung cancer. Cancer Causes Control 20, 925–933 (2009). https://doi.org/10.1007/s10552-009-9296-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-009-9296-0