Abstract
Purpose
Breast cancer is more likely attributed to a combination of genetic variations and lifestyle factors. Both one-carbon metabolism and diet-related factors could interfere with the carcinogenesis of breast cancer (BC), but whether diet consumed underlie a specific metabolism pathway could influence the impact of genetic variants on breast cancer risk remains equivocal.
Methods
A case–control study of the Chinese female population (818 cases, 935 controls). 13 SNPs in eight one-carbon metabolism-related genes (MTHFD1, TYMS, MTRR, MAT2B, CDO1, FOLR1, UNG2, ADA) were performed. Diet was assessed by a validated food-frequency questionnaire. We examined the associations of the adherence to the Mediterranean dietary pattern (MDP) and single-nucleotide polymorphisms (SNPs) of one-carbon metabolism with breast cancer risk. We constructed an aggregate polygenic risk score (PRS) to test the additive effects of genetic variants and analyzed the gene–diet interactions.
Results
High adherence (highest quartile) to the MDP decreased the risk of breast cancer among post- but not premenopausal women, respectively (OR = 0.54, 95% CI = 0.38 to 0.78 and 0.90, 0.53 to 1.53). Neither of the polymorphisms or haplotypes was associated with breast cancer risk, irrespective of menopause. However, a high PRS (highest quartile) was associated with more than a doubling risk in both post- and premenopausal women, respectively (OR = 1.95, 95% CI = 1.32 to 2.87 and 2.09, 1.54 to 2.85). We found a gene–diet interaction with adherence to the MDP for aggregate PRS (P-interaction = 0.000) among postmenopausal women. When adherence to the MDP was low (< median), carries with high PRS (highest quartile) had higher BC risk (OR = 2.80, 95% CI = 1.55 to 5.07) than low PRS (lowest quartile), while adherence to the MDP was high (≥ median), the association disappeared (OR = 1.57, 95% CI = 0.92 to 2.66).
Conclusion
High adherence to the MDP may counteract the genetic predisposition associated with one-carbon metabolism on breast cancer risk in postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary risk factors of breast cancer (BC) include overweight or obesity, physical inactivity, exogenous hormone intake (use of oral contraceptive and hormone replacement therapy), reproduction condition (late age at first birth and low parity), family history of cancer and unhealthy diet [1,2,3,4,5,6]. Besides, these modifiable risk factors, having a genetic predisposition increases a women’s lifetime risk of BC [7, 8], as inherited mutations in BRCA1 and BRCA2 genes [9]. However, these genetic changes account for only a small proportion of breast carcinogenesis (< 10%) [10]. For most cases, BC is more likely attributed to a combination of genetic variations and environmental or lifestyle factors. Thus, elucidating the gene–environment interactions is essential to unveil the molecular mechanisms involved in breast carcinogenesis.
A dietary pattern is a combination of lifestyle and environmental factors and is related to an individual’s health outcome. A well-balanced dietary practice, such as the Mediterranean dietary pattern (MDP), is identified to reduce the risk of a variety of diseases [11]. The traditional Mediterranean diet is characterized by the liberal use of olive oil; the high consumption of vegetables, fruits, nuts, legumes and unprocessed cereals; the moderate amounts of fish and wine; the low intake of dairy products (with the exception of cheeses), red meat and meat products [12]. A number of epidemiological studies have suggested that this dietary pattern had substantial health benefits and displayed inverse associations with multiple cancer types [13]. Since the development of cancer is generally determined by the interplay between extrinsic and intrinsic factors, the role of underlying metabolism pathways associated with the genetic variants might help explain the discrepancies in the published studies, inconsistent results of the association between the gene polymorphism and cancer risk [14,15,16,17,18,19].
One-carbon metabolism is a complicated metabolic network composed of cascade reactions based on the transfer of one-carbon units, which is required by nucleotide synthesis, DNA replication, repair and methylation. The inadequate intake of nutrients in the daily diet could destroy the normal physiological process of one-carbon metabolism, associated with the disruption of DNA replication and repair as well as aberrant DNA methylation patterns, eventually leading to carcinogenesis [20]. Some epidemiological studies provided some promising results, which focused on MTHFR (rs1801131, rs1801133) and MTR (rs1805087), intake of folate and vitamin B6/B12 and BC risk [21, 22]. Also, genetic polymorphisms in the one-carbon metabolism pathway genes encoding functional enzymes and co-enzymes have been suggested to influence individuals’ susceptibility to cancer, such as Methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR) and thymidylate synthase (TYMS) [23,24,25]. However, in addition to these most common polymorphisms, the role of genetic variants in other one-carbon metabolism genes and their interactions with diet regarding BC risk remain largely unexplored, especially in the Asian female population.
Hence, we selected a series of key genes that play a role in the one-carbon metabolic pathway [25], and picked SNP sites that were previously suggested to be related to the risk of other cancers [26, 27]. We aim to evaluate the association between genetic polymorphisms of one-carbon metabolism and the risk of BC among Chinese women and whether adherence to the Mediterranean dietary pattern could modify the association of genetic variants with BC risk.
Materials and methods
Subjects
Subjects were from the Chinese Wuxi Exposure and Breast Cancer Study (2013–2014), a population-based case–control study on the role of biology, diet, lifestyle, and environmental factors in the etiology of BC in Asian women. The subjects were all women who lived in Wuxi city, Jiangsu Province, China, for more than five years. Newly diagnosed breast cancer patients within one year were selected as the case group according to the local cancer registration system. All cases were identified according to the International Classification of Diseases for Oncology (ICD-10, code C50), excluding patients with secondary or recurrent BC. For those with multiple incident cancers, only included those with BC as the first original malignancy diagnosed. Controls were matched to the cases in a ratio of 1:1 by the same residence area and age (range of ± 5 years), excluding individuals with any cancer history. The study protocol was approved by the Institutional Review Boards of Jiangsu CDC, and informed consent was obtained from all subjects. Blood samples were collected from both cases and controls.
Data on diet
The usual diet was assessed by a validated, semi-quantitative food-frequency questionnaire (FFQ), which included 149 items along with the recipes commonly used in China. Nutrient and energy intake were calculated through the Chinese Food Composition Database (2018, 6th version). Dietary intake assessment included whether the food was consumed, consumption frequency (times of per day/week/month/year) and the average amount of food consumption at each time. The 149 food items in the FFQ were classified into 18 predefined food groups based on similarities in nutrient profile and culinary usage. The validity of the FFQ has been proved in the previous study [28].
Mediterranean diet scale
The alternate Mediterranean diet score (aMED) established by Fung et al. [29] includes nine dietary components and ranges from 0 to 9 scores (minimum to maximum conformity). One point is given to each subject when the food intake is equal to or above the median intake of controls for the following seven components regarded as healthy: grains, fruits, vegetables, legumes, nuts, fish, and monounsaturated fat–saturated fat ratio. One point is given when the intake amount of a subject is less than the median intake of unhealthy food such as red meat or processed meat, or alcohol consumption within 5–25 g/day for women as a specified range. The higher the score obtained from the questionnaire, the greater the adherence to the Mediterranean dietary pattern (MDP).
Lifestyle, anthropometric, medical history and reproductive history data
Demographic, lifestyle characteristics, menstrual and reproductive events, dietary intake, disease history and physical activity-related data were collected from a structured questionnaire, through in-person interviews conducted by trained interviewers. Anthropometric measures were obtained by trained personnel following the protocol. Physical activity was measured by referencing the Global Physical Activity Questionnaire [30]. Postmenopause was defined as an absence of menstruation in the past 12 months.
Genotyping assays
A total of 18 single-nucleotide polymorphisms (SNPs) involved in 12 one-carbon metabolism genes including Methylenetetrahydrofolate dehydrogenase 1 (MTHFD1), Thymidylate Synthetase (TYMS), methionine synthase reductase (MTRR), methionine adenosyltransferase 1A (MAT1A), methionine adenosyltransferase 2B (MAT2B), folate receptor 1 (FOLR1), cystathionine-\(\upbeta\)-synthase (CBS), glutaminase (GLS), DNA methyltransferases 3B (DNMT3B), uracil N-glycosylase 2 (UNG2), adenosine deaminase (ADA) and cysteine dioxygenase (CDO1), were selected for genotyping analysis. Genomic DNA was extracted from 200 μl of peripheral blood using the QIAamp DNA Blood Mini Kit (QIAGEN, Germany) following the manufacturer’s instructions. Purified DNA was evaluated and quantified by agarose gel electrophoresis and spectrophotometer methods. Experimental design and SNP genotyping were carried out by the Sequenom MassARRAY platform. The call rate for each SNP among all the samples was > 95%. In addition, five percent of samples were randomly selected and then repeatedly genotyped with a concordant rate of 100%.
Statistical analysis
Chi-square tests were carried out to examine Hardy–Weinberg equilibrium (HWE) in the control group. Linkage disequilibrium between SNPs was calculated as D’ and r2 values. The SNPs with strong linkage disequilibrium will be constructed as haplotypes for further analysis.
Polygenic risk score (PRS) is a useful way of summarizing the effects of genetic variants, the weighted sum of the risk allele counts across one-carbon metabolism pathway was calculated, where the weight for each associated individual SNP is determined by the adjusted log OR of its association with breast cancer risk.
Unconditional logistic regression methods were used to assess: (1) associations between SNP polymorphisms and BC risk (statistical significance should be after Benjamini & Yekutieli correction for multiple testing). (2) Associations between PRS and BC risk. (3) Associations between adherence to the MDP and BC risk. The effect size of association was assessed after adjusting the potential confounders, included are age at diagnosis for cases or enrollment for controls (by years), area (urban, rural), education (ordered as illiterate and primary, middle and high school, university and above), tobacco smoking (no, or yes: including smoking and second-hand smoking \(\ge\) 3 day/week), moderate physical activity (minutes/day), oral contraceptives use (no, or yes: current use or ever use), hormone replacement therapy (no, or yes: current use or ever use), family history of breast cancer (no, or yes: in a first-degree relative), history of benign breast disease (no, or yes: including lactation mastitis, plasma cell mastitis, cyclomastopathy, fibroadenoma of breast, galactocele), age at menarche (by years), parity (0, 1, 2,\(\mathrm{or }\ge\) 3), age at first full-term delivery (by years), breastfeeding (no, or yes), body mass index (BMI; in kg/m2).
To examine the role of the Mediterranean dietary pattern in the etiology of breast cancer, we test the interaction between the Mediterranean diet score and the duration of Mediterranean diet intake on breast cancer risk. To examine the synergistic effect of diet-genes on breast cancer risk, we test the interaction between the SNPs polymorphisms or their PRS and adherence to the Mediterranean dietary pattern. The likelihood ratio test was used to test interactions.
All analyses were performed with R version 4.0.2 (The R Project for Statistical Computing, USA; http://www.r-project.org/).
Results
From Nov 2013 to Nov 2014, a total of 1410 newly diagnosed breast cancer cases were identified in Wuxi City, 1072 cases meeting the inclusion criteria and 818 of them were recruited in this study. 1072 controls were screened and 935 of them were recruited. Of the 818 cases and 935 controls, the demographic characteristics and anthropometric measures of the subjects stratified by menopausal status are presented in Supplemental Table 1.
Characteristics and frequencies of one-carbon metabolism genes
Genotype and allele frequencies of the 18 SNPs in twelve one-carbon metabolism pathway-related genes (MTHFD1, TYMS, MTRR, MAT1A, MAT2B, CDO1, FOLR1, CBS, GLS, DNMT3B, UNG2, ADA) among cases and controls are shown in Table 1. Because there is no genetic variation (minor allele frequency less than 5% was found in the MTHFD1 T>C (rs2230491), MAT1A T>C (rs10887718), CBS T>C (rs11701048), GLS T > C (rs12185688) and DNMT3B G>A (rs13045669), they will be excluded in the following analyses. The results presented in this study were based on 13 SNPs in the eight one-carbon metabolism pathway-related genes (MTHFD1 G>A (rs11627387), MTHFD1 T>C (rs2281603), MTHFD1 G>A (rs8003567), TYMS A>T (rs10502289), TYMS T>G (rs2298582), TYMS G>A (rs11664283), MTRR G>C (rs16879334), MTRR T>C (rs2287780), MAT2B C>A (rs4869087), CDO1 G>C (rs34869), FOLR1 T>G (rs10501409), UNG2 G>A (rs231622), ADA G>A (rs244072)). The genotype frequencies of the SNPs included in the control group did not deviate from Hardy–Weinberg equilibrium (HWE).
Associations between single-nucleotide polymorphisms (SNPs) and breast cancer risk
We did not found any significant association between individual single-nucleotide polymorphisms (SNPs) and pre-or postmenopausal BC risk, irrespective of an additive model (MM versus Mm versus mm) or a dominant model (MM versus Mm + mm). The genotype can be a major allele homozygote (MM), a heterozygote (Mm), or a minor allele homozygote (mm), results shown in Supplemental Table 2.
For linkage disequilibrium analysis, three considerable degree of linkage disequilibrium were observed between the MTRR T>C (rs227780) and MTRR G>C (rs16879334) (D’ = 0.99, r2 = 1), MTHFD1 G>A (rs8003567) and MTHFD1 G>A (rs11627387) (D’ = 0.96, r2 = 0.35) and MTHFD1 G>A (rs8003567) and MTHFD1 G>A (rs2281603) (D’ = 0.93, r2 = 0.13), Fig. 1. Haplotypes with a frequency greater than 0.03 were constructed in the case group and control group, but no significant difference was found in the distribution of haplotypes between cases and the controls, results shown in Supplemental Table 3.
Logarithm-transformed P values for the association between breast cancer risk and single-nucleotide polymorphisms (SNPs) of one-carbon metabolism genes and pattern of linkage disequilibrium for tagging SNPs genotyped in carbon metabolism genes. The heatmap was constructed using a custom R script with the LDheatmap package in R version 4.0.2 software
Associations between adherence to the Mediterranean dietary pattern and breast cancer risk
We found a consistent trend of decreasing BC risk with increasing aMED score, with the whole population in the high aMED score category (highest quartile) having 39% reduction in risk of breast cancer (OR = 0.61, 95% CI = 0.50 to 0.76). However, the significant association was only among post- (OR = 0.54, 95% CI = 0.38 to 0.78, P-trend = 0.001), but not premenopausal women (OR = 0.90, 95% CI = 0.53 to 1.53, P-trend = 0.824), Table 2.
A significant interaction was found between the Mediterranean diet score and the duration of Mediterranean diet intake on breast cancer risk in postmenopausal women (P-interaction = 0.020). Long-term (\(\ge\) median) adherence to MDP (highest quartile of aMED) significantly reduces the risk of breast cancer in postmenopausal women, compared to short-term (< median) and lowest quartile of aMED (OR = 0.41, 95% CI = 0.24 to 0.68), Supplemental Table 4. We collected the estrogen receptor information of 439 of 818 cases, including 301 estrogen receptor-positive and 138 estrogen receptor-negative. We found the inverse association between MDP and breast cancer risk was greater and easier to detect among ER− tumors, Supplemental Table 5.
Associations between PRS and breast cancer risk
The PRS was normally distributed had a strong positive association with BC risk, Fig. 2, a relatively high PRS (highest quartile) was associated with more than a doubling in the risk of breast cancer in the whole population (OR = 2.09, 95% CI = 1.54 to 2.85, P-trend = 0.000). When stratification of menopause, the association among premenopausal women was slightly stronger than that for postmenopausal women, respectively (OR = 2.30, 95% CI = 1.31 to 4.03, P-trend = 0.000 and OR = 1.95, 95% CI = 1.32 to 2.87, P-trend = 0.001), Table 2.
Associations between Polygenic risk score (PRS), adherence to the Mediterranean dietary pattern (MDP) and breast cancer risk, stratification by menopause. a–c PRS density distribution in predicting breast cancer risk. d–f PRS quartiles in predicting breast cancer risk. g–i Adherence to MDP affects the association between PRS and breast cancer risk
Gene–diet interaction between one-carbon metabolism genes and adherence to the Mediterranean dietary pattern in determining breast cancer risk
In the interaction analyses, the wild-type genotype of the SNPs at the lowest quartile values of aMED was used as the reference group. We found a nominal statistical significance of the relevant interaction between MTHFD1 G>A (rs8003567) polymorphisms with postmenopausal breast cancer risk based on the additive and dominant genotypic effects (P-interaction = 0.0260 for additive genotypic effects and P-interaction = 0.0465 for dominant genotypic effects, Supplemental Table 6. However, this interaction was no longer significant under the Benjamini & Yekutieli correction for multiple comparisons, Supplemental Table 7.
However, there was a significant interaction between adherence to the MDP and cumulative PRS in determining breast cancer risk (P-interaction = 0.006), Table 3. The association or not of these polymorphisms combination with breast cancer risk depended on the degree of adherence to the MDP. When adherence to the MDP was low (\(<\) median), carriers with more genetic variants (highest quartile of PRS) had a higher risk of breast cancer (OR = 2.91, 95% CI = 1.80 to 4.67) than that with low PRS (lowest quartile). However, when adherence to the MDP was high (\(\ge\) median), the association was declining (OR = 1.72, 95% CI = 1.14 to 2.60).
Further analysis for stratification of menopausal status, we found the relevant interaction was only among post- but not premenopausal women, respectively (P-interaction = 0.000 and P-interaction = 0.411). When adherence to the MDP was low (< median score), carries with high PRS (highest quartile) had higher BC risk (OR = 2.80, 95% CI = 1.55 to 5.07) than low PRS (lowest quartile), while adherence to the MDP was high (\(>\) median), the association disappeared (OR = 1.57, 95% CI = 0.92 to 2.66). When we used individual Mediterranean dietary components instead of MDP, we didn’t find any significant interaction between the specific foods and PRS on breast cancer risk, Supplemental Table 8.
Discussion
In this study, among 13 SNPs involved eight one-carbon metabolism pathway-related genes, none of them were statistically significantly associated with the pre- or postmenopausal BC risk. However, we observed a high PRS had more than a doubling breast cancer risk irrespective of individual’s menopause. These results were similar to previous studies; most low penetrance single-nucleotide polymorphisms (SNPs) individually have a relatively weak association with the risk of breast cancer while examined the risk in relation to the combination of polymorphism have reported a much stronger association [14,15,16,17,18,19]. Interestingly, when stratification of menopause, we found the association between the PRS and risk of BC among premenopausal women was somewhat stronger than that for postmenopausal women, which also in agreement with existing evidence which indicates that familial/genetic BC typically occurs at an earlier age than sporadic BC [31].
A number of previous studies using MED indices have consistently shown an inverse association of high adherence to the MDP with the risk of BC [32]. However, the effect modification of menopausal status and estrogen receptor status were not consistent [33, 34]. In the current study, we found that adherence to the MDP could reduce post- but not premenopausal breast cancer risk. The possible differential effect by menopausal status may be due to a stronger influence of genetic factors and early life events in premenopausal breast cancer [34]. Additionally, we found the inverse association between MDP and breast cancer risk is stronger among ER- tumors than ER+ tumors. There is an explanation that the influence of dietary factors may be more difficult to detect in ER+ tumors because of the strong influence of hormonal factors [35]. Taken overall, our results are consistent with the previous review study [36]. The Mediterranean diet is characterized by foods rich in fiber and antioxidants, such as flavonoids, vitamins and carotenoids. Epidemiological evidence strongly suggested that long-standing consumption of plant polyphenols- rich diets could help against breast cancer initiation and proliferation, especially the anti-inflammatory and antioxidant effects of diet [34]. The underlying mechanisms by which the Mediterranean dietary pattern modulated BC risk were previously identified as the decrease of endogenous estrogens [37], neutralization of free radicals to prevent DNA damage [38] as well as reduction of oxidative stress [39]. This also partly explains the heterogeneity we observed in menopause and estrogen receptors. The ovaries are the predominant site of estrogen synthesis in the premenopausal period, the contribution of adipocytes to the circulating pool of estrogens is negligible. Besides, evidence suggests that changes in fat distribution associated with menopause cause fat to be redistributed toward the abdominal region, with a preferential increase in visceral fat after menopause. As metabolic perturbations related to hormonal are induced particularly by abdominal adiposity, the adverse effect of inflammatory on BC may be much weaker during the period of premenopausal than postmenopausal. Additionally, exposure to endogenous estrogen is one of the strongest risk factors for breast cancer and it has less influence on ER- tumors than ER+ tumors, which results in the influence of dietary factors may be easier to detect among ER-tumors [34, 36]. In total, our results of association of MDP and BC risk supports the hypothesis that food components and their combinations in the MDP play an etiologic role related to the risk of breast cancer, which enhances the evidence of its role in non-Mediterranean country populations.
However, the main finding and novelty of our results were that we found the association between one-carbon metabolism genes and BC risk depend on the diet consumed combination. Specifically, when the dietary pattern departed from an overall MDP, the effect of gene variants were significantly associated with BC risk, while a good adherence to MDP blunted this association. This gene–diet interaction was robust among the whole population and, in particular, postmenopausal women. As far as we know, this is the first time that a significant interaction between the one-carbon metabolism gene and diet in determining BC risk has been reported in the Chinese female population.
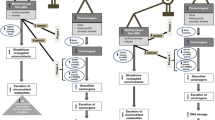
The nutrients associated with one-carbon metabolism could not naturally be manufactured by the human body, which means they need to be obtained from foods. The disruption on the one-carbon metabolism pathway could interfere with DNA-repair, DNA replication, and gene expression regulation, which could enhance the deleterious effect of genetic variants [40, 41]. That has motivated lots of studies focusing on the potential link between the nutrients associated with the one-carbon metabolism pathway and carcinogenesis [42, 43]. A previous study showed that SNPs of one-carbon metabolism gene have interactions with folate intake to affect the BC risk [44]. However, most previous studies focus on the effect of individual nutrients rather than a combination of foods [45], which have been limited in interpreting the high degree of intercorrelation among various nutrients. These associations were always weak because it is hard to attribute effects to single independent component foods [41]. In this context, we choose a ‘prior’ dietary pattern instead of individual nutrients, which could better capture specific diet characteristics and cumulative effects of nutrients. The Mediterranean dietary pattern is characterized by high consumption of vegetables, fruit, legumes, and fish, rich in folate, choline, vitamins and methionine. Sufficient levels of micronutrients play an important role in the one-carbon metabolism [46, 47], because specific enzymes and co-enzymes in one-carbon metabolism require ample quantities of dietary micronutrients (e.g., folate, methionine and other specific amino acids and B2, B6 and B12 and other vitamins), as substrates to achieve their biological functions [48, 49]. In addition, one-carbon metabolism is interconnected to the biological processes of DNA methylation and DNA synthesis [50], Fig. 3. Both processes are thought to play key roles in carcinogenesis [51, 52]. DNA methylation is an epigenetic mechanism by which cells regulate gene expression, which involves the addition of a methyl (-CH3) to the 5-carbocytosine residue, predominantly in the cytosine guanine dinucleotide (CPGs). Dietary micronutrients involved in one-carbon metabolism play an essential role in DNA methylation, such as folic acid, choline, betaine, riboflavin, vitamins B6 and B12, and the amino acid methionine (Fig. 3) [40, 53]. Especially folic acid (or called vitamin B9), whose role is crucial in the DNA methylation process, producing the methyl group donor, S-adenosylmethionine. A recent study in rural African women support that one-carbon nutrient may affect methylation levels, dietary intake of one-carbon metabolites and cofactors in diet fluctuates with seasons. The concentration of biomarkers of maternal carbon metabolism nutrients during pregnancy was associated with the methylation of metastable epi-alleles in DNA from birth infant’s lymphocytes and hair follicles. Specifically, plasma concentrations of riboflavin and vitamin B6 indicate this association [54], and previous studies [55] had shown a positive correlation between the two biomarkers and carefully measured dietary intake. However, a recent big cross-sectional study that included 5186 adults does not found any log-linear association between the intake of one-carbon metabolic nutrients and individual CpG methylation. The relationship between nutrients and DNA methylation is complicated, and there is no unified conclusion now, needs more in-depth study.
In addition to the robust interaction between MPD and PRS, we found a nominally significant interaction result in MTHFD1 G>A (rs8003567), which also implies that one-carbon metabolism genes may be related to diet and BC risk by affecting DNA methylation. The MTHFD1 gene product is a multifunctional enzyme possessing the activities of methylene-THF dehydrogenase, methenyl-THF cyclohydrolase and formyl-THF synthetase in the one-carbon metabolism pathway [56]. It usually catalyzes sequential and reversible reactions in multiple conversion of tetrahydrofolate (THF), the active form of folate, into 5,10-methylene-THF, which is essential for the de novo purine and thymidylate synthesis as well as the supply of one-carbon units for subsequent DNA methylation. The deficiency or dysregulation of the MTHFD1 enzyme may influence cell division and global methylation pattern, eventually contributing to tumorigenesis [56, 57]. Since rs8003567 is located in the intronic region of the MTHFD1 gene and no disease-related studies on SNPs have been reported before, another possible explanation cannot be excluded that there are additional functional genetic variants in linkage disequilibrium with these two SNPs that modify BC risk in Chinese female population. However, the interpretation of a nominally significant interaction should be cautious, because the corrected P values for multiple comparisons is no longer significant; the gene–diet interaction obtained may be a false-positive result. Thus, replication of the findings in other independent studies is needed before the firm conclusions can be drawn.
The strength of our study is that we focus on the genetic variants in the one-carbon metabolism pathway associated with BC risk linking a specific dietary pattern. Most of the previous studies historically focused on individual nutrients [58, 59] and individual SNP, which could not capture the complicated interrelationships and cumulative effects between nutrients and genetic variants [60]. Thus, examining the risk in relation to combinations of SNPs and diet may be more predictive for the in vivo situation [61, 62] and interpretative for disease risk and biological mechanism. Several limitations should also be taken into account in our study. First, data were collected from a case–control study, which might be partially influenced by the biases inherent in case–control designs, as selection bias, recall bias, residual confounding, and reverse causality, etc. Second, since the number of cases and controls enrolled in this project is relatively small, the associations we founded require replication in other larger sample independent studies. Further work should assess associations of BC risk and the concentrations of these nutrients in plasma associated with one-carbon metabolism and DNA methylation.
Conclusion
In conclusion, our finding demonstrates that adherence to the Mediterranean dietary pattern (MDP) may attenuate the deleterious effect of genetic factors on the risk of breast cancer among women of Chinese descent, in particular, postmenopausal women.
References
Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, Thomson CA, Caan BJ, Tinker LF, Urrutia RP, Knudtson J, Anderson GL (2015) Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol 1(5):611–621
Trinh T, Eriksson M, Darabi H, Bonn SE, Brand JS, Cuzick J, Czene K, Sjolander A, Balter K, Hall P (2015) Background risk of breast cancer and the association between physical activity and mammographic density. Breast Cancer Res (BCR) 17:50
Ellingjord-Dale M, Vos L, Tretli S, Hofvind S, Dos-Santos-Silva I, Ursin G (2017) Parity, hormones and breast cancer subtypes - results from a large nested case-control study in a national screening program. Breast Cancer Res (BCR) 19(1):10
Bethea TN, Rosenberg L, Hong CC, Troester MA, Lunetta KL, Bandera EV, Schedin P, Kolonel LN, Olshan AF, Ambrosone CB, Palmer JR (2015) A case–control analysis of oral contraceptive use and breast cancer subtypes in the African American Breast Cancer Epidemiology and Risk Consortium. Breast Cancer Res (BCR) 17:22
Brewer HR, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ (2017) Family history and risk of breast cancer: an analysis accounting for family structure. Breast Cancer Res Treat 165(1):193–200
Deschasaux M, Julia C, Kesse-Guyot E, Lecuyer L, Adriouch S, Mejean C, Ducrot P, Peneau S, Latino-Martel P, Fezeu LK, Fassier P, Hercberg S, Touvier M (2017) Are self-reported unhealthy food choices associated with an increased risk of breast cancer? Prospective cohort study using the British Food Standards Agency nutrient profiling system. BMJ Open 7(6):e013718
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130
Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J ClinOncol 25(11):1329–1333
Torres D, Bermejo JL, Rashid MU, Briceno I, Gil F, Beltran A, Ariza V, Hamann U (2017) Prevalence and penetrance of BRCA1 and BRCA2 germline mutations in Colombian breast cancer patients. Sci Rep 7(1):4713
Song M, Lee KM, Kang D (2011) Breast cancer prevention based on gene–environment interaction. MolCarcinog 50(4):280–290
Soltani S, Jayedi A, Shab-Bidar S, Becerra-Tomas N, Salas-Salvado J (2019) Adherence to the Mediterranean diet in relation to all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. AdvNutr 10(6):1029–1039
Trichopoulou A, Martinez-Gonzalez MA, Tong TY, Forouhi NG, Khandelwal S, Prabhakaran D, Mozaffarian D, de Lorgeril M (2014) Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med 12:112
Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, Boffetta P, Trichopoulos D (2008) Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. Br J Cancer 99(1):191–195
Shieh Y, Hu D, Ma L, Huntsman S, Gard CC, Leung JW, Tice JA, Vachon CM, Cummings SR, Kerlikowske K, Ziv E (2016) Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat 159(3):513–525
Wen W, Shu XO, Guo X, Cai Q, Long J, Bolla MK, Michailidou K, Dennis J, Wang Q, Gao YT, Zheng Y, Dunning AM, Garcia-Closas M, Brennan P, Chen ST, Choi JY, Hartman M, Ito H, Lophatananon A, Matsuo K, Miao H, Muir K, Sangrajrang S, Shen CY, Teo SH, Tseng CC, Wu AH, Yip CH, Simard J, Pharoah PD, Hall P, Kang D, Xiang Y, Easton DF, Zheng W (2016) Prediction of breast cancer risk based on common genetic variants in women of East Asian ancestry. Breast Cancer Res 18(1):124
Vachon CM, Pankratz VS, Scott CG, Haeberle L, Ziv E, Jensen MR, Brandt KR, Whaley DH, Olson JE, Heusinger K, Hack CC, Jud SM, Beckmann MW, Schulz-Wendtland R, Tice JA, Norman AD, Cunningham JM, Purrington KS, Easton DF, Sellers TA, Kerlikowske K, Fasching PA, Couch FJ (2015) The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju397
Cuzick J, Brentnall AR, Segal C, Byers H, Reuter C, Detre S, Lopez-Knowles E, Sestak I, Howell A, Powles TJ, Newman WG, Dowsett M (2017) Impact of a panel of 88 single nucleotide polymorphisms on the risk of breast cancer in high-risk women: results from two randomized tamoxifen prevention trials. J ClinOncol 35(7):743–750
Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, Tyrer JP, Chen TH (2019) Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet 104(1):21–34
Mavaddat N, Pharoah PD, Michailidou K, Tyrer J, Brook MN, Bolla MK, Wang Q, Dennis J, Dunning AM, Shah M (2015) Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv036
Miranti EH, Stolzenberg-Solomon R, Weinstein SJ, Selhub J, Mannisto S, Taylor PR, Freedman ND, Albanes D, Abnet CC, Murphy G (2017) Low vitamin B12 increases risk of gastric cancer: a prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. Int J Cancer 141(6):1120–1129
Ma E, Iwasaki M, Junko I, Hamada GS, Nishimoto IN, Carvalho SM Jr, Motola J, Laginha FM, Tsugane S (2009) Dietary intake of folate, vitamin B6, and vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: a case–control study in Brazilian women. BMC Cancer 9:122
Platek ME, Shields PG, Marian C, McCann SE, Bonner MR, Nie J, Ambrosone CB, Millen AE, Ochs-Balcom HM, Quick SK, Trevisan M, Russell M, Nochajski TH, Edge SB, Freudenheim JL (2009) Alcohol consumption and genetic variation in methylenetetrahydrofolate reductase and 5-methyltetrahydrofolate-homocysteine methyltransferase in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev 18(9):2453–2459
Wu JL, Zhou SX, Zhao R, Zhang X, Chang K, Gu CY, Gan HL, Dai B, Zhu Y, Zhang HL, Shi GH, Qu YY, Zhao JY, Ye DW (2016) MTHFR c.677C>T inhibits cell proliferation and decreases prostate cancer susceptibility in the Han Chinese population in Shanghai. Sci Rep 6:36290
Shao HB, Ren K, Gao SL, Zou JG, Mi YY, Zhang LF, Zuo L, Okada A, Yasui T (2018) Human methionine synthase A2756G polymorphism increases susceptibility to prostate cancer. Aging 10(7):1776–1788
Ducker GS, Rabinowitz JD (2017) One-carbon metabolism in health and disease. Cell Metab 25(1):27–42
Han SS, Sue LY, Berndt SI, Selhub J, Burdette LA, Rosenberg PS, Ziegler RG (2012) Associations between genes in the one-carbon metabolism pathway and advanced colorectal adenoma risk in individuals with low folate intake. Cancer Epidemiol Biomarkers Prev 21(3):417–427
Cheng TY, Makar KW, Neuhouser ML, Miller JW, Song X, Brown EC, Beresford SA, Zheng Y, Poole EM, Galbraith RL, Duggan DJ, Habermann N, Bailey LB, Maneval DR, Caudill MA, Toriola AT, Green R, Ulrich CM (2015) Folate-mediated one-carbon metabolism genes and interactions with nutritional factors on colorectal cancer risk: Women’s Health Initiative Observational Study. Cancer 121(20):3684–3691
Zhao W, Hasegawa K, Chen J (2002) The use of food-frequency questionnaires for various purposes in China. Public Health Nutr 5(6A):829–833
Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB (2005) Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J ClinNutr 82(1):163–173
Armstrong T, Bull F (2006) Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health 14(2):66–70
Easton DF (2002) Familial risks of breast cancer. Breast Cancer Res 4(5):179–181
van den Brandt PA, Schulpen M (2017) Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer 140(10):2220–2231
Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D (2010) Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J ClinNutr 92(3):620–625
Buckland G, Travier N, Cottet V, Gonzalez CA, Lujan-Barroso L, Agudo A, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, May A, Bueno-de-Mesquita HB, BvanDuijnhoven FJ, Key TJ, Allen N, Khaw KT, Wareham N, Romieu I, McCormack V, Boutron-Ruault M, Clavel-Chapelon F, Panico S, Agnoli C, Palli D, Tumino R, Vineis P, Amiano P, Barricarte A, Rodriguez L, Sanchez MJ, Chirlaque MD, Kaaks R, Teucher B, Boeing H, Bergmann MM, Overvad K, Dahm CC, Tjonneland A, Olsen A, Manjer J, Wirfalt E, Hallmans G, Johansson I, Lund E, Hjartaker A, Skeie G, Vergnaud AC, Norat T, Romaguera D, Riboli E (2013) Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer 132(12):2918–2927
Fung TT, Hu FB, McCullough ML, Newby P, Willett WC, Holmes MD (2006) Diet quality is associated with the risk of estrogen receptor–negative breast cancer in postmenopausal women. J Nutr 136(2):466–472
Coughlin SS, Stewart J, Williams LB (2018) A review of adherence to the Mediterranean diet and breast cancer risk according to estrogen- and progesterone-receptor status and HER2 oncogene expression. Ann Epidemiol Public Health. https://doi.org/10.33582/2639-4391/1002
Carruba G, Granata OM, Pala V, Campisi I, Agostara B, Cusimano R, Ravazzolo B, Traina A (2006) A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Nutr Cancer 56(2):253–259
Visioli F, Grande S, Bogani P, Galli C (2004) The role of antioxidants in the Mediterranean diets: focus on cancer. Eur J Prev 13(4):337–343
Mitjavila MT, Fandos M, Salas-Salvado J, Covas MI, Borrego S, Estruch R, Lamuela-Raventos R, Corella D, Martinez-Gonzalez MA, Sanchez JM, Bullo M, Fito M, Tormos C, Cerda C, Casillas R, Moreno JJ, Iradi A, Zaragoza C, Chaves J, Saez GT (2013) The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. ClinNutr 32(2):172–178
Locasale JW (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13(8):572–583
Kim YI (2004) Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev 13(4):511–519
Weinstein SJ, Stolzenberg-Solomon R, Pietinen P, Taylor PR, Virtamo J, Albanes D (2006) Dietary factors of one-carbon metabolism and prostate cancer risk. Am J ClinNutr 84(4):929–935
Milne RL, Fletcher AS, MacInnis RJ, Hodge AM, Hopkins AH, Bassett JK, Bruinsma FJ, Lynch BM, Dugue PA, Jayasekara H, Brinkman MT, Popowski LV, Baglietto L, Severi G, O’Dea K, Hopper JL, Southey MC, English DR, Giles GG (2017) Cohort profile: The Melbourne collaborative cohort study (Health 2020). Int J Epidemiol 46(6):1757–1757i
Kakkoura MG, Sokratous K, Demetriou CA, Loizidou MA, Loucaides G, Kakouri E, Hadjisavvas A, Kyriacou K (2017) Mediterranean diet-gene interactions: a targeted metabolomics study in Greek-Cypriot women. MolNutr Food Res. https://doi.org/10.1002/mnfr.201600558
Lissowska J, Gaudet MM, Brinton LA, Chanock SJ, Peplonska B, Welch R, Zatonski W, Szeszenia-Dabrowska N, Park S, Sherman M, Garcia-Closas M (2007) Genetic polymorphisms in the one-carbon metabolism pathway and breast cancer risk: a population-based case-control study and meta-analyses. Int J Cancer 120(12):2696–2703
Park JY, Nicolas G, Freisling H, Biessy C, Scalbert A, Romieu I, Chajes V, Chuang SC, Ericson U, Wallstrom P, Ros MM, Peeters PH, Mattiello A, Palli D, Maria HJ, Amiano P, Halkjaer J, Dahm CC, Trichopoulou A, Orfanos P, Teucher B, Feller S, Skeie G, Engeset D, Boutron-Ruault MC, Clavel-Chapelon F, Crowe F, Khaw KT, Vineis P, Slimani N (2012) Comparison of standardised dietary folate intake across ten countries participating in the European Prospective Investigation into Cancer and Nutrition. Br J Nutr 108(3):552–569
Woodside JV, McCall D, McGartland C, Young IS (2005) Micronutrients: dietary intake v. supplement use. Proc NutrSoc 64(4):543–553
Lucock M (2000) Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab 71(1–2):121–138
Stevens VL, McCullough ML, Pavluck AL, Talbot JT, Feigelson HS, Thun MJ, Calle EE (2007) Association of polymorphisms in one-carbon metabolism genes and postmenopausal breast cancer incidence. Cancer Epidemiol Biomarkers Prev 16(6):1140–1147
Xu X, Chen J (2009) One-carbon metabolism and breast cancer: an epidemiological perspective. J Genet Genomics 36(4):203–214
Maruti SS, Ulrich CM, Jupe ER, White E (2009) MTHFR C677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: a nested case–control study. Breast Cancer Res 11(6):R91
Lewis SJ, Harbord RM, Harris R, Smith GD (2006) Meta-analyses of observational and genetic association studies of folate intakes or levels and breast cancer risk. J Natl Cancer Inst 98(22):1607–1622
Crider KS, Yang TP, Berry RJ, Bailey LB (2012) Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. AdvNutr 3(1):21–38
Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, Fulford AJ, Guan Y, Laritsky E, Silver MJ, Swan GE, Zeisel SH, Innis SM, Waterland RA, Prentice AM, Hennig BJ (2014) Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun 5:3746
Dominguez-Salas P, Moore SE, Cole D, Da CKA, Cox SE, Dyer RA, Fulford AJ, Innis SM, Waterland RA, Zeisel SH, Prentice AM, Hennig BJ (2013) DNA methylation potential: dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am J ClinNutr 97(6):1217–1227
MacFarlane AJ, Perry CA, McEntee MF, Lin DM, Stover PJ (2011) Mthfd1 is a modifier of chemically induced intestinal carcinogenesis. Carcinogenesis 32(3):427–433
Ding K, Jiang J, Chen L, Xu X (2018) Methylenetetrahydrofolate dehydrogenase 1 silencing expedites the apoptosis of non-small cell lung cancer cells via modulating DNA methylation. Med SciMonit 24:7499–7507
Matejcic M, de Batlle J, Ricci C, Biessy C, Perrier F, Huybrechts I, Weiderpass E, Boutron-Ruault MC, Cadeau C, His M, Cox DG, Boeing H, Fortner RT, Kaaks R, Lagiou P, Trichopoulou A, Benetou V, Tumino R, Panico S, Sieri S, Palli D, Ricceri F, Bueno-de-Mesquita HB, Skeie G, Amiano P, Sanchez MJ, Chirlaque MD, Barricarte A, Quiros JR, Buckland G, van Gils CH, Peeters PH, Key TJ, Riboli E, Gylling B, Zeleniuch-Jacquotte A, Gunter MJ, Romieu I, Chajes V (2017) Biomarkers of folate and vitamin B12 and breast cancer risk: report from the EPIC cohort. Int J Cancer 140(6):1246–1259
Kim SJ, Zuchniak A, Sohn KJ, Lubinski J, Demsky R, Eisen A, Akbari MR, Kim YI, Narod SA, Kotsopoulos J (2016) Plasma folate, vitamin B-6, and vitamin B-12 and breast cancer risk in BRCA1- and BRCA2-mutation carriers: a prospective study. Am J ClinNutr 104(3):671–677
Turati F, Carioli G, Bravi F, Ferraroni M, Serraino D, Montella M, Giacosa A, Toffolutti F, Negri E, Levi F, La Vecchia C (2018) Mediterranean DIET and s. Nutrients 10(3):326
Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV (2010) Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J ClinNutr 91(5):1294–1302
Gerber M (2003) Biofactors in the Mediterranean diet. ClinChem Lab Med 41(8):999–1004
Acknowledgements
We are grateful to all study participants for their contributions. We thank the entire data collection team. Breast cancer cases and healthy controls in this study were collected by Wuxi Center for Disease Control and Prevention, Jiangsu Provincial Center for Disease Control and Prevention.
Funding
This study was supported by World Cancer Research Fund (2011/RFA/473) and Wuxi Young Medical Talents (QNRC035).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study was approved by the ethical committee of the Jiangsu Center for Disease Control and Prevention (Jiangsu, China).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, S., Zhu, Z., Zhou, J. et al. Associations of one-carbon metabolism-related gene polymorphisms with breast cancer risk are modulated by diet, being higher when adherence to the Mediterranean dietary pattern is low. Breast Cancer Res Treat 187, 793–804 (2021). https://doi.org/10.1007/s10549-021-06108-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06108-8