Abstract
Background
PEG-rhG-CSF reduces neutropenia and improves chemotherapy safety. In China’s registration trial (CFDA: 2006L01305), we assessed its efficacy and safety against rhG-CSF, and prospectively explored its value over multiple cycles of chemotherapy.
Methods
In this open-label, randomized, multicenter phase 3 study, breast cancer patients (n = 569) were randomized to receive PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, or rhG-CSF 5 µg/kg/d after chemotherapy. The primary endpoints were the incidence and duration of grade 3/4 neutropenia during cycle 1. Secondary endpoints included the incidence and duration of grade 3/4 neutropenia during cycles 2–4, the incidence of febrile neutropenia, and the safety.
Results
A once-per-cycle PEG-rhG-CSF at either 100 µg/kg or 6 mg was not different from daily injections of rhG-CSF for either incidence or duration of grade 3/4 neutropenia. Interestingly, a substantial difference was noted during cycle 2, and the difference became bigger over cycles 3–4, reaching a statistical significance at cycle 4 in either incidence (P = 0.0309) or duration (P = 0.0289) favoring PEG-rhG-CSF. A significant trend toward a lower incidence of all-grade adverse events was noted at 129 (68.98%), 142 (75.53%), and 160 (82.47%) in the PEG-rhG-CSF 100 µg/kg and 6 mg and rhG-CSF groups, respectively (P = 0.0085). The corresponding incidence of grade 3/4 drug-related adverse events was 2/187 (1.07%), 1/188 (0.53%), and 8/194 (4.12%), respectively (P = 0.0477). Additionally, PFS in metastatic patients preferred PEG-rhG-CSF to rhG-CSF despite no significance observed by Kaplan–Meier analysis (n = 49, P = 0.153).
Conclusions
PEG-rhG-CSF is a more convenient and safe formulation and a more effective prophylactic measure in breast cancer patients receiving multiple cycles of chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy is still a major strategy for breast cancer. In daily practice, chemotherapy-induced neutropenia increases the risk of potentially life-threatening infections and febrile neutropenia (FN), which requires hospitalization for intravenous antibiotics and generally causes dose reductions below the optimal drug level or delays in subsequent chemotherapy cycles [1].
Granulocyte colony stimulating factor (G-CSF), stimulating the production of neutrophil precursors, enhancing the function of mature neutrophils, and ameliorating neutropenia and its complications [2], has been used to decrease the incidence of myelosuppression caused by cytotoxic chemotherapy. The first recombinant human granulocyte colony stimulating factor (rhG-CSF) approved for clinical practice is filgrastim with a short plasma half-life of about 3–4 h, requiring daily subcutaneous (s.c.) injections. It has been proved that filgrastim administration increases WBC counts and decreases the duration of neutropenia, days of hospitalization, and the number of culture-confirmed infections [3, 4]. Furthermore, prophylactic G-CSF is routinely recommended by current treatment guidelines for patients receiving chemotherapy regimens associated with 20% or higher risk of FN [5,6,7,8,9,10,11]. Selective use of G-CSFs in patients at increased risk for neutropenic complications may, however, enhance the cost effectiveness [12, 13].
The covalent attachment of polyethylene glycol (PEG) significantly extends the half-life to about 42–62 h after a single injection per chemotherapy cycle to achieve the same effect as multiple daily injections of rhG-CSF. PEG-modification rhG-CSF, with a similar biological activity to rhG-CSF, is eliminated mainly via neutrophil receptor-mediated endocytosis and degradation [14]. Thus, it remains in the pharmacological range and only drops during neutrophil recovery. Potential benefits of PEG-rhG-CSF over rhG-CSF include fewer injections, better compliance, and decreased burden for both patients and healthcare professionals [15]. In addition, PEG-rhG-CSF may perform better in support of patients through a course of multiple cycles of chemotherapy [16, 17]. However, its value remains unclear.
The aim of this study was to evaluate the efficacy and safety of both 100 µg/kg and fixed 6 mg dose of PEG-rhG-CSF per cycle of chemotherapy, compared with daily administration of rhG-CSF, in provision of neutrophil support for breast cancer patients receiving myelosuppressive chemotherapy, and prospectively explore its value over multiple cycles of chemotherapy.
Patients and methods
Study population
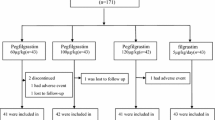
This is an open-label, multicenter, randomized, active-controlled, phase IIIb trial. The institutional review boards or ethics committees of the participating centers approved the protocol. Written informed consent was obtained from each patient before the study-related procedure was performed. Forty-two centers in China were designed to enroll 540 breast cancer patients from May 2014 to January 2015, but actually 569 patients were randomized into the study. Of 569 patients, 557 (97.89%) received at least one dose of the study drug and 12 (2.11%) withdrew from the study. Of 557 patients, 9 (1.58%) had major protocol violations, with the remaining 548 patients in per-protocol set (PPS) for efficacy analyses. All 569 randomized patients were included in full analysis set (FAS) and safety set (SS) for efficacy and safety analyses. Table 1 provides the inclusion and exclusion criteria for this study.
Study design
The primary objective of this trial was to evaluate whether once-per-cycle PEG-rhG-CSF was as safe and effective as multiple daily dose of rhG-CSF in breast cancer patients receiving four cycles of myelosuppressive chemotherapy. As a result, the sample size of the study was based on a noninferiority design. Eligible patients were randomly assigned in a 1:1:1 ratio to receive either one of the three intervention arms, i.e., (1) a single-dose s.c. injection of PEG-rhG-CSF 100 µg/kg, (2) a single-dose s.c. injection of PEG-rhG-CSF 6 mg, and (3) a daily dose of rhG-CSF 5 µg/kg.
If patients in the study groups experienced FN and/or ANC < 0.5 × 109/L for longer than 3 days, a dose of rhG-CSF 5 µg/kg was permitted to continue daily until an ANC ≥ 5.0 × 109/L or for a maximum of 14 days, whichever occurred first. Otherwise, it was not permitted to receive rhG-CSF or other hemogram-impacted treatment such as radiotherapy. Patients randomized to the PEG-rhG-CSF group received a single 100 µg/kg or a fixed 6 mg s.c. injection on day 3 of each cycle onward (48 h after completion of chemotherapy).
On day 1 of each cycle, patients received an i.v. bolus epirubicin of 100 mg/m2 followed 1 h later by an i.v. bolus cyclophosphamide of 600 mg/m2 (EC regimen), or an i.v. bolus epirubicin of 75 mg/m2 followed 1 h later by a 1-h infusion of docetaxel of 75 mg/m2(TC regimen), or a 1-h infusion of docetaxel of 75 mg/m2 followed 1 h later by an i.v. bolus cyclophosphamide of 600 mg/m2(ET regimen). Chemotherapy was repeated every 3 weeks for up to 4 cycles. Dose reduction was permitted only when the patients experienced grade 4 thrombocytopenia, grade 4 anemia, severe cardiac disorders, or other situations considered unsuitable to be continued by investigators.
Prophylactic antibiotics were not permitted during the study. Systemically, antibiotics were allowed only for an ANC ≤ 0.5 × 109/L, FN, infection, or suspected infection with an increased temperature of ≥ 38 °C.
Blood samples were collected prior to drug injection on days 3, 5, 7–11, 13, 15, 17, and 21 of cycle 1, and on days 5, 7, 9, 11, 13, and 21 of subsequent cycles. Plasma for antibody analysis was collected before premedication and at the end of both cycle 2 and cycle 4.
Efficacy and safety measurements
The primary efficacy endpoints were the incidence and duration of grade 3/4 neutropenia in cycle 1 based on both FAS and PPS. The secondary efficacy endpoints included the incidence and duration of grade 3/4 neutropenia in cycles 2–4 and the incidence of FN in each cycle. FN is defined as an ANC < 0.5 × 109/L or an ANC < 1.0 × 109/L with a trend to drop below 0.5 × 109/L in the following 48 h, concurrent with a single oral temperature of ≥ 38.3 °C, or a sustained temperature of ≥ 38 °C for at least 1 h.
Safety was assessed by the incidence of adverse events using preferred terms designated by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE version 4.0) based on safety set (SS). Specific antibodies against rhG-CSF or PEG-rhG-CSF were also included in safety assessment.
Statistical analyses
Statistical analyses were carried out using statistical analysis system (SAS) software, version 9.2. The significant level for all statistical tests was set at 0.05 with 95% two-sided confidence intervals (CIs). The basic characteristics and outcomes of study patients were compared across three interventional arms using Chi-square (χχ 2) test for categorical data and analysis of variance (ANOVA) or Kruskal–Wallis test for normally and nonnormally distributed continuous data, respectively. Treatment group differences in the incidence of grade 3/4 neutropenia were calculated by Cochran–Mantel–Haenszel (CMH) Chi square (χχ 2) based on FAS and PPS. The FAS population comprised all randomized patients, and the PPS population comprised all randomized patients without any major protocol violation. The results from these analyses for FAS did not differ materially from that for PPS.
For safety analyses, changes in laboratory values and vital signs were recorded and summarized using descriptive statistics, including mean, standard deviation, median, minimum, and maximum values.
Additionally, progression-free survival (PFS) was analyzed by Kaplan–Meier analysis between the PEG-rhG-CSF and rhG-CSF groups. PFS was defined as the time from registration to the earliest of death due to any cause or disease progression. Patients who were known to be alive were censored at the last follow-up visit.
Results
Patients
Of the 569 patients enrolled into this trial, 187 patients were randomized to PEG-rhG-CSF 100 µg/kg, 188 to PEG-rhG-CSF 6 mg, and 194 to rhG-CSF 5 µg/kg/day. The study flow diagram for patient enrollment, allocation, and follow-up is shown in Fig. 1. The mean ages across the three interventional arms were 47.12 ± 8.81, 49.40 ± 8.84, and 49.22 ± 9.24 years, respectively. The patient population was predominantly women and Asian with a good ECOG performance status (0 or 1). The three arms were balanced for other demographic factors and disease status at baseline, except for age, height, plus rate and disease history. Table 2 (placed at the end of the manuscript) shown below shows the comparison of the characteristics of the study patients. In general, there was no statistical difference in clinical characteristics with respect to breast cancer-associated baseline.
All the patients received at least one dose of the assigned study drug and were included in both efficacy and safety analyses. 548 (96.30%) patients were eligible for efficacy analyses of the primary end point to evaluate the robustness of the FAS results.
Efficacy
Incidence and duration of grade 3/4 neutropenia in cycle 1
The incidence of grade 3/4 neutropenia for the FAS was 44.39%, 49.47%, and 48.45%, and for the PPS, it was 45.56, 49.45, and 49.46%, in the PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, and rhG-CSF 5 µg/kg/day groups, respectively. There were no significant differences between three intervention arms (P = 0.5892 for FAS, P = 0.6802 for PPS). Likewise, the mean ± SD duration of grade 3/4 neutropenia showed no statistically significant differences (P = 0.2512 for FAS, P = 0.5189 for PPS), which was 0.96 ± 1.29 days in the PEG-rhG-CSF 100 µg/kg group and 1.19 ± 1.43 days in the PEG-rhG-CSF 6 mg group, compared with 1.10 ± 1.44 days in the rhG-CSF 5 µg/kg/day group for the FAS, and for the PPS, it was 0.99 ± 1.30, 1.18 ± 1.43, and 1.11 ± 1.45, respectively. Although the incidence and duration of grade 3/4 neutropenia in the PEG-rhG-CSF 100 µg/kg group tended to be lower, both PEG-rhG-CSF 100 µg/kg and PEG-rhG-CSF 6 mg were noninferior to rhG-CSF 5 µg/kg/day.
Incidence and duration of grade 3/4 neutropenia in subsequent cycles
The incidence and duration of grade 3/4 neutropenia for cycles 2 to 4 based on the FAS are demonstrated in Table 3 and Fig. 2, which tended to be of more and more difference at cycles 2–3, and reached a statistically significant difference at cycle 4 (P = 0.0289), indicating that PEG-rhG-CSF performs even better than rhG-CSF in support of patients through the course of cytotoxic chemotherapy.
Incidence of grade 3/4 neutropenia in each group for all cycles. The incidence and duration of grade 3/4 neutropenia for cycles 2–4 based on the FAS were demonstrated, which tended to be of more and more difference at cycles 2–3, and reached a statistically significant difference at cycle 4 (P = 0.0289)
Febrile neutropenia in all cycles
Over the course of the trial, the cumulative incidence of FN was reported only for cycles 1–2. 6, 8, and 6 patients in the PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, and rhG-CSF 5 µg/kg/day groups experienced FN, and the FN rate for each group showed no difference.
Safety
Adverse events
Adverse events (AEs) were reported in 129 (68.98%), 142 (75.53%), and 142 (75.53%) patients from the PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, and rhG-CSF 5 µg/kg/day groups, respectively. There were significant differences in the overall safety profile across arms (P = 0.0085). Most adverse events were attributable to complications of myelosuppressive chemotherapy and were of mild or moderate intensity. Common AE profiles over all cycles across the three arms are summarized in Table 4. Serious adverse events (SAEs) were noted in 12/187 (6.42%), 3/188 (1.60%), and 12/194 (6.19%) patients from the PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, and rhG-CSF 5 µg/kg/day groups, respectively (P = 0.0333). A total of 26 events had an unrelated or unlikely relationship to PEG-rhG-CSF or rhG-CSF, and 1 was considered unassessable. Of these SAEs, 24 patients experienced grade 4 neutropenia, including 9, 3, and 12 in the PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, and rhG-CSF 5 µg/kg/day groups, respectively. The other three patients experienced asthma recurrence, hemorrhoidal hemorrhage, and thymosin-induced anaphylaxis, respectively.
Adverse drug reactions
The incidence of grade 3/4 adverse drug reactions was 3/187 (1.60%), 2/188 (1.06%), and 8/194 (4.12%) in the PEG-rhG-CSF 100 µg/kg, PEG-rhG-CSF 6 mg, and rhG-CSF groups, respectively, indicating that PEG-rhG-CSF was well tolerated compared with rhG-CSF (P = 0.0477, Table 5). Of adverse reactions over all cycles across the three arms, the higher reported nonhematologic adverse events included nausea, vomiting, constipation, anorexia, fatigue, and liver dysfunction.
Antibody formation
No patient developed binding or neutralizing antibodies against drugs in any arms. All patients were observed expected transient neutropenia, and recovered their ANC during the treatment period.
Progression-free survival (PFS)
A total of 49 metastatic patients who completed at least three cycles of chemotherapy were analyzed by Kaplan–Meier analysis. 28 patients were administered PEG-rhG-CSF 100 µg/kg or PEG-rhG-CSF 6 mg, and 21 patients with rhG-CSF 5 µg/kg/day. The median follow-up was 15.23 months. Median PFS was 8.13 months in the rhG-CSF group and it was not yet estimable in the PEG-rhG-CSF group until more follow-up has occurred. As shown in Fig. 3, two survival lines intersected at the beginning and gradually separated with an increasing difference. This might suggest that metastatic patients preferred PEG-rhG-CSF to rhG-CSF despite no significance observed by Kaplan–Meier analysis (n = 49, P = 0.153).
Kaplan–Meier plot of progression-free survival between the PEG-rhG-CSF and rhG-CSF groups. Median PFS was 8.13 months in the rhG-CSF group and it was not yet estimable in the PEG-rhG-CSF group until more follow-up has occurred. Two survival lines intersected at the beginning and gradually separated with an increasing difference
Discussion
This randomized study revealed that PEG-rhG-CSF was at least equivalent to rhG-CSF in efficacy and even showed better performance in subsequent cycles. In cycle 1, incidence or duration of grade 3/4 neutropenia showed no difference. Interestingly, incidence or duration of grade 3/4 neutropenia in cycle 2 showed a substantial difference, and the difference became bigger over cycles 3 and 4, reaching statistical significance at cycle 4 in either incidence (P = 0.0309) or duration (P = 0.0289). This suggested that PEG-rhG-CSF had a better performance over rhG-CSF in support of patients through a course of cytotoxic chemotherapy. These findings were rarely reported by other long-acting rhG-CSF [18, 19]. However, it was not occasional. In a previous study published on JCO 2002 [16], significant differences were observed in cycles 2–4 between pegfilgrastim and filgrastim with respect to the duration of grade 4 neutropenia and the incidence of FN. Recently, a meta-analysis showed that lipegfilgrastim, another long-acting filgrastim, was associated with significant reductions in risk of severe neutropenia and febrile neutropenia in cycles 2–4 [17]. These results suggested additional clinical benefits of the longer-acting form for patients who underwent multi-cycle chemotherapy. The underlying mechanism of such findings was unclear. Holmes et al. presumed that constant stimulation of neutrophils and neutrophil precursors in bone marrow and blood may play a role in the improved efficacy noted [16]. As PEG-rhG-CSF eliminated mainly via neutrophil receptor-mediated endocytosis and degradation, its metabolites may stimulate cytokines or interact with cytokine cross-talk in neutrophil cells, resulting in secondary effects on hematopoietic cells with a long-lasting subsequent impact on neutrophils.
As for FN, the cumulative incidence of FN over the course of the study was only reported for cycles 1–2 in three interventional arms that were less than expected, indicating that the prophylactic use of G-CSF was warranted to reduce the risk of febrile neutropenia.
Safety profile was generally similar between PEG-G-CSF and reference G-CSF; however, the results of adverse drug reactions showed that PEG-rhG-CSF was well tolerated compared with rhG-CSF (P = 0.0477). The incidence of grade 3/4 adverse drug reactions was observed in three patients in the PEG-rhG-CSF 100 µg/kg group, two patients in the PEG-rhG-CSF 6 mg group, and 8 patients in the rhG-CSF group. With respect to adverse events, most of them were attributable to complications of myelosuppressive chemotherapy, were of mild or moderate intensity, and showed no significant difference. Of SAEs over all cycles, nine patients in the PEG-rhG-CSF 100 µg/kg group, three patients in the PEG-rhG-CSF 6 mg group, and 12 patients in the rhG-CSF 5 µg/kg/day group experienced severe neutropenia. Three patients in the PEG-rhG-CSF 100 µg/kg group experienced asthma recurrence, hemorrhoidal hemorrhage, and thymosin-induced anaphylaxis, respectively. Of these SAEs, a total of 26 events had an unrelated or unlikely relationship to PEG-rhG-CSF or rhG-CSF, and 1 was considered unassessable. Moreover, in accordance with the low immunogenic potential of rhG-CSF, immunogenic response to PEG-rhG-CSF assessed showed no increased risk of developing anti-PEG-rhG-CSF antibodies.
Additionally, PFS was explored between the PEG-rhG-CSF and rhG-CSF groups. The survival lines of PEG-rhG-CSF and rhG-CSF intersected at the beginning and gradually separated with an increasing difference, indicating that metastatic patients preferred PEG-rhG-CSF to rhG-CSF despite no significance observed by Kaplan–Meier analysis (n = 49, P = 0.153). G-CSF has been associated with multiple immune effects, including the stimulation of neutrophil-mediated cytotoxicity of lymphoma cells. Neutrophils have been described as potent cytotoxic effectors, able to produce many cytotoxic molecules, and exert direct tumoricidal activity [20, 21]. Paradoxically, high doses of G-CSF might induce immune suppression. The immune boosting phenomenon appears to be dose dependent and occurs preferably at lower doses of G-CSF. PEG-rhG-GSF, a long-acting form of G-CSF, observed of lower incidence and shorter duration of grade 3/4 neutropenia over multiple cycles of chemotherapy, presumed constant and mild stimulation of neutrophils and neutrophil precursors in bone marrow and/or blood, may manipulate immunological response in a positive way and thus prolong PFS in breast cancer patients. However, this study was neither designed nor powered to assess PFS, and the number of PFS events was limited. Further evaluation involving more metastatic patients with longer follow-up will be needed.
In conclusion, compared with rhG-CSF, PEG-rhG-CSF is a more convenient and safe formulation and a more effective prophylactic measure in breast cancer patients receiving multiple cycles of chemotherapy.
Abbreviations
- PEG-rhG-CSF:
-
PEG-modification recombinant human granulocyte colony stimulating factor
- rhG-CSF:
-
Recombinant human granulocyte colony stimulating factor
- EC:
-
Epirubicin 100 mg/m2 and cyclophosphamide 600 mg/m2
- ET:
-
Epirubicin 75 mg/m2 and docetaxel 75 mg/m2
- TC:
-
Docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2
- FN:
-
Febrile neutropenia
- s.c.:
-
Subcutaneous
- PEG:
-
Polyethylene glycol
- PPS:
-
Per-protocol set
- FAS:
-
Full analysis set
- SS:
-
Safety set
- NCI:
-
National Cancer Institute
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CIs:
-
Confidence intervals
- ANOVA:
-
Analysis of variance
- CMH:
-
Cochran–Mantel–Haenszel
- AEs:
-
Adverse events
- SAEs:
-
Serious adverse events
- PFS:
-
Progression-free survival
- Hb:
-
Hemoglobin
- WBC:
-
White blood count
- ANC:
-
Absolute neutrophil count
- PLT:
-
Platelet count
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- TBIL:
-
Total bilirubin
- Scr:
-
Serum creatinine
- ULN:
-
Upper limit of normal
References
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100:228–237. https://doi.org/10.1002/cncr.11882
Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G (1996) Filgrastim (r-metHuG-CSF): the first 10 years. Blood 88:1907–1929
Gabrilove JL, Jakubowski A, Scher H, Sternberg C, Wong G, Grous J, Yagoda A, Fain K, Moore MA, Clarkson B et al (1988) Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med 318:1414–1422. https://doi.org/10.1056/NEJM198806023182202
Sheridan WP, Morstyn G, Wolf M, Dodds A, Lusk J, Maher D, Layton JE, Green MD, Souza L, Fox RM (1989) Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet 2:891–895
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL (2011) Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404. https://doi.org/10.1186/1471-2407-11-404
Crawford J, Caserta C, Roila F, Group EGW (2010) Hematopoietic growth factors: eSMO clinical practice guidelines for the applications. Ann Oncol 21(Suppl 5):v248–251. https://doi.org/10.1093/annonc/mdq195
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for R, Treatment of C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Crawford J, Rodgers GM (2014) Treatment strategies for myeloid growth factors and intravenous iron: when, what, and how? J Natl Compr Canc Netw 12:821–824
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J clin oncol 24:3187–3205. https://doi.org/10.1200/JCO.2006.06.4451
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J clin oncol 24:3187–3205. https://doi.org/10.1200/JCO.2006.06.4451
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors: version 2. 2014
Ravangard R, Bordbar N, Keshavarz K, Dehghani M (2017) Pegfilgrastim versus filgrastim for primary prophylaxis of febrile neutropenia in patients with non-Hodgkin’s lymphoma: a cost-effectiveness study. Asian Pac J Cancer Prev 18:2703–2707. https://doi.org/10.22034/APJCP.2017.18.10.2703
Akpo EIH, Jansen IR, Maes E, Simoens S (2017) Cost-utility analysis of lipegfilgrastim compared to pegfilgrastim for the prophylaxis of chemotherapy-induced neutropenia in patients with stage ii-iv breast cancer. Front Pharmacol 8:614. https://doi.org/10.3389/fphar.2017.00614
Aapro M, Boccia R, Leonard R, Camps C, Campone M, Choquet S, Danova M, Glaspy J, Hus I, Link H, Sliwa T, Tesch H, Valero V (2017) Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. https://doi.org/10.1007/s00520-017-3842-1
Weycker D, Bensink M, Wu H, Doroff R, Chandler D (2017) Risk of chemotherapy-induced febrile neutropenia with early discontinuation of pegfilgrastim prophylaxis based on real-world data from 2010 to 2015. Curr Med Res Opin 33:2115–2120. https://doi.org/10.1080/03007995.2017.1386638
Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, Glaspy J, Moore M, Meza L, Wiznitzer I, Neumann TA, Hill LR, Liang BC (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J clin oncol 20:727–731. https://doi.org/10.1200/JCO.2002.20.3.727
Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, Glaspy J, Moore M, Meza L, Wiznitzer I, Neumann TA, Hill LR, Liang BC (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J clin oncol 20:727–731. https://doi.org/10.1200/JCO.2002.20.3.727
Vose JM, Crump M, Lazarus H, Emmanouilides C, Schenkein D, Moore J, Frankel S, Flinn I, Lovelace W, Hackett J, Liang BC (2003) Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J clin oncol 21:514–519. https://doi.org/10.1200/JCO.2003.03.040
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, Siena S, Lalisang RI, Samonigg H, Clemens MR, Zani V, Liang BC, Renwick J, Piccart MJ, International Pegfilgrastim 749 Study G (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30:459–489. https://doi.org/10.1146/annurev-immunol-020711-074942
Albanesi M, Mancardi DA, Jonsson F, Iannascoli B, Fiette L, Di Santo JP, Lowell CA, Bruhns P (2013) Neutrophils mediate antibody-induced antitumor effects in mice. Blood 122:3160–3164. https://doi.org/10.1182/blood-2013-04-497446
Acknowledgements
The authors thank all the researchers and patients who participated in this study. All the authors contributed to enrolling breast cancer patients and drafting or revising the manuscript. All the authors reviewed and approved the final version of the manuscript, and approved for publication. This study was funded by Shangdong New Time Pharmaceutical Co., LTD. PEG-rhG-CSF was provided by the funding source. Employees of the funding source were involved in the collection and assembly of data, performing statistical analysis, analyzing and interpreting data, and approving the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest were disclosed.
Informed consent
All patients provided written informed consent.
Additional information
Jie Xie and Jun Cao are joint first authors.
Rights and permissions
About this article
Cite this article
Xie, J., Cao, J., Wang, Jf. et al. Advantages with prophylactic PEG-rhG-CSF versus rhG-CSF in breast cancer patients receiving multiple cycles of myelosuppressive chemotherapy: an open-label, randomized, multicenter phase III study. Breast Cancer Res Treat 168, 389–399 (2018). https://doi.org/10.1007/s10549-017-4609-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4609-6