Abstract
Purpose
This multi-center, randomized, phase III study was conducted to demonstrate the non-inferiority of DA-3031 compared with daily filgrastim in patients during the first cycle of chemotherapy for breast cancer in terms of the duration of severe neutropenia (DSN).
Methods
Seventy-four patients with breast cancer who were receiving combination chemotherapy with docetaxel, doxorubicin, and cyclophosphamide (TAC) were enrolled. All participants were randomized to receive either daily subcutaneous injections of filgrastim 100 μg/m2/day for up to 10 days or a single subcutaneous injection of DA-3031 at fixed doses of 6 mg on day 2 of each chemotherapy cycle.
Results
The mean duration of grade 4 (G4) neutropenia in cycle 1 was 2.08 ± 0.85 days for the filgrastim group and 2.28 ± 1.14 days for the DA-3031 group. The difference between groups was 0.2 ± 1.10 days (95 % confidence interval (CI) = −0.26, 0.66), which supported non-inferiority. No statistically significant differences were observed in nadir absolute neutrophil count (ANC) (154.34/mm3 and 161.75/mm3 for the filgrastim and DA-3031 groups, respectively; P = 0.8414) or in time to ANC recovery (10.03 ± 0.75 and 9.83 ± 1.56 days in the filgrastim and DA-3031 groups, respectively; P = 0.0611) during cycle 1. Serious AEs occurred in six (15.8 %) patients receiving filgrastim and in ten (27.8 %) patients receiving DA-3031; however, none was determined to be related to the study drug.
Conclusions
DA-3031 and daily filgrastim are similar in regard to DSN and safety in breast cancer patients receiving TAC chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hematologic toxicities, including myelosuppression, are the most important dose-limiting factors in cytotoxic chemotherapy. Patients with severe neutropenia often develop clinically important complications, including an increased risk for opportunistic infections and sepsis. As a result, patients with early stage cancers may receive adjuvant chemotherapy at a decreased relative dose intensity (RDI), which compromises treatment efficacy [1, 2]. In patients with advanced or recurrent cancers, severe infectious complications can be a major cause of morbidity and can deteriorate the quality of life [3].
Combination chemotherapy with docetaxel, doxorubicin, and cyclophosphamide (TAC) is a standard of care for both patients with high-risk early and advanced HER-2 negative breast cancers [4, 5]. However, implementation of TAC chemotherapy in patient care has been limited by the high incidence of febrile neutropenia. Based on the clinical benefit of granulocyte-colony stimulating factor (G-CSF) in the prevention of febrile neutropenia and subsequent intravenous antibiotic treatment or hospitalization in the setting of TAC chemotherapy, prophylactic use of G-CSF is recommended by the guidelines of the American Society of Clinical Oncology, the European Organization for Research and Treatment of Cancer, and the National Comprehensive Cancer Network [6, 7].
Advances in recombinant technology introduced a pegylated form of G-CSF via the addition of polyethylene glycol (PEG) to original filgrastim, resulting in a long plasma half-life by single injection per chemotherapy cycle [8]. This convenient biologic agent has led to better compliance in patients and a decreased burden for clinicians, but its high cost and accessibility limit its routine use in practice [9].
DA-3031 (tripegfilgrastim; Dong-A ST, Seoul, Korea) is composed of three isomers, the Lys35, MetN-terminal, and Lys17-mono-PEGylated filgrastim, which are manufactured by conjugation of a 23 kDa polyethylene glycol (amine PEGylation) to one of three conjugate sites (N-terminal, Lys17, or Lys35) of filgrastim. A phase II trial showed that a fixed dose of 6 mg DA-3031 has comparable efficacy to that of daily injections of filgrastim in ameliorating grade 4 neutropenia in patients receiving TAC chemotherapy [10].
The primary objective of this study was to demonstrate the non-inferiority of DA-3031 compared with daily filgrastim in patients during the first cycle of chemotherapy for breast cancer in terms of the duration of severe neutropenia (DSN).
Patients and methods
Patients
The study protocol was approved by the institutional review board of each participating center and the Ministry of Food and Drug Safety. All patients gave written informed consent before any study-related procedure was performed. Patients were eligible if they were at least 18 years of age; had no prior chemotherapy; were eligible for six cycles of TAC for the treatment of high-risk stage II, III, or IV breast cancer; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; had an absolute neutrophil count (ANC) ≥ 1.5 × 109/L and a platelet count ≥ 100 × 109/L; and had adequate renal, hepatic (i.e., bilirubin <1.5× the upper limit of normal and aspartate aminotransferase [AST], alanine transaminase [ALT], or both <1.5× the upper limit of normal concomitant with alkaline phosphatase <2.5× the upper limit of normal), and normal cardiac function.
Exclusion criteria included history of participation in any investigational drug trial within 30 days before informed consent, previous exposure to filgrastim or pegfilgrastim, pregnant or breast-feeding women, treatment with systemic antibiotics within 72 h of chemotherapy, prior bone marrow or stem-cell transplantation, or prior radiation therapy within 4 weeks of informed consent.
Study drug
Patients who were randomized to filgrastim received daily subcutaneous injections of filgrastim 100 μg/m2/day beginning approximately 24 h after chemotherapy and continued until ANC was documented to be 5 × 109/L after nadir, or for up to 10 days. Patients who were randomized to the DA-3031 group received a single subcutaneous injection of DA-3031 at fixed doses of 6 mg on day 2 of each chemotherapy cycle approximately 24 h after completion of chemotherapy.
Study design
This was a randomized, multi-center, open-label, phase III study that compared the efficacy and safety of once-per-cycle DA-3031 with that daily filgrastim. Eligible patients were randomized in a 1:1 ratio to receive either daily subcutaneous filgrastim 100 μg/m2 or a single fixed dose of DA-3031 6 mg. Patients were assigned to treatment groups using a block randomization method according to the participating institution.
Chemotherapy
Patients received chemotherapy on day 1 of each cycle, which consisted of doxorubicin 50 mg/m2, cyclophosphamide 500 mg/m2, and docetaxel 75 mg/m2, which were infused in that order. Chemotherapy was repeated every 3 weeks for up to six cycles.
Endpoints
The primary endpoint was the duration of grade 4 neutropenia (defined as ANC < 0.5 × 109/L) in chemotherapy cycle 1 of the full analysis set. Secondary endpoints were the depth of ANC nadir, time to ANC recovery to ≥2 × 109/L in the first cycle of chemotherapy, rate of febrile neutropenia, and number of patients requiring intravenous antibiotics during six cycles of chemotherapy.
Safety was assessed by the incidence of adverse events (AEs) using preferred terms designated by the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0) and graded according to the National Cancer Institute’s Common Toxicity Criteria (version 4.0).
Statistical analysis
The planned sample size for this study was approximately 74 patients. With 37 patients in each group, the sample size was calculated to have 90 % power to establish the non-inferiority of DA-3031 compared with filgrastim (Leucostim) at the 2.5 % significance level, assuming that the standard deviation for the duration of grade 4 neutropenia was 2.5 days and the non-inferiority margin was 2 days. The sample size also assumed that the true mean difference between treatment groups equaled zero and the dropout rate was 10 %.
The efficacy analysis included all randomized patients who took at least one dose of the study drug and had at least one post-baseline measurement. The safety analysis included all randomized patients who received at least one dose of the study drug and any safety data were collected.
For the primary endpoint, the treatment group difference in duration of grade 4 neutropenia was analyzed using a 95 % two-sided confidence interval (CI). DA-3031 was considered non-inferior to filgrastim (Leucostim®) if, in cycle 1, the upper limit of the 95 % two-sided confidence interval for the difference in mean duration of grade 4 neutropenia was less than the pre-specified non-inferiority margin of 2 days. The secondary endpoints were summarized as the mean, standard deviation (SD), minimum, median, and maximum for continuous data and as counts and percentages for categorical data. For continuous variables, the inter-group comparisons were performed using the two-sample t test or Wilcoxon rank sum test, and for categorical variables, the inter-group comparisons were performed using the chi-square test or Fisher’s exact test.
All significance tests were two tailed with a nominal significance level of 0.05, and the statistical analysis was performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
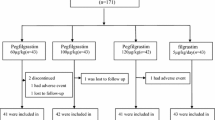
Between February 2012 and February 2013, a total of 83 Korean patients with breast cancer were screened at ten participating sites in Korea. Seventy-four patients were randomized to receive either filgrastim (n = 38) or DA-3031 (n = 36). Seventy-three patients finished the first cycle treatment; one patient in the DA-3031 group withdrew from the study. Figure 1 shows the progress of patients through the phases of the study. As shown in Table 1, the baseline characteristics of patients in each group were well balanced.
Efficacy
The primary endpoint, the mean duration of G4 neutropenia in cycle 1, was 2.08 ± 0.85 days for the filgrastim group and 2.28 ± 1.14 days for the DA-3031 group in the full analysis set (Table 2). The difference between groups was 0.2 ± 1.00 days (95 % CI = −0.26, 0.66) which supported non-inferiority (pre-specified non-inferiority margin of 2 days), and similar results were seen in the per-protocol set.
Similarly, differences in secondary endpoints were not significant (Table 3). First, nadir ANC during the first cycle of treatment was 154.34/mm3 in the filgrastim group and 161.75/mm3 in the DA-3031 group (P = 0.8414). Second, time to ANC recovery in cycle 1 was 10.03 ± 0.75 and 9.83 ± 1.56 days in the filgrastim and DA-3031 groups, respectively. Third, three patients (7.9 %) in the filgrastim group developed febrile neutropenia during all chemotherapy cycles compared with six patients (17.1 %) in the DA-3031 group (P = 0.2963). During all chemotherapy cycles, 14 patients in the filgrastim group (36.8 %) and 13 patients in the DA-3031 group (37.1 %) required hospitalization for intravenous antibiotic treatment (P = 0.9788).
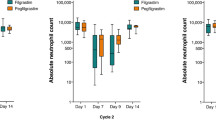
The duration of severe neutropenia during all treatment cycles was comparable between the two treatment groups (Fig. 2a). Mean time to ANC recovery showed a similar pattern in the two treatment groups, except in the second and third cycle treatments (Fig. 2b). The dose intensities of chemotherapy were also similar between patients who received filgrastim and those who received DA-3031 for all cycles (Fig. 2c).
Any antibodies to filgrastim or DA-3031 were not newly detected during treatment in this study. One patient in the filgrastim group was positive for anti-G-CSF antibody at baseline, but negative conversion was reported on six consecutive tests with treatment.
Safety
All patients experienced AEs and the safety profile of DA-3031 was similar to that of filgrastim in this study (Table 4). Severe (G3 or higher) AEs were reported in 94.7 % of filgrastim patients and 97.2 % of DA-3031 patients. However, most AEs were attributed to cytotoxic chemotherapy; 6 (15.8 %) patients in the filgrastim group and 2 (5.6 %) patients in the DA-3031 group were reported as study drug-related AEs. Most of the study drug-related AEs were related to musculoskeletal pain in the back, muscles, and extremities. None of the AEs related to musculoskeletal system led to the discontinuation of study participation and were managed by analgesics. Serious AEs occurred in six (15.8 %) patients receiving filgrastim and in ten (27.8 %) patients receiving DA-3031, while none was determined to be related to the study drug. One patient treated with DA-3031 died from disease progression during the study.
Discussion
The data of this study demonstrated the non-inferiority of a single fixed dose of DA-3031, a tripegfilgrastim, versus daily injection of filgrastim in patients who receive TAC chemotherapy for breast cancer. The mean duration of G4 neutropenia in cycle 1 was 2.28 days for the DA-3031 group and 2.08 days for the filgrastim group, supporting non-inferiority in the full analysis set. This data were consistent with the results from the previous phase II study that determined dosing using the same study drug [10]. In addition, the analysis for all secondary endpoints was comparable between the DA-3031- and filgrastim-treated groups, and no additional safety issue was identified.
Although the DSN in cycle 1 was the primary endpoint to evaluate the efficacy of the study drug, the incidences of febrile neutropenia and hospitalization are more clinically meaningful endpoints. In this study, a fixed dose of 6 mg tripegfilgrastim, DA-3031, showed comparable clinical parameters of efficacy to reference daily filgrastim; the incidences of febrile neutropenia and intravenous antibiotic use in all cycles were similar (Table 3). Reportedly, the incidence of febrile neutropenia induced by TAC chemotherapy exceeds 24 % without prophylactic G-CSF in patients with early breast cancer [11, 12]. However, with the use of prophylactic G-CSF, the incidence of febrile neutropenia over six cycles of TAC treatment was significantly decreased to 7–16 % [13]. Moreover, prophylactic use of G-CSF significantly reduced the incidence of non-hematologic toxicities including asthenia, anorexia, myalgia, nail disorders, and stomatitis by an unknown mechanism [12]. Consistent with previous studies, our study showed an incidence of febrile neutropenia of 7.9 % in the filgrastim group and 17.1 % in the DA-3031 group, but the numeric difference was not statistically significant. Furthermore, mean dose intensities of chemotherapy were comparable between treatment groups (Fig. 2c); dose reduction of chemotherapy was required in five patients (13.2 %) and four patients (11.1 %) in filgrastim and DA-3031 groups, respectively.
The mean DSN in cycle 1 in our study (2.28 and 2.08 days for the DA-3031 group and filgrastim group, respectively) was longer than those of other studies were. A Korean study using pegteograstim or reference pegfilgrastim also showed a mean DSN of 1.84–1.96 days in patients receiving TAC chemotherapy [14]. In a recent German study, however, the mean DSN in cycle 1 ranged from 1.17 to 1.20 days after daily injection of a biosimilar or reference filgrastim in patients receiving TAC chemotherapy for early breast cancers [15]. Potential reasons for the difference in the mean DSN among studies might include an ethnic difference between Asian and non-Asian patients, as shown in an integrated analysis [16]. Given the divergent myelotoxicity in Asian patients, more individualized supportive care including pegfilgrastim might be of more benefit to the patients receiving docetaxel-based chemotherapy.
The use of biosimilar pegfilgrastim is now widely accepted based on studies that showed similar efficacies and safety profiles in clinical use. As the number of patients who participated in this pivotal study is relatively small, further safety and efficacy data should be collected after extrapolation to other diseases. In addition, we used daily subcutaneous injections of filgrastim as the reference drug instead of the pegylated form, because no pegylated G-CSF was available at the time of study initiation in Korea. Our data also has limitation as we used the approved dosage of filgrastim in Korea, 100 μg/m2, which is lower than the FDA-recommended dose (5 μg/kg/day). DA-3031 is now being used in clinical practice after approval by the Korea health authority. Further clinical studies and vigilant post-marketing surveillance of efficacy and safety in the real world were in progress.
This study demonstrates that DA-3031 and daily filgrastim are similar in regard to the duration of severe neutropenia and safety in breast cancer patients receiving TAC chemotherapy. The new pegfilgrastim, DA-3031, could contribute towards better accessibility to treatment in the future.
References
Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, Ferree CR, Muss HB, Green MR, Norton L, Frei E 3rd (1998) Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 90(16):1205–1211
Wood WC, Budman DR, Korzun AH, Cooper MR, Younger J, Hart RD, Moore A, Ellerton JA, Norton L, Ferree CR et al (1994) Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med 330(18):1253–1259. doi:10.1056/NEJM199405053301801
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106(10):2258–2266. doi:10.1002/cncr.21847
Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of intergroup trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol 21(8):1431–1439. doi:10.1200/JCO.2003.09.081
Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, Saleh M, Sehdev S, Provencher L, Semiglazov V, Press M, Sauter G, Lindsay MA, Riva A, Buyse M, Drevot P, Taupin H, Mackey JR (2011) Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol 29(29):3877–3884. doi:10.1200/JCO.2010.28.5437
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO, American Society of Clinical Oncology (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3212. doi:10.1200/JCO.2015.62.3488
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for Research and Treatment of Cancer (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32. doi:10.1016/j.ejca.2010.10.013
Zamboni WC (2003) Pharmacokinetics of pegfilgrastim. Pharmacotherapy 23(8 Pt 2):9S–14S
Aapro M, Crawford J, Kamioner D (2010) Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer 18(5):529–541. doi:10.1007/s00520-010-0816-y
Park KH, Sohn JH, Lee S, Park JH, Kang SY, Kim HY, Park IH, Park YH, Im YH, Lee HJ, Hong DS, Park S, Shin SH, Kwon HC, Seo JH (2013) A randomized, multi-center, open-label, phase II study of once-per-cycle DA-3031, a biosimilar pegylated G-CSF, compared with daily filgrastim in patients receiving TAC chemotherapy for early-stage breast cancer. Investig New Drugs 31(5):1300–1306. doi:10.1007/s10637-013-9973-4
Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, Guevin R, Howell A, Fornander T, Hainsworth J, Coleman R, Vinholes J, Modiano M, Pinter T, Tang SC, Colwell B, Prady C, Provencher L, Walde D, Rodriguez-Lescure A, Hugh J, Loret C, Rupin M, Blitz S, Jacobs P, Murawsky M, Riva A, Vogel C, Breast Cancer International Research Group 001 Investigators (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352(22):2302–2313. doi:10.1056/NEJMoa043681
Martín M, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, Rodríguez-Lescure A, Grosse R, Calvo L, Fernandez-Chacón C, Roset M, Antón A, Isla D, del Prado PM, Iglesias L, Zaluski J, Arcusa A, López-Vega JM, Muñoz M, Mel JR (2006) Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol 17(8):1205–1212. doi:10.1093/annonc/mdl135
Aapro M, Schwenkglenks M, Lyman GH, Lopez Pousa A, Lawrinson S, Skacel T, Bacon P, von Minckwitz G (2010) Pegfilgrastim primary prophylaxis vs. current practice neutropenia management in elderly breast cancer patients receiving chemotherapy. Crit Rev Oncol Hematol 74(3):203–210. doi:10.1016/j.critrevonc.2009.06.004
Lee KH, Kim JY, Lee MH, Han HS, Lim JH, Park KU, Park IH, Cho EK, Yoon SY, Kim JH, Choi IS, Park JH, Choi YJ, Kim HJ, Jung KH, Kim SY, Oh DY, Im SA (2016) A randomized, multicenter, phase II/III study to determine the optimal dose and to evaluate the efficacy and safety of pegteograstim (GCPGC) on chemotherapy-induced neutropenia compared to pegfilgrastim in breast cancer patients: KCSG PC10-09. Support Care Cancer 24(4):1709–1717. doi:10.1007/s00520-015-2963-7
Blackwell K, Semiglazov V, Krasnozhon D, Davidenko I, Nelyubina L, Nakov R, Stiegler G, Singh P, Schwebig A, Kramer S, Harbeck N (2015) Comparison of EP2006, a filgrastim biosimilar, to the reference: a phase III, randomized, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol 26(9):1948–1953. doi:10.1093/annonc/mdv281
Yano R, Konno A, Watanabe K, Tsukamoto H, Kayano Y, Ohnaka H, Goto N, Nakamura T, Masada M (2013) Pharmacoethnicity of docetaxel-induced severe neutropenia: integrated analysis of published phase II and III trials. Int J Clin Oncol 18(1):96–104. doi:10.1007/s10147-011-0349-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the institutional review board of each participating center and the Ministry of Food and Drug Safety. All patients gave written informed consent before any study-related procedure was performed.
Rights and permissions
About this article
Cite this article
Park, K.H., Lee, S., Park, J.H. et al. A randomized, multi-center, open-label, phase III study of once-per-cycle DA-3031, a pegylated G-CSF, in comparison with daily filgrastim in patients receiving TAC chemotherapy for breast cancer. Support Care Cancer 25, 505–511 (2017). https://doi.org/10.1007/s00520-016-3429-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3429-2