Abstract
To estimate the 15-year survival following a diagnosis of stage I breast cancer among women who carry a BRCA1 mutation and to determine predictors of mortality, including the use of chemotherapy. Patients were 379 women with stage I breast cancer for whom a BRCA1 mutation had been identified, in herself or in a close family member. Patients were followed for up to 15 years from the initial diagnosis of breast cancer. Survival rates were estimated for women by age, tumor size (≤1 cm; >1 cm), ER status (±), and by chemotherapy (yes/no). 42 women died of breast cancer in the follow-up period (11.2 %). Survival rates were similar for women with cancers of size 0–1.0 cm and size 1.1–2.0 cm. Of the 267 women in the study who used chemotherapy, 21 had died (7.9 %) compared to 21 deaths among 112 women who did not receive chemotherapy (18.8 %; p = 0.002). The 15-year survival was 89.4 % for women who received chemotherapy and was 73.1 % for women who did not receive chemotherapy (p = 0.08; log rank). The adjusted hazard ratio for death following a diagnosis of stage I breast cancer associated with chemotherapy was 0.53 (95 % CI 0.28–1.07; p value 0.06) after adjusting for age of diagnosis, tumor size, and estrogen receptor status. This was statistically significant only among women with ER-negative breast cancers (HR = 0.28; 95 % CI 0.10–0.79; p = 0.02). BRCA1 positive women who are treated for stage I breast cancer with chemotherapy have better survival than those who do not receive chemotherapy. The difference cannot be explained by other prognostic factors. All women with invasive breast cancer and a BRCA1 mutation should be considered to be candidates for chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The choice of whether or not to give chemotherapy to a woman with breast cancer is based primarily on the tumor size, grade, hormone receptor status, and nodal status [1]. Among host factors, young age is an adverse prognostic factor and may also be considered in the decision whether or not to give chemotherapy [2]. A second host factor, BRCA1 mutation status, may also be relevant [3]. Foulkes et al. [4] found little relationship between tumor size and nodal status in BRCA1 carriers and others have suggested that the clinical outcome after a diagnosis of invasive breast cancer in a woman with a BRCA1 mutation is not dependent on tumor size [5, 6]. Several authors suggest that the benefit from chemotherapy in women with a BRCA1 mutation is present for women with breast cancers of any size [6, 7]. It is generally accepted that chemotherapy is indicated for young women with large or node-positive breast cancers, in particular if they are of high grade and/or estrogen receptor negative, but chemotherapy is not recommended for all women with stage I cancers. However, no study has systematically addressed the question of whether or not chemotherapy is indicated for women with stage I breast cancers and a BRCA1 mutation. We merged four datasets of patients with a BRCA1 mutation and a small (<2 cm) node-negative breast cancer and we estimated the 15-year survival rate in this cohort of patients. We evaluated the influences of tumor size, ER status, patient age, and the use of chemotherapy on mortality, singly and in combination.
Methods
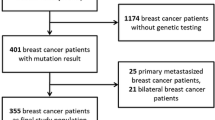
Subjects were eligible for the present study if they were diagnosed with stage I invasive breast cancer (2.0 cm or less and node-negative) and carried a deleterious mutation in BRCA1. For a small number of patients (n = 17; North American cohort only), genetic testing was done on a close relative but not on the patient herself and she was assumed to be positive. Each patient had had a nodal dissection or sentinel node biopsy that was reported as negative (patients without node dissection or sentinel node biopsy were excluded). ER status was categorized as positive, negative, or missing. ER status was determined locally, based on medical records.
To maximize the number of study subjects, four different databases were merged:
-
(1)
North American Study The parent study was of women with stage I or stage II breast cancer, diagnosed at age 65 or below, between 1975 and 2000 from five clinical centers in Canada or the United States. A total of 615 families with a BRCA1 or BRCA2 mutation, which, in aggregate, contained 1,820 breast cancer cases were reviewed. Living and deceased women were eligible, but those with a prior diagnosis of cancer (including breast cancer) or those who resided outside of North America were excluded. It was not necessary to be a proven carrier of the mutation found in the family to be included in the study; however, affected women who were known to be non-carriers were excluded. 1,259 women were eligible for the parent study and we were able to obtain information for 852. Of these, 209 of the women had stage I breast cancer and were eligible for the current study. Of the 209 women, 192 were proven to be a gene carrier and 17 women had not been tested. Each of these untested women had a >95 % chance of being a mutation carriers based on BRCAPRO analysis. The study population is described in more detail in reference [8].
-
(2)
Poland, Pomeranian Medical University Patients were eligible for the parent study if they had a new diagnosis of stage I–IV invasive breast cancer, at or below age 50, that was pathologically confirmed by core biopsy or fine-needle aspiration biopsy [9]. The dates of diagnosis were between 1996 and 2006. Patients with a previous diagnosis of cancer in the contralateral breast or of another cancer were ineligible. 4,734 patients were identified and contributed from 17 hospitals situated throughout Poland. The 4,734 cases represent approximately 15 % of all patients in this age group diagnosed throughout Poland during this period. Genetic testing was conducted on all patients for three Polish BRCA1 founder mutations and 409 women were found to carry a mutation. Of these, 109 were diagnosed with a cancer that was node-negative and <2 cm and were included in the current study. Patients for whom more than 3 years had elapsed from initial diagnosis were ineligible. Clinical variables were obtained by review of the medical records. Follow-up (mortality) was obtained by linkage to the vital statistics registry of Poland. The average time from diagnosis to genetic testing was 0.7 years.
-
(3)
Beth Israel Deaconess A total of 66 women with breast cancer and a known deleterious BRCA1 mutation were identified through the Beth Israel Deaconess Medical Center’s Cancer Risk and Prevention Program. Dates of genetic testing for this cohort ranged from 1997–2010 and testing was performed by Myriad Genetics. Pathological and clinical variables were obtained by review of medical records, with IRB approval. Of the 66 patients, 26 qualified for the current analysis. The average time from diagnosis to genetic testing was 0.6 years.
-
(4)
Memorial Sloan Kettering A total of 361 women with breast cancer and a known deleterious BRCA1 mutation were identified through the databases of the Clinical Genetics Database at Memorial Sloan Kettering Cancer Center. Of these, 37 qualified for the current study. Dates of genetic testing for this cohort ranged from 1995 and 2010. The average time from diagnosis to genetic testing was 0.2 years. Pathological and clinical variables were obtained by review of medical records, with IRB approval.
Chemotherapy was recorded, based on a review of medical records. In total, 267 of 379 patients (70.4 %) received chemotherapy. Chemotherapy was recorded as yes/no. Neither the type nor the duration of chemotherapy were recorded. A small number of patients (largely from Poland) received neo-adjuvant chemotherapy.
Statistical analysis
A number of survival analyses were performed. We considered each woman to be at risk for death from the date of the first surgical procedure until the last date of follow-up or until death from breast cancer, death from another cause, date of last follow-up, or 15 years from diagnosis. Survival curves were constructed using the Kaplan–Meier method and compared for subgroups of women defined by age (≤40 or >40), size (≤1 cm vs >1 cm), ER status, and by chemotherapy (yes/no). ER was coded as positive, negative and missing, with negative being the reference category. The log rank test was used to evaluate statistical significance of the univariate comparisons. Adjusted hazard ratios were estimated using the Cox proportional hazards model, implemented in SAS. p values below 0.05 were considered to be statistically significant.
Results
The characteristics of the 379 breast cancer patients are presented in Table 1. The mean age of diagnosis was 43 years. The patients were followed from diagnosis for a mean of 9.2 years (range 0.2–15 years). 42 subjects (11.1 %) died during the follow-up period. Of those that died, the mean time to death was 7.0 years from diagnosis (range 1.3–14.7 years).
The 15-year survival rate was similar for women with cancers of 0.1–1.0 cm (75.0 %) and for women with cancers 1.1–2.0 cm (81.7 %; p = 0.6) (Fig. 1). The adjusted hazard rate for larger, versus smaller cancers was 1.39 (95 % CI 0.67–2.90; p = 0.38). Survival rates did not differ by age of diagnosis (Supplementary Fig. 1) or by ER status (Supplementary Fig. 2). In the multivariable analysis (Table 2) none of these three variables was predictive of mortality.
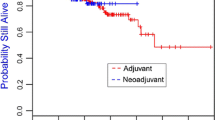
In contrast, the use of chemotherapy was a significant predictor of survival in this analysis. Of the 267 women in the study who used chemotherapy, 21 had died (7.9 %) compared to 21 deaths among 112 women who did not receive chemotherapy (18.8 %; p = 0.002). The 15-year survival was 89.4 % for women who received chemotherapy and was 73.1 % for women who did not receive chemotherapy (p = 0.08; log rank) (Fig. 2). The hazard ratio for death following a diagnosis of stage I breast cancer associated with the use of chemotherapy was 0.53 (95 % CI 0.28–1.07; p value 0.06) after adjusting for age of diagnosis, size, and estrogen receptor status. The difference in mortality could not be explained by a different risk profile in the two groups of patients (Table 1); the women who received chemotherapy were on average younger at diagnosis (42.2 vs 43.3 years), had larger tumors (1.4 vs 1.2 cm), and had tumors that were more likely to be ER-negative (62 vs 38 %). However, they did have a shorter period of follow-up (8.7 vs 10.8 years).
Of the women with ER-negative cancers, 82 % received chemotherapy, compared to 63 % of women with ER-positive cancers. Among ER-negative women, the 15-year survival rates were 93.1 and 51.4 % for women with and without chemotherapy, respectively (HR = 0.39; p = 0.06). Among ER-positive women, the 15-year survival rates were 87.7 and 77.5 % for women with and without chemotherapy, respectively (HR = 0.60; p = 0.39). In this cohort, of the 112 patients who did not get chemotherapy, 21 had died (Table 1). Of these, two patients had cancers that were ER-positive and less than one or equal to 1 cm and 19 patients had cancers that were ER-negative or greater than 1 cm. Characteristics of the deceased patients are presented in Table 3.
Discussion
In this study of stage I breast cancers, chemotherapy was associated with a large reduction in breast cancer mortality in BRCA1 carriers (HR = 0.53; 95 % CI 0.28–1.07) that was of marginal statistical significance (p = 0.06). The reduction in mortality reached statistical significance in the subgroup of women with ER-negative breast cancers (HR = 0.28; 95 % CI 0.10–0.79; p = 0.02) but not in women with ER-positive cancers (HR = 0.60; 95 % CI 0.23–1.60). In the general breast cancer population, the use of chemotherapy is not associated with an equally large survival advantage for women with stage I tumors. For example, we reviewed the survival experience of stage I breast cancer patients in the Henrietta Banting Database at Women’s College Hospital. These patients were treated at a single center in Toronto from 1986 to 1998 (discussed in detail in ref [10]). In the Banting database, the 15-year survival of patients treated with chemotherapy was 91.1 % and of patients not treated with chemotherapy was 90.7 % (Fig. 3).
Ours is a confirmatory study; it has been proposed by Robson et al. [7] in 2004, in a study of 56 carrier women and 440 controls, that the survival of women with a BRCA1 or BRCA2 mutation was inferior to that of non-carriers, but the survival difference was abrogated if chemotherapy was given. In a study of Israeli Jewish women, Rennert et al. [5] observed that tumor size was not a predictor of mortality in BRCA1 carriers and that, among women with small breast cancers, the use of chemotherapy was associated with a reduction in the 10-year mortality rates. Our study confirms these early reports but our study is much larger and includes non-Jewish women. Recently, Goodwin et al. [6] reviewed the outcomes of 92 women with breast cancer and a BRCA1 mutation in an international study. In their study, 13 of 48 (27 %) of the women with stage I breast cancer and a BRCA1 mutation experienced a distant recurrence after a mean of 7.9 years, compared to 144 of 958 women (15 %) without a mutation (p = 0.02) (85 % of the patients received chemotherapy). They did not see an association with tumor size or grade and survival and concluded that “Caution should be exercised in withholding adjuvant chemotherapy in the face of favorable tumor characteristics in BRCA1 and BRCA2 mutation carriers.” Although none of these studies on its own is definitive, there is a high degree of consistency among all studies to date and we agree with the opinion expressed by Goodwin and colleagues.
In a study of triple-negative breast cancer patients (all stages), all treated with chemotherapy, Bayraktar et al. [11] found similar 10-year survival rates for women who did and who did not have a BRCA1 or BRCA2 mutation. In this study, the median follow-up was very short (3.4 years) and there were only nine deaths recorded in the BRCA positive group. A second study of triple-negative breast cancers treated with chemotherapy had a similar result; there was no survival difference for carriers and non-carriers [12]. However, one cannot conclude based on these two studies that the survival experiences of carriers and non-carriers is similar, given that all patients in both series received chemotherapy, and in the present study, the adverse survival for mutation carriers was seen only among women who did not receive chemotherapy. In a further study of the MD Anderson cohort, Arun et al. [13] reported that BRCA1 carriers were sensitive to neo-adjuvant chemotherapy; they reported a pathologic complete response rate of 46 % in BRCA1 mutation carriers, compared to 22 % in non-carriers (p = 0.001). All of the BRCA1 carriers who achieved a pathologic complete response were alive at 5 years.
The strengths of our study include the large sample size and the long follow-up period. We included four centers with different patient ascertainment schemes. In the Polish series, all women with breast cancer diagnosed with breast cancer under age 50 in one of the participating hospitals were offered genetic testing within 1 year of diagnosis and study subjects were selected from those with a positive test on the basis of size and nodal status. In the BIDMC and MSKCC series, for some patients the genetic test result was given after the diagnosis of breast cancer, raising the possibility of survivorship bias. For this reason, women who were tested three or more years after breast cancer were excluded. In these three centers, only women who were proven mutation carriers were included. In the North American series, women were ascertained systematically on the basis of pedigree review among families with a BRCA1 mutation and all women who had breast cancer and a >95 % probability of having a mutation, based on BRCAPRO, were included.
We did not include women with BRCA2 mutations, because the number of patients with a BRCA2 mutation available for study was small. The choice of treatment was not randomised and there were baseline imbalances in the treatment groups for several prognostic indicators. We did not have details on the type of chemotherapy, or the number of cycles given nor on other treatments, such as endocrine treatment or another type of surgery. It is possible that the response to chemotherapy differs according to regimen and we are unable to address that here. However, based on the distribution of other prognostic variables, and on standard patterns of practice, the women who received chemotherapy would be likely to have, on average, a worse prognosis than women who did not receive chemotherapy (Table 1). Similarly, we did not have information on tumor grade, but the majority of BRCA1-associated breast cancers are high grade and it is likely that a woman with a high grade tumor would be more likely to receive chemotherapy than a woman with a low grade tumor. Nevertheless, in spite of this relatively unfavorable risk profile, they did better in terms of survival. This contrasts with the survival experience of women in the non-carrier setting. For example, in the Henrietta Banting database, the crude 15-year survival rates were almost identical among women who did and who did not receive chemotherapy—this is commonly the case and reflects a balancing of survival benefit of chemotherapy with the adverse prognostic features of cancers in women typically given chemotherapy.
Further studies are needed, in particular with regard to the choice of chemotherapy. We did not record the specific type of chemotherapy (nor the number of cycles received) in the present study. In a recent study from Poland, Byrski et al. [9] reported a much higher response rate to neo-adjuvant cisplatinum than to conventional types of chemotherapy in BRCA1 carriers, but the effect of cisplatinum (or other chemotherapy) on cancer mortality has not been evaluated. Arun et al. [13] reported a high rate of pathologic complete response among women given anthracycline-based neoadjuvant chemotherapy.
Our data have important implications for MRI screening as well. We have recently shown that participation in an annual MRI screening program is associated with a down-staging of breast cancers in BRCA1 carriers [14] and some might argue that chemotherapy could be avoided for some women with screen-detected stage I breast cancer. However, the results of the current study suggest that it is not prudent to withhold chemotherapy from these women until follow-up studies of MRI cohorts, with mortality as an endpoint, are completed.
The evidence in favor of treating all women with breast cancer and a BRCA1 mutation can be summarized as follows: Among women with stage I breast cancer and a BRCA1 mutation who do not receive chemotherapy, the 15-year survival is 73.1 % and is inferior to that of non-carriers with stage I breast cancer who do not receive chemotherapy. Among women with stage I breast cancer and a BRCA1 mutation who receive chemotherapy, the 15-year survival is 89.4 % and is similar to that of non-carriers with stage I breast cancer who receive chemotherapy. Among women with stage I breast cancer and a BRCA1 mutation, neither tumor size, ER status nor age of diagnosis was predictive of mortality and none of these conventional risk factors can be used to define a subgroup at low risk of recurrence. However, there were relatively few ER-positive breast cancer patients in this study and the data are not strong enough to make a clear recommendation for this subgroup. Nevertheless, the 15-mortality from breast cancer in women with ER+ breast cancers who were not treated with chemotherapy was 19 %—in general, this risk is sufficiently high enough to justify chemotherapy, but the mortality estimate is based on a small number of events.
The current NCCN guidelines state that chemotherapy should be considered for triple-negative tumors that are 5 mm in size or greater [15]. Based on the data presented here, we concur with these guidelines for BRCA1 carriers and would extend this recommend to include ER-positive patients with 5 mm breast cancers as well. The current data are not sufficient to extend the recommendation to node-negative cancers of one to 4 mm in BRCA1 carriers. In this study, there were ten patients with node-negative cancers of 0.1–0.4 cm who did not get chemotherapy and none of these patients have died.
The data here are based on a compilation of several studies and is the largest study to date to address the issue of chemotherapy in stage I breast cancer in BRCA1 carriers. It is hoped that other prospective studies will be done on similar cohorts in order to define accurately the impact of chemotherapy on recurrence for women with a BRCA1 mutation and to help determine which chemotherapy regimen is optimal.
References
Senn HJ, Thürlimann B, Goldhirsch A, Wood WC, Gelber RD, Coates AS (2003) Comments on the St. Gallen Consensus 2003 on the primary therapy of early breast cancer. Breast 12:569–582
Bharat A, Aft RL, Gao F, Margenthaler JA (2009) Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J Surg Oncol 100:248–251
Narod SA, Foulkes WD (2004) BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 4:665–676
Foulkes WD, Metcalfe K, Hanna W, Lynch HT, Ghadirian P, Tung N et al (2003) Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer 98:1569–1577
Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS et al (2007) Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med 357:115–123
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM et al (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30:19–26
Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR et al (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res 6:R8–R17
Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, Olopade OI, Eisen A, Weber B, McLennan J, Sun P, Foulkes WD, Narod SA (2004) Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol 22:2328–2335
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M et al (2010) Pathological complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28:375–379
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK et al (2011) Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat 130:145–153
Lee LJ, Alexander B, Schnitt SJ, Comander A, Gallagher B, Garber JE et al (2011) Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 117:3093–3100
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L et al (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol 29:3739–3746
Warner E, Hill K, Causer P, Plewes D, Jong R, Yaffe M et al (2011) Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 29:1664–1669
National Cancer Comprehensive Network (2011) NCNN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, Inc. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 20 Dec 2012
Acknowledgments
We thank Fonds de la recherche en santé du Québec (FRSQ), the Canadian Breast Cancer Research Alliance and the Canadian Breast Cancer Foundation (Ontario Chapter) for their support. Also supported by the Polish Ministries of Science and Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Narod, S.A., Metcalfe, K., Lynch, H.T. et al. Should all BRCA1 mutation carriers with stage I breast cancer receive chemotherapy?. Breast Cancer Res Treat 138, 273–279 (2013). https://doi.org/10.1007/s10549-013-2429-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2429-x