Abstract
Background
The impact of various breast-cancer treatments on patients with a BRCA2 mutation has not been studied. We sought to estimate the impact of bilateral oophorectomy and other treatments on breast cancer-specific survival among patients with a germline BRCA2 mutation.
Methods
We identified 664 women with stage I–III breast cancer and a BRCA2 mutation by combining five different datasets (retrospective and prospective). Subjects were followed for 7.2 years from diagnosis to death from breast cancer. Tumour characteristics and cancer treatments were patient-reported and derived from medical records. Predictors of survival were determined using Cox proportional hazard models, adjusted for other treatments and for prognostic features.
Results
The 10-year breast-cancer survival for ER-positive patients was 78.9% and for ER-negative patients was 82.3% (adjusted HR = 1.23 (95% CI, 0.62–2.45, p = 0.55)). The 10-year breast-cancer survival for women who had a bilateral oophorectomy was 89.1% and for women who did not have an oophorectomy was 59.0% (adjusted HR = 0.45; 95% CI, 0.28–0.72, p = 0.001). The adjusted hazard ratio for chemotherapy was 0.83 (95% CI, 0.65–1.53: p = 0.56).
Conclusions
For women with breast cancer and a germline BRCA2 mutation, positive ER status does not predict superior survival. Oophorectomy is associated with a reduced risk of death from breast cancer and should be considered in the treatment plan.
Similar content being viewed by others
Background
The choice of treatment for a patient with early breast cancer takes into account tumour features, including tumour size, grade, hormone-receptor status, HER2 status, axillary nodal status and patient preferences. Evidence is emerging that knowledge of inherited mutations in predisposing genes, such as BRCA1 and BRCA2, is also important.1,2,3,4 The majority of BRCA2-associated breast cancers are ER-positive, and in the general population, ER positivity is a favourable prognostic factor, compared to ER negativity early in the course of the disease.5 However, recent studies indicate that positive ER status and low tumour grade may not predict a good outcome in BRCA2 carriers.6,7
A beneficial effect of oophorectomy on breast-cancer survival has been seen in BRCA1 mutation carriers1,2,3 with hazard ratios ranging from 0.4 to 0.6. It is important to confirm that oophorectomy is helpful in the treatment of breast cancer in patients with a BRCA2 mutation. Many of the patients are premenopausal, and oophorectomy will curtail fertility and induce surgical menopause, which increases risks for osteoporosis, cardiovascular disease and possibly cognitive dysfunction.8 In general, hormone-replacement therapy is not advised for women with ER-positive breast cancer. It is not likely that a randomised clinical trial will be conducted in BRCA2 mutation carriers; therefore, we must rely on large, well-conducted observational studies. Our primary goal was to assess the efficacy of various treatments in reducing breast-cancer mortality in the BRCA2 patient population. We combined data from five different databases and conducted a multicentre historical cohort study of women with breast cancer and a BRCA2 mutation, resident in North America, Europe and Australia, to identify the impact of various treatments (chemotherapy, oophorectomy, hormonal therapy, contralateral mastectomy and radiotherapy) on survival.
Methods
Study subjects
Patients were eligible for the study if they had a diagnosis of stage I–III invasive breast cancer that was pathologically confirmed and carried a germline BRCA2 mutation. We excluded patients with known metastatic disease at diagnosis. Age at diagnosis was restricted to 25 and 70 years and year of diagnosis was between 1990 and 2019. Patients were eligible if they had genetic testing within 5 years of the diagnosis of breast cancer (2 years in Australia) and were found to be BRCA2-positive. Patients were also eligible if they developed breast cancer after genetic testing (i.e., in the follow-up period). Patients with a previous diagnosis of cancer in the same or contralateral breast or cancer at another site were excluded. We wished to study the impact of oophorectomy after diagnosis, and therefore patients who had a bilateral oophorectomy prior to their breast-cancer diagnosis were excluded. The indication for oophorectomy was not recorded.
Patient sources
North America
Eligible study subjects included female BRCA2 mutation carriers from 67 participating centres in Canada and the United States who were enrolled in a multicentre, longitudinal cohort study. All subjects sought testing for BRCA1 and BRCA2 mutations because of a personal or family history of breast and/or ovarian cancer. Mutation detection was performed using a range of techniques, but all nucleotide sequences were confirmed by direct sequencing of DNA. The study was approved by the institutional ethics review boards of the respective host institutions and all study subjects provided written informed consent. All subjects completed a baseline research questionnaire at the time of a clinic appointment or at their home at a later date. Subjects were eligible if they were cancer-free at the time of study enrolments (the baseline questionnaire) and reported a new diagnosis of invasive breast cancer in one of the follow-up questionnaires. They were also eligible if they had been diagnosed with breast cancer in the 5-year period prior to genetic testing. The questionnaires requested detailed information on family and personal history of cancer, reproductive and medical histories as well as medication use. Follow-up questionnaires are completed every 2 years thereafter to update exposure information and to capture incident disease and deaths from all causes. Information on diagnoses and treatment of breast cancer was collected from the questionnaires and from review of medical records. Hormone-receptor status of the tumour and other pathologic features were abstracted from the pathology report and/or medical record review. Cause and date of death was obtained by the collaborating investigator at each of the participating sites. This was determined by patient record, by correspondence with the treating physician or by next of kin.
Poland
A BRCA2 mutation is present in approximately 2% of the familial breast-cancer cases in Poland.9 Since 1996, the team has collected blood samples for DNA extraction from unselected women with newly diagnosed breast cancer in various clinical centres across Poland. From 1996 to 2001, this was done primarily for young breast-cancer patients (age of diagnosis <50), and from 2002 onwards, included breast cancer patients diagnosed at all ages. In total, DNA samples from approximately 13 000 breast-cancer patients were collected between 1996 and 2014. The patients were tested for the presence of two founder mutations in BRCA2. The patients in the study were those who were found to be positive for one of the two mutations. The mean time from diagnosis to blood testing was 1.4 years. Patients had no prior diagnosis of breast cancer or another cancer. Information on clinical presentation and treatments received was retrieved from the medical records. Vital status and date of death was retrieved by linkage to the Vital Statistics Database of the Polish Ministry of Administration and Internal Affairs. Cause of death was determined by medical record review and interview with the treating physician.
Australia
Between 1997 and 2008, more than 6000 women enrolled in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab).10 They have been systematically followed up with a mailed questionnaire at 3-yearly intervals as part of the kConFab Follow-Up Study.11 Study participants are predominantly from families with multiple cases of breast and/or ovarian cancer and were recruited from one of 16 Familial Cancer Centres across Australia and New Zealand. At the time of enrolment, blood was collected for BRCA1 and BRCA2 mutation analysis. The baseline and 3-yearly follow-up questionnaires ask about demographics, cancer risk factors, cancer diagnoses, surgeries (treatment and prophylactic) and cancer treatments.12 Breast cancers are verified by obtaining pathology reports and medical records. Vital status and cause of death was obtained by relative report or from the death registry. The kConFab Follow-up Study includes 508 women with a BRCA2 germline mutation with a mean of 11.3 years of follow-up; 278 of these were affected with Stage I–III invasive breast cancer either before baseline or during follow-up. Patients were included if there was no prior history of cancer, if they were between 25 and 70 years of age and if less than 2 years had elapsed between the date of breast cancer and the date of genetic testing.
United Kingdom
The source of patients was a clinical database of BRCA1 and BRCA2 mutation carriers initiated in Manchester in 1999. This includes all carriers of pathogenic mutations in BRCA2 identified through genetic testing in the Manchester region. The regional testing laboratory opened in 1996. Data include all treatment details relating to breast and ovarian cancer and the associated pathology. Vital status is determined through NHS systems.
Italy
The source of patients was the clinical database of BRCA2 mutation carriers treated at the outpatient clinic of the San Gerardo hospital in Milan between 2007 and 2019. Data were retrieved for women who had identified a mutation prior to 2018. All patients participated in an ongoing cohort study and all indicated their willingness to be followed. Each of the 57 patients enrolled in the study was contacted via email or by phone, and details about the pathological examination of breast cancer were recorded. Details about treatment received were collected from the patients and from review of clinic notes and hospital records.
The nature of patient selection and the testing process varied by centre (see above), but all patients carried a confirmed pathogenic mutation in BRCA2. For each case, the medical record was reviewed, and information on age of diagnosis, tumour size (cm) and nodal status (positive/negative) was obtained. Information was also obtained from the questionnaires (patient- reported outcomes). Grade, oestrogen-receptor status (positive/negative), progesterone-receptor status (positive/negative) and HER2 status (positive/negative) was recorded as assigned by the pathologist associated with the hospital of treatment. Chemotherapy, tamoxifen, aromatase inhibitors and radiotherapy were recorded as yes/no. Most studies did not routinely record the specific chemotherapy formulation or the timing of administration (neoadjuvant or adjuvant) or use of ovarian-suppression therapy. Oophorectomy was recorded (yes/no) as well as the date of oophorectomy. In general, most operations are bilateral salpingo-oophorectomy (TAH-BSO). Hysterectomy is done in some centres but not all. Oophorectomies that were done for the treatment of ovarian cancer, metastatic breast cancer or other cancer were considered as no oophorectomy. We recorded the date of contralateral mastectomy; some were done as initial surgical treatment and some were done at a later date. We recorded the dates of all contralateral breast cancers.
Statistical analysis
Survival analysis was conducted using Cox proportional hazard models; subjects were followed from the date of diagnosis (or date of genetic testing, whichever came last) until age 80, the date of completion of the last questionnaire or date last known alive, ovarian cancer, pancreatic cancer, death from breast cancer or death from another cause. Women for whom the cause of death was missing were censored as death from another cause. To adjust for potential survivorship bias that might result because genetic testing was done after diagnosis, follow-up was left-truncated at the date of genetic testing. We estimated the hazard ratio for breast-cancer-specific death associated with radiotherapy (yes/no), chemotherapy (yes/no), tamoxifen/AI use and oophorectomy. Oophorectomy and contralateral mastectomy were treated as time-dependent covariates. The covariates included tumour size (0–1.9 cm, 2.0–4.9 cm and 5.0 cm+), nodal status (negative/positive), ER status (+/−), PR status (+/−/missing), HER2 (+/−/missing) and tumour grade (I/II vs. III). The analysis was repeated restricting the study set to ER-positive breast-cancer patients. The analysis was also repeated for subgroups according to age at diagnosis (<40; 40–50 ≥ 50 years).
A secondary matched analysis was done specifically to study the effect of oophorectomy on survival. In this study, each woman who had an oophorectomy was matched to a woman who had not. Pairs were generated, matched on date of birth (within 4 years), year of genetic test (within 2 years) and country. Further, to be matched to an exposed woman, the unexposed women had to have an age of the last follow-up older than age at the date of oophorectomy in the matched exposed woman. The unexposed control had an age at diagnosis younger than that of the exposed woman. Both subjects were followed for death from the date of oophorectomy in the case. The date of oophorectomy in the exposed woman was used as the date of the initial follow-up for the control. The hazard ratio generated for the matched pairs was adjusted for tumour size, nodal status and ER status using a Cox proportional hazard model.
We also assessed time from diagnosis to oophorectomy as a predictor of mortality. The average time elapsed from diagnosis to oophorectomy was 3.2 years. In total, 82 of the oophorectomies were done within the first year following diagnosis. We assessed the hazard ratio in the Cox model for three subgroups of patients based on time from breast surgery to oophorectomy (less than 1 year, 1–3 years and 3 or more years), compared to women who never had an oophorectomy. We also conducted an analysis comparing women who had an oophorectomy in the first year with all other women (no oophorectomy and later oophorectomy).
Results
Summary information for the 664 patients is presented in Table 1 and divided by centre in Supplementary Table 1. The mean age of diagnosis was 44.5 years (range 26–70 years); 68.5% of cancers were ER-positive and 41.9% were lymph-node-positive. The median time of follow-up was 6.3 years (range 0.1–27.9 years). Among those with ER status reported, the proportion that was ER-positive was 83% for women aged less than 40 years at diagnosis, 81% for women 40–50, 79% for women 50–60 and 76% for women of age 60 years and above. Among the ER-positive cancers, 49.9% were node-positive, and among the ER-negative cancers, 37.8% were node-positive (p = 0.03 for difference).
The majority of the patients with ER-positive breast cancer received endocrine therapy: tamoxifen alone (269, 66.4%) an AI alone (43, 10.6%) or both (18, 4.4%). When women with missing data were excluded, 419 (71.8%) of all patients received chemotherapy (neoadjuvant or adjuvant) and 248 (67.0%) received both endocrine therapy and chemotherapy. About 46.2% had a contralateral mastectomy and 56.8% had a bilateral oophorectomy after diagnosis. The proportion of women who had an oophorectomy was similar for women with ER-positive (58.7%) and ER-negative breast cancer (55.1%).
Overall, there were 112 deaths in the cohort; of these, 91 were from breast cancer and nine were from causes other than breast cancer (Supplementary Table 2). For 12 women, the cause of death was missing. Both tumour size and axillary nodal status were prognostic, but ER status and tumour grade were not (Supplementary Table 3 and Table 2 and Figs. 1a, b).
Tamoxifen/endocrine therapy
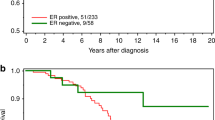
Among the 455 ER-positive patients, the 10-year survival for those who had taken any endocrine therapy was 78.8% and for those who had taken no endocrine therapy was 82.8% (adjusted HR = 1.48, 95% CI, 0.69–3.20;, p = 0.31). The adjusted hazard ratio for any endocrine therapy (tamoxifen or AI) versus no endocrine therapy was 1.48 (95% CI, 0.69–3.20, p = 0.31). The adjusted hazard ratio for tamoxifen (vs. no endocrine therapy) was 1.57 (95% CI, 0.72–3.41, p = 0.25). The adjusted hazard ratio for AI alone versus no endocrine therapy was 1.23 (95% CI, 0.30–5.04, p = 0.77). Figure 1c illustrates the crude survival of the women who did and did not receive endocrine therapy.
Chemotherapy
Among all 664 patients, the adjusted hazard ratio for chemotherapy (yes/no) was 0.83 (95% CI, 0.65–1.53, p = 0.56). Among the 455 ER-positive women, the adjusted hazard ratio for chemotherapy was 1.05 (95% CI, 0.49–2.24, p = 0.90), and among the 107 ER-negative women, the hazard ratio for chemotherapy was 0.48 (95% CI, 0.08–3.01, p = 0.43).
Contralateral mastectomy
About 307 of the patients had a contralateral mastectomy; the prevalence ranged from 16.1% in Poland to 67.1% in Australia. The mean interval from initial to contralateral surgery was 2.0 years (range 0–20 years). The adjusted hazard ratio for breast-cancer death associated with contralateral mastectomy (time-dependent) was 0.71 (95% CI, 0.44–1.15, p = 0.17).
Radiotherapy
Among the 664 women, the adjusted hazard ratio for radiotherapy (yes/no) on breast-cancer mortality was 0.81 (95% CI, 0.41–1.59, p = 0.54).
Oophorectomy
About 377 of the patients had an oophorectomy. For 11 subjects, an oophorectomy was performed for the treatment of ovarian cancer and these were considered non-oophorectomies for purposes of the analysis (patients were censored for ovarian cancer). About 82 oophorectomies were done within a year of diagnosis and 295 were done within 1 or more years after diagnosis.
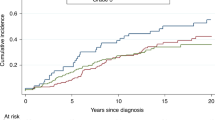
The overall 10-year survival rate was 78.6% for all 625 women in the cohort. The 10-year survival rate was 89.7% for women who had an oophorectomy and was 59.0% for those who did not have an oophorectomy (Fig. 2a). The 10-year survival rate was 87.5% for 82 women who had an oophorectomy in the first year post diagnosis.
Among all patients, the adjusted hazard ratio for oophorectomy (time-dependent) was 0.45 (95% CI, 0.28– 0.72, p = 0.0009) for breast-cancer-specific death and was 0.44 (95% CI, 0.29–0.67, p = 0.0001) for all-cause death.
Among women under age 50 at diagnosis, the adjusted hazard ratio for oophorectomy and breast-cancer-specific death was 0.51 (95% CI, 0.30–0.87, p = 0.01) and for women over 50 at diagnosis it was 0.33 (95% CI, 0.15–0.74, p = 0.01).
The adjusted hazard ratio for oophorectomy was 0.28 (95% CI, 0.06–1.41, p = 0.13) for ER-negative patients and was 0.48 (95% CI, 0.25–0.86, p = 0.01) for ER-positive patients.
The adjusted hazard ratio for oophorectomy was 0.32 (95% CI, 0.12–0.80, p = 0.02) for women with small node-negative breast cancers (<2 cm) and was 0.48 (95% CI, 0.19–1.22, p = 0.12) for women with breast cancer that had node-positive breast cancer or greater than 2 cm.
The adjusted hazard ratio for oophorectomy was 0.55 (95% CI, 0.30–1.00, p = 0.05) for those who had chemotherapy and was 0.25 (95% CI, 0.07–0.88, p = 0.0.03) for those who did not have chemotherapy.
The adjusted hazard ratio for those who had an oophorectomy in the first year after diagnosis was 0.56 (95% CI, 0.26–1.21, p = 0.14) compared to those who never had an oophorectomy.
The adjusted hazard ratio for those who had an oophorectomy in the first year after diagnosis was 0.77 (95% CI, 0.37–1.63, p = 0.49) compared to all others (those who never had an oophorectomy and those who had an oophorectomy at a later time).
We generated 149 matched pairs for the matched analysis (Supplementary Table 4). In the matched analysis, the adjusted hazard ratio associated with oophorectomy was 0.52 (95% CI, 0.27–0.99, p = 0.04) (Fig. 2b).
Discussion
The goal of our study was to assess the associations of various treatments in reducing breast-cancer mortality in the BRCA2 patient population. We observed a decrease in the proportion of cancers that were ER-positive with age of diagnosis (rather than the expected increase) and observed that ER-positive cancers were more likely to be node-positive than ER-negative cancers. These associations have also been seen in an earlier study from our group13 and in the Nordic BRCA2 mutation carrier study.14 Moreover, positive ER status was not predictive of better survival, compared to negative ER status.
Of the four treatments studied, oophorectomy appeared to be the most effective. The adjusted hazard ratio for breast-cancer-specific death associated with oophorectomy was 0.45 (95% CI, 0.28–0.72, p = 0.0009). The results were similar for the analysis of the 149 matched pairs (HR 0.52, 95% CI, 0.27–0.99). Surprisingly, the association with oophorectomy was present in women with ER-negative breast cancer, but there were only 107 ER-negative patients and the association did not reach statistical significance. It is important that possible sources of bias be considered. Oophorectomy might occur years after the diagnosis; therefore, it is important that we corrected for immortal time bias by including oophorectomy in survival models as a time-dependent covariate. We also adjusted for potential confounders, including tumour size, nodal status and contralateral mastectomy. We excluded women who had known metastatic disease at diagnosis. It is also possible that some women developed distant recurrence early in the follow-up period and that these women did not have an oophorectomy for this reason. This could bias the result towards longer survival for the oophorectomy group. However, among the women with stage I breast cancer, the hazard ratio associated with oophorectomy was 0.32 (95% CI, 0.12–0.80) and it is unlikely for these women that the choice of oophorectomy or not was based on prognosis.
Unfortunately, we did not have the dates of distant recurrence for the women in this cohort, and we cannot rule out the possibility of residual bias because of early recurrence. For this reason, we conducted a separate analysis for oophorectomy done in the first year post diagnosis. This analysis is that which closely emulates a randomised trial, where the intervention (oophorectomy) would be done in the first year of treatment. In this subgroup, the association was still significant but when we compared women with an oophorectomy in the first year with all other women (the most conservative analysis), the association was attenuated. Further, to ensure that women with and without an oophorectomy were as similar as possible, we confirmed our results in a separate matched analysis, matched on date of birth (within 4 years), year of genetic test (within 2 years) and country of residence. A protective association of oophorectomy on breast-cancer mortality was also seen in our earlier studies of BRCA1-associated breast cancer.1,2,3 It is important that these observations be confirmed in other large studies with robust methods.
There are other limitations to the study. Missing values for several prognostic factors might limit our ability to adjust for potential confounding. We had limited data on HER2 status although HER2 positivity is rare among BRCA2 carriers.15 We did not distinguish between neoadjuvant and adjuvant chemotherapy, and we did not have the dates of distant recurrences. In cases where neoadjuvant chemotherapy was given, the size and nodal status was based on clinical parameters and pre-treatment imaging. Genetic testing was done for some women after diagnosis, and therefore we did a left-censored survival analysis from the date of genetic testing.
The effect of chemotherapy in BRCA2 mutation carriers was similar to that observed in studies of non-carriers, and there is no indication from the data presented here that the decision to prescribe chemotherapy should depend on BRCA2 carrier status.
We saw little evidence for the benefit of tamoxifen or an aromatase inhibitor in ER-positive patients in the current study (adjusted hazard ratio: any vs. neither 1.48, 95% CI, 0.69–3.20, p = 0.31). This could be because those treated with endocrine therapy had more aggressive cancers than those who did not get treated, but the association was adjusted for age and conventional prognostic factors. A similar lack of the effect of tamoxifen was seen in an earlier study by Goodwin et al.16 It is not clear if tamoxifen or aromatase inhibitors are effective treatments for ER-positive breast cancer in BRCA2 mutation carriers. The mean follow-up in this cohort was 7.2 years and this may be insufficient to characterise the effect of a given treatment in ER-positive breast cancers. Nevertheless, it is surprising that oophorectomy appeared to be effective in this patient cohort, which did not show a beneficial effect of anti-hormonal therapy.
Increasingly, premenopausal women with breast cancer are offered ovarian suppression with a gonadotropin-releasing hormone agonist, particularly if they wish to maintain fertility, and regardless of their mutation status. We did not evaluate the effect of medical ovarian suppression on breast cancer survival in the BRCA2 carrier population to see if medical ovarian suppression is a viable alternative to oophorectomy. In the SOFT trial,17 ovarian function suppression given for 5 years in addition to tamoxifen improved 8-year disease-free survival from 78.9% to 83.2% (p = 0.009), but this approach has not been studied in mutation carriers.
The majority of patients in the study were stage II or III (85%) and few were aware of their mutation status at the time of diagnosis. It is hoped that as genetic testing becomes more widespread, a greater proportion of patients will be diagnosed in MRI surveillance programmes and this will result in more stage I breast cancers. It will be important to revisit predictors of survival for women with MRI-detected breast cancer and for women with stage I cancers in both BRCA2 and BRCA1 carriers.
In conclusion, we found that oophorectomy is associated with a reduced risk of death from breast cancer in BRCA2 carriers with breast cancer and should be considered in the treatment plan. Oophorectomy is also of value in preventing the second primary ovarian cancer. Further studies on the benefits of oophorectomy and of other endocrine therapies in this group of women are warranted.
Change history
13 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41416-022-02130-9
References
Byrski, T., Gronwald, J., Huzarski, T., Grzybowska, E., Budryk, M., Stawicka, M. et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 28, 375–379 (2010).
Narod, S. A., Huzarski, T., Gronwald, J., Byrski, T., Marczyk, E., Cybulski, C. et al. Predictors of survival for breast cancer patients with a BRCA1 mutation. Breast Cancer Res. Treat. 168, 513–521 (2018).
Metcalfe, K., Lynch, H. T., Foulkes, W. D., Tung, N., Kim-Sing, C., Olopade, O. I. et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 1, 306–313 (2015).
Metcalfe, K., Gershman, S., Ghadirian, P., Lynch, H. T., Snyder, C., Tung, N. et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 348, g226 (2014).
Jayasekara, H., MacInnis, R. J., Chamberlain, J. A., Dite, G. S., Leoce, N. M., Dowty, J. G. et al. Mortality after breast cancer as a function of time since diagnosis by estrogen receptor status and age at diagnosis. Int. J. Cancer 145, 3207–3217 (2019).
Jonasson, J. G., Stefansson, O. A., Johannsson, O. T., Sigurdsson, H., Agnarsson, B. A., Olafsdottir, G. H. et al. Oestrogen receptor status, treatment and breast cancer prognosis in Icelandic BRCA2 mutation carriers. Br. J. Cancer 115, 776–783 (2016).
Metcalfe, K., Lynch, H. T., Foulkes, W. D., Tung, N., Olopade, O. I., Eisen, A. et al. Oestrogen receptor status and survival in women with BRCA2-associated breast cancer. Br. J. Cancer 120, 398–403 (2019).
Shuster, L. T., Gostout, B. S., Grossardt, B. R. & Rocca, W. A. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 14, 111–116 (2008).
Cybulski, C., Kluźniak, W., Huzarski, T., Wokołorczyk, D., Kashyap, A., Rusak, B. et al. The spectrum of mutations predisposing to familial breast cancer in Poland. Int. J. Cancer 145, 3311–3320 (2019).
Mann, G. J., Thorne, H., Balleine, R. L., Butow, P. N., Clarke, C. L., Edkins, E. et al. Analysis of cancer risk and BRCA1 and BRCA2 mutation prevalence in the kConFab familial breast cancer resource. Breast Cancer Res. 8, R12 (2006).
Phillips, K. A., Butow, P. N., Stewart, A. E., Chang, J. H., Weideman, P. C., Price, M. A. et al. Predictors of participation in clinical and psychosocial follow-up of the kConFab Breast Cancer Family Cohort. Fam. Cancer 4, 105–113 (2005).
Phillips, K. A., Milne, R. L., Buys, S., Friedlander, M. L., Ward, J. H., McCredie, M. et al. Agreement between self-reported breast cancer treatment and medical records in a population-based breast cancer family registry. J. Clin. Oncol. 23, 4679–4686 (2005).
Foulkes, W. D., Metcalfe, K., Sun, P., Hanna, W. M., Lynch, H. T., Ghadirian, P. et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin. Cancer Res. 10, 029–2034 (2004).
Olafsdottir, E., Borg, A., Jensen, M. B., Gerdes, A. M., Johansson, A. L. V., Barkardottir, R. B. et al. Breast cancer survival in Nordic BRCA2 mutation carriers—unconventional association with oestrogen receptor status. Br. J. Cancer https://doi.org/10.1038/s41416-020-01056-4 (2020).
Bane, A. L., Beck, J. C., Bleiweiss, I., Buys, S. S., Catalano, E., Daly, M. B. et al. BRCA2 mutation-associated breast cancers exhibit a distinguishing phenotype based on morphology and molecular profiles from tissue microarrays. Am. J. Surg. Pathol. 31, 121–128 (2007).
Goodwin, P. J., Phillips, K. A., West, D. W., Ennis, M., Hopper, J. L., John, E. M. et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J. Clin. Oncol. 30, 19–26 (2012).
Francis, P. A., Pagani, O., Fleming, G. F., Walley, B. A., Colleoni, M., Láng, I. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379, 122–137 (2018).
Acknowledgements
Our sincere gratitude for the valuable contributions of the women who participated in this study, without whom this research would not be possible. We would like to acknowledge the following individuals for helping with data collection: Sara Lazzarin, Debora Vicini and Federica Sina.
Other members of the Polish Hereditary Breast Cancer Consortium
Błasińska-Morawiec Maria16, Chosia Maria17, Drosik Kazimierz18, Gozdecka-Grodecka Sylwia19, Goźdź Stanisław20, Grzybowska Ewa21, Jeziorski Arkadiusz22, Karczewska Aldona23, Kordek Radzisław22, Synowiec Agnieszka24, Kozak-Klonowska Beata25, Lamperska Katarzyna26, Lange Dariusz27, Mackiewicz Andrzej19, Mituś Jerzy Władysław28, Niepsuj Stanislas29, Oszurek Oleg17, Gugała Karol30, Morawiec Zbigniew16, Mierzwa Tomasz31, Posmyk Michał32, Ryś Janusz33, Szczylik Cezary34, Uciński Michał17, Urbański Krzysztof35, Waśko Bernard36, Wandzel Piotr37
Other members of the kConFab Follow-Up Study Team
Michael Friedlander38, Sue Anne McLachlan39, Stephanie Nesci40, Sandra Picken40, Sarah O’Connor40, Lucy Stanhope40
Other members of Hereditary Breast Cancer Clinical Study Group
Andrea Eisen41, Kevin Sweet42, Raymond Kim43, William Foulkes44, Pal Moller45, Susan Neuhausen46, Carey Cullinane47, Charis Eng48, Peter Ainsworth49, Fergus Couch50, Christian Singer51, Beth Karlan52, Wendy McKinnon53, Marie Wood53
Author information
Authors and Affiliations
Consortia
Contributions
Conception, design and study supervision: S.A.N. Acquisition of data: J.G., J.L., T.H., Z.H., C.F., K.M., L.S., D.Z. and M.E. Analysis of clinical data: R.F., J.L., C.C., D.Z., J.W. and N.T. Statistical analysis and critical review: P.S., R.L.M. and K.M. Interpretation of the results and writing of the original draft: D.G.E., S.A.N. and K.A.P. All authors revised the paper and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the following institutional ethics review boards: Women’s College Hospital Ethics Board, Research Ethics Committee of the Pomeranian Medical University, Central Manchester Research Ethics Committee (10/H1008/24 and 11/H1003/3), Peter MacCallum Cancer Centre Human Research Ethics Committee (97/27) and the Ethical Committee Brianza. All study subjects provided written informed consent. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Individual person’s data not included.
Data availability
All data relevant to the study are included in the paper.
Competing interests
Steven A. Narod is a board member of BJC. All other authors declare no conflict of interest.
Funding information
This work was supported by grants to kConFab and the kConFab Follow-Up Study from Cancer Australia (809195, 1100868), the Australian National Breast Cancer Foundation (IF 17), the National Health and Medical Research Council (454508, 288704 and 145684), the National Institute of Health USA (1RO1CA159868), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia and the Cancer Foundation of Western Australia. K.A.P. is an Australian National Breast Cancer Foundation Fellow (PRAC17-004). The work is supported by the Peter Gilgan Center for Research on Women’s Cancers and the Canadian Institute of Health Research (FDN 154275). D.G.E. is supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007).
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Polish Breast Cancer Consortium, kConFab Follow-Up Study Team and Hereditary Breast Cancer Clinical Study Group are listed above Acknowledgements.
The original online version of this article was revised: The original version of this article unfortunately contained a mistake in an author affiliation. The correct affiliations of author Tomasz Huzarski are: Department of Genetics and Pathology, International Hereditary Cancer Center, Pomeranian Medical University, Szczecin, Poland and University of Zielona Góra, Zielona Góra, Poland.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evans, D.G., Phillips, KA., Milne, R.L. et al. Survival from breast cancer in women with a BRCA2 mutation by treatment. Br J Cancer 124, 1524–1532 (2021). https://doi.org/10.1038/s41416-020-01164-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-01164-1
- Springer Nature Limited
This article is cited by
-
ER-positive and BRCA2-mutated breast cancer: a literature review
European Journal of Medical Research (2024)
-
Estrogen receptor-positive breast cancer and adverse outcome in BRCA2 mutation carriers and young non-carrier patients
npj Breast Cancer (2023)
-
PREDICT validity for prognosis of breast cancer patients with pathogenic BRCA1/2 variants
npj Breast Cancer (2023)
-
The impact of oophorectomy on survival from breast cancer in patients with CHEK2 mutations
British Journal of Cancer (2022)
-
Adjuvant olaparib — should all patients with breast cancer have genetic testing?
Nature Reviews Clinical Oncology (2021)