Abstract
Breast-conserving surgery (BCS) provides equivalent survival outcomes to unilateral mastectomy. There is no survival advantage to bilateral mastectomy in average risk breast cancer. Among a cohort of breast cancer patients expected to be candidates for BCS, we examined choice of surgery and factors associated with it. A prospective cohort study of unilateral clinical Stage I breast cancer patients treated at National Comprehensive Cancer Network centers from 2000 to 2009 was performed. The proportion of patients who initially underwent mastectomy versus BCS and time to definitive surgery and chemotherapy were examined. Of 10,249 patients, 23 % underwent mastectomy as an initial surgery. No decline in the use of mastectomy as initial surgery was found. There was significant institutional variation, with rates of initial mastectomy ranging from 14 to 30 % (adjusted odds ratio: 0.42–1.38). Tumor characteristics were associated with surgical option, but with small absolute differences. Of those who received initial mastectomy, 22 % had bilateral mastectomy, with an increase over time (2000:13 % vs. 2009:30 %) and substantial institutional variation (11–34 %). Women treated with initial mastectomy had longer median times from diagnosis to complete definitive surgery (6 vs. 4 weeks) and to start of adjuvant chemotherapy (12 vs. 11 weeks). Among Stage I breast cancer, the overall use of mastectomy did not change significantly over 10 years; however, an increasing proportion of women with unilateral cancer had bilateral mastectomy, and there was wide variation in type of surgery by institution. Further studies to assess reasons for the observed wide variation are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than three decades ago, several randomized controlled clinical trials showed similar survival outcomes for breast-conservative surgery (BCS) in association with adjuvant radiotherapy versus mastectomy. This led to the 1990 National Institute of Health recommendation of BCS as the preferable surgical treatment for unilateral breast cancer [1–6]. Recent population-based data suggest that there may be better outcomes with BCS and that bilateral mastectomy is unlikely to be associated with any significant survival advantage over BCS with adjuvant radiotherapy for the treatment of unilateral breast cancer [7]. Nevertheless, in the last decade, rates of mastectomy and bilateral mastectomy remained stable and may be rising [8–20].
Data for initial surgical options focused on women who meet medical criteria BCS are limited. One such group is women with Stage I disease who, by definition, have tumors under 2 cm in size and are most often amenable to BCS. A recent study from the National Cancer Data Base which included over 1,000,000 patients with Stage I–III breast cancer suggested an increase in the rate of mastectomy and particularly of bilateral mastectomy from 2003 to 2011, with steeper increases in women with node-negative disease [20]. In contrast, analyses using the Surveillance, Epidemiology, and End Results Medicare (SEER) dataset looking at definitive surgery trends among breast cancer patients found declines in mastectomy rates for Stage I disease in recent years [21, 22].
We used the National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database [23], which collects granular tumor, patient, and treatment information on women who received care at participating institutions, to examine in an academic cohort of patients with Stage I breast cancer the initial choice of surgery and patient, tumor, and health system factors potentially associated with the surgical option. In addition, we also examined the type of definitive local therapy (BCS with or without radiotherapy, vs. unilateral or bilateral mastectomy) and timeliness of breast cancer care in relation to the type of initial surgery.
Methods
Study design and data source
This was a prospective cohort study performed in the NCCN Breast Cancer Outcomes Database. Patients were included in the database if they received all or some of their treatment at a reporting center; those with one-time consultations were not included. Eight centers contributed data to this analysis: City of Hope National Medical Center; University of Texas MD Anderson Cancer Center (MADCC); Fox Chase Cancer Center; Dana-Farber Cancer Institute; Roswell Park Cancer Institute; H. Lee Moffitt Cancer Center; University of Michigan Cancer Center; and Ohio State University. All centers adhered to the data collection procedures and definitions developed by the NCCN Breast Cancer Outcomes Database. Data were subjected to rigorous quality assurance [24].
Institutional review boards (IRBs) from participating centers approved data collection, transmission, and storage protocols. At centers where the IRB required signed informed consent for data collection, only patients who provided consent were included in the database; elsewhere, the IRB granted a waiver of signed informed consent.
We identified women with a first invasive unilateral breast cancer diagnosed and presented at an NCCN cancer center between January 2000 through December 2009 (n = 24,931). We only included women with clinical Stage I breast cancer (n = 11,585). We restricted the cohort to those who did not receive neoadjuvant treatment (n = 11,391). Lastly, we excluded patients with unknown hormone receptor (HR) and human epidermal receptor 2 (HER2) status (n = 523) and those who were not treated with definitive surgery (n = 619). A cohort of 10,249 patients with newly diagnosed unilateral clinical Stage I breast cancer was included in the analysis (Fig. 1).
Outcomes of interest: initial surgical treatment
Our primary outcome of interest was initial surgical treatment defined as initial surgery for breast cancer, as BCS or mastectomy (unilateral or bilateral), based on the first surgical procedure performed on the ipsilateral breast. The initial surgery was identified after reviewing surgeries that occurred after diagnosis and prior to start of adjuvant systemic and radiation therapy. For those who did not receive any adjuvant therapy, we also excluded any surgeries that occurred more than 365 days after the patient’s diagnosis date. Type of definitive local therapy was defined as the last surgery performed within 365 days after diagnosis or end of adjuvant therapy, as BCS (with or without radiotherapy) and mastectomy (unilateral or bilateral). A woman having a breast-conserving operation, followed by mastectomy on a later date but within 365 days of diagnosis, would be classified as having BCS for initial surgical treatment and mastectomy as definitive local therapy. This might occur if the initial BCS showed unexpected extensive cancer or if the patient elected to have mastectomy.
As a secondary outcome, we examined both type of definitive local therapy and timeliness of breast cancer care focusing on time to definitive surgery and time to chemotherapy. Time to definitive surgery was defined as time from diagnosis to last surgery and time to chemotherapy was defined as time from diagnosis to initiation of chemotherapy.
Variables of interest
Variables of interest included age at diagnosis, body mass index (BMI), Charlson comorbidity score [25, 26], race, insurance at presentation, median household income, clinical stage, grade, histology, tumor subtype, pre-operative use of breast magnetic resonance imaging (MRI), year of diagnosis, and center. Variables were categorized as in Table 1.
The following variables are abstracted by chart review: age at diagnosis, height and weight, clinical stage, insurance at presentation, median household income, and clinical stage. Stage was defined according to the version of the American Joint Committee on Cancer (AJCC) TNM staging applicable at the time of diagnosis. Data on race/ethnicity and comorbidity score came from patient surveys collected at initial presentation to the NCCN center. Comorbidity score was grouped into scores of 0, 1, and ≥2 [27, 28]. Information on pathologic tumor size, nodal status, grade, HR, and human epidermal growth factor receptor 2 (HER2) status is abstracted from pathology reports. HR is considered positive if the estrogen receptor (ER) and/or progesterone receptor (PR) are positive. For HER2 classification, the fluorescence in situ hybridization result was used, if available. If only immunohistochemistry was available, 3+, “high positive,” or “positive NOS” were considered HER2+, while 2+, 1+, 0, or “negative” were considered HER2−; 0.6 % (n = 66) of the patients were “positive NOS.” Tumor grade is categorized as high (according to histologic grade, or, if not available, by nuclear grade) or low–intermediate grade; 2 % of patients (N = 205) with unknown grade were included in the low–intermediate grade category.
Statistical analysis
The clinicopathological features at time of diagnosis were summarized descriptively by initial surgical treatment. Type of definitive treatment, timeliness of breast cancer care (time to definitive surgery and time to chemotherapy), and the remaining treatment variables were also summarized descriptively at time of diagnosis by initial surgical treatment. The Cochran–Armitage test for trend was used to test for changes over time in the proportion of patients receiving initial mastectomy and the proportion of patients receiving bilateral mastectomy. The Wilcoxon rank-sum test was used to test for differences in time from diagnosis to definitive surgery and time from diagnosis to initiation of adjuvant chemotherapy, between types of initial surgical treatment.
A multivariable logistic regression model was fitted for the outcome of initial surgical treatment (mastectomy vs. BCS). All variables of interest were included in the multivariable model regardless of statistical significance as presented in Table 1. Six percent of patients (N = 570) had unknown income, and these missing values were imputed as the median income among patients with the same center, age, and race.
All p values presented are two-sided tests of statistical significance at 0.05. Statistical analyses were conducted using Stata version 11.2 (StataCorp LP, College Station, TX).
Results
Type of initial surgery received
Among 10,249 patients with unilateral clinical Stage I breast cancer, 2361 (23 %) underwent initial mastectomy. 7888 (77 %) underwent BCS as initial surgery. No statistically significant time trend was observed in this cohort regarding type of initial surgery (p trend = 0.34) (Fig. 2).
The choice of initial surgery was associated with several factors, including patient, tumor, care, and institutional characteristics (Table 1). Among patient characteristics, age, BMI, and income were significantly associated with type of initial surgery. Older patients were significantly less likely to undergo mastectomy compared with younger patients (17 % among patients ≥70 years of age versus 30 % of patients aged <50 years, adjusted odds ratio [OR] = 0.59, 95 % confidence interval (CI) 0.47–0.73); those with higher BMI (>30 kg/m2) were less likely to undergo mastectomy compared with those normal BMI (18.5 to <25 kg/m2) (20 % of patients with higher BMI vs. 28 % with normal BMI had initial mastectomy (adjusted OR 0.68, 95 % CI 0.59–0.77). Finally, those with higher incomes were less likely to have mastectomy, but absolute differences were small (22 % patients in highest quintile versus 25 % in lowest quintile; adjusted OR 0.76, 95 % CI 0.64–0.89). Family history or BRCA status was not available in the dataset.
Regarding tumor characteristics, tumor size was not consistently associated with choice of surgery. Among patients with T1mic tumors, 32 % elected mastectomy, compared to 18 % of those with T1b and 25 % of those with T1c. Exploratory analyses suggested that this may be associated with a higher proportion of multifocal disease among patients with T1mic tumors. Thirty-eight percent of patients with HER2+/HR− tumors had initial mastectomy versus 22 to 27 % among other subtypes (adjusted OR 1.47 lower odds of having mastectomy if a patient had a HER2+/HR− tumor versus having HER2+/HR+ tumors, 95 % CI 1.13–1.90). Exploratory analyses suggested that this may be associated with a higher proportion of extensive intraductal component and multifocal disease among patients with HER2+/HR− tumors (Table A1 in Supplementary Material). Small absolute differences (≤5 %) were seen in the proportion of patients undergoing mastectomy with high- versus low-grade tumors (25 vs. 22 %, adjusted OR 1.21, 95 % CI 1.08–1.36) and with ductal vs. lobular tumors (27 versus 23 %, adjusted OR 1.43, 95 % CI 1.21–1.69).
We also examined the association between the use of pre-operative breast MRI and rates of mastectomy and found that the use of pre-operative breast MRI was associated with statistically significantly higher rate of initial mastectomy (32 vs. 22 %, adjusted OR 1.80, 95 % CI 1.56–2.08).
Finally, we noted significant institutional variation in the type of initial surgery received, with rates of initial mastectomy ranging from 14 to 30 %. The OR of receiving initial mastectomy compared with center A ranged between 0.42 and 1.38; p < 0.001 (Table 1).
Type of definitive local treatment received and timing to definitive surgery and to chemotherapy
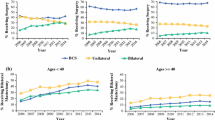
Of those who received initial mastectomy, 22 % received bilateral mastectomy, with an increase in its use over time (13 % in 2000 vs. 30 % in 2009, p trend < 0.001) and considerable inter-institutional variability (11–34 %) (Fig. 3).
1—Type of definitive treatment among patients treated initially with mastectomy. a Proportion of patients receiving unilateral or bilateral mastectomy by year of diagnosis. b Proportion of patients receiving unilateral and bilateral mastectomy by institution. 2—Type of definitive treatment among patients treated initially with breast-conservative surgery. a Proportion of patients receiving breast-conservative surgery with or without radiation, unilateral or bilateral mastectomy by year of diagnosis. b Proportion of patients receiving breast-conservative surgery with or without radiation, unilateral or bilateral mastectomy by institution. MAST mastectomy, BCS breast-conservative surgery, MRI magnetic resonance imaging
Of those initially receiving BCS, the vast majority also received radiotherapy (6747; 86 %). 523 (7 %) did not receive radiotherapy, 614 (8 %) were ultimately converted to unilateral mastectomy, and 144 (2 %) to bilateral mastectomy. No noteworthy time trend changes were found among this group, but there was significant (Chi squared p < 0.001) institutional variability (among those who initially received BCS, institutional conversion for unilateral mastectomy ranged from 3 to 10 % and the conversion to bilateral mastectomy from 0 to 3 %, Fig. 3).
Of note, pre-operative MRI was performed in 11 % of patients who underwent BCS as a definitive surgery, 17 % of those of had unilateral mastectomy and 21 % of those of underwent bilateral mastectomy.
Timeliness of breast cancer care
The time from diagnosis to definitive surgery was longer in the group initially treated with mastectomy. The median time from diagnosis to definitive surgery was 4 weeks in the group who received breast-conservative surgery versus 6 weeks in the group treated with mastectomy (p < 0.001). Among those who initially had BCS and were converted to mastectomy, the median time to definitive surgery was 8 weeks. Forty-three percent of patients were treated with chemotherapy; among them, the median time to chemotherapy (from diagnosis) was 12 weeks in the group initially treated with mastectomy and 11 weeks in the BCS group (p < 0.001) (Table 2).
Discussion
In this large study (N = 10,249) of surgical patterns across eight academic institutions in the United States, we observed that almost one quarter of women presenting with clinical Stage I breast cancer underwent mastectomy as their initial procedure. Furthermore, among women undergoing mastectomy, we identified an increase in the proportion of women electing contralateral prophylactic mastectomy over time. In this dataset, limited to clinically node-negative patients with tumors 2 cm or less, there was no consistent direction of association between tumor size and type of surgery. Absolute differences in type of surgery by histology (ductal vs. lobular) or grade (low–intermediate vs. high), while statistically significant, were small. Key factors significantly associated with type of initial breast surgery included institution and use of pre-operative breast MRI, and both of these factors also seem to impact definitive surgery.
With the endorsement of BCS as an appropriate and preferable treatment for early breast cancer and the overall perception that ‘breast surgery should be limited to the proper minimum’ [29], many assumed that the use of mastectomy among BCS candidates would continue to decline. [1–6] Nevertheless, recently some reports document an increase in mastectomy rates and an associated rise in bilateral mastectomy rates [13, 15, 19, 30, 31]. In fact, the few prior studies that specifically evaluated surgical trends among patients with unilateral Stage I breast cancer showed conflicting results, with some showing continuing decline in mastectomy rates and others showing increased rates of mastectomy among this population [20–22]. Our study, which used a large dataset of the US academic centers to examine surgical options among patients with clinically small breast cancers and looked at both initial and definitive surgical options, did not show a decline in mastectomy rates as initial choice of surgery, with almost one quarter of women presenting with clinical Stage I breast cancer undergoing mastectomy as their initial procedure. Thirty percent of these patients were found to have either multifocal disease or an extensive intraductal component on final pathology which suggests that in some cases this may have been a clinically appropriate procedure. Nevertheless, our analyses suggested that among patients treated with mastectomy there was an increase in the use of bilateral mastectomy over time, and, by 2009, 30 % of those patients treated with mastectomy had bilateral mastectomy.
Recently, a study using the SEER dataset examined rates of mastectomy among patients with Stage I disease. This identified an association between patient characteristics such as race, marital status, and geographic region with choice of treatment but, interestingly, suggested a paradox of tumor size and surgical choice in those patients with microinvasion who had a higher probability of having a mastectomy [22]. Our study confirms these results. We found significant associations with the treating institution and some patient characteristics. Although some of the expected tumor-related factors (such as histology, grade, subtype) also seemed to influence type of surgery, the absolute differences between groups were small. We did not find a consistent direction of association between tumor size and type of surgery. As in the SEER analyses, we found a high rate of mastectomy in T1mic tumors, which in our dataset may be partially explained by a higher proportion of multifocal disease among those with T1mic tumors.
Nevertheless, in aggregate, these findings suggest that medical necessity and pathological factors are probably not the only driving forces for the decision making in a population of patients with small tumors. Arguing in favor of this hypothesis is also the strength of the observed institution effect. After adjusting for patient and tumor characteristics, the OR of undergoing initial mastectomy compared with center A ranged between 0.42 and 1.38. In addition, there appeared to be a strong institutional effect on the use of bilateral mastectomy with rates ranging from 11 to 34 % of all mastectomies across the 8 institutions. Prior studies suggested geographical variation on treatment choices [14, 16, 17, 22, 32]. Our study identified substantial institutional variation; however, it cannot distinguish between variation associated with provider or care differences versus regional variations in patients’ culture and preferences. In fact, a recent study which looked at breast surgery patient decision making suggested that patients’ preferences may be important decision drivers, with, for example, a reasonable number of patients wanting to proceed with bilateral mastectomy for “peace of mind,” even acknowledging that it does not have any survival advantage associated and can bring along higher short- and long-term complications [33].
Finally, this study also calls into question the impact of surgical choice on the time to other treatment milestones. Patients treated with mastectomy had a median of a 2-week delay in time to definitive surgery when compared with patients treated with BCS, with 25 % of mastectomy patients waiting at least 8 weeks to definitive surgery. In addition, as shown in other studies, surgical procedures also impact time to chemotherapy. [34] In our study, patients treated with initial mastectomy also experienced a median 1-week delay from diagnosis to chemotherapy initiation when compared with patients treated with BCS.
Our study is a comprehensive description of surgical options used in Stage I breast cancer among the US academic institutions. We acknowledge several limitations. First, this study was limited to patients who presented to academic centers, and therefore we are not capturing the trends in the US community practices, where most of the care is provided. Nevertheless, many of the trends observed have similarly been observed in other datasets [13, 15, 19, 30, 31]. Second, the NCCN database does not capture reasons for surgical treatment such as family history or BRCA germ-line status information and therefore we could not determine which patients were appropriate candidates for BCS. Third, this study had the inability to directly question patients and surgeons regarding factors influencing their decision making. Fourth, we observed considerable institutional variability in practice, but could not account for provider-level variation and we cannot distinguish between variation driven by institutional factors versus geographic factors relating to potential differences in patient preferences.
In conclusion, in a contemporary cohort of patients presenting with clinical Stage I, unilateral breast cancer, we demonstrated that a substantial minority of patients (23 %) undergo mastectomy, which in some cases may be clinically appropriate (e.g., multifocal disease). No decline in the use of mastectomy was found over time. Moreover, we noted a significant increase in the proportion of patients treated with mastectomy electing contralateral prophylactic mastectomy. Notably, significant institutional variations emerged as a key component in the choice of BCS versus mastectomy. We also highlighted that surgical choice can impact time from diagnosis to definitive surgery or chemotherapy. We believe that these data stress the need for dedicated studies to understand the surgical decision-making process, in order to ensure that patients are given the information and tools they need to make informed decisions in the setting of accurate perceptions of risks and benefits.
References
Blichert-Toft M, Rose C, Andersen JA et al (1992) Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr 11:19–25
Lichter AS, Lippman ME, Danforth DN Jr et al (1992) Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol 10:976–983
Sarrazin D, Le MG, Arriagada R et al (1989) Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol 14:177–184
Veronesi U, Cascinelli N, Mariani L et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
van Dongen JA, Voogd AC, Fentiman IS et al (2000) Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92:1143–1150
Clarke M, Collins R, Darby S et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366:2087–2106
Kurian AW, Lichtensztajn DY, Keegan TH et al (2014) Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA 312:902–914
Du X, Freeman DH Jr, Syblik DA (2000) What drove changes in the use of breast conserving surgery since the early 1980s? The role of the clinical trial, celebrity action and an NIH consensus statement. Breast Cancer Res Treat 62:71–79
Lazovich D, Solomon CC, Thomas DB et al (1999) Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer 86:628–637
Katipamula R, Degnim AC, Hoskin T et al (2009) Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 27:4082–4088
Gomez SL, Lichtensztajn D, Kurian AW et al (2010) Increasing mastectomy rates for early-stage breast cancer? Population-based trends from California. J Clin Oncol 28:e155–e157 (author reply e158)
Tuttle TM, Habermann EB, Grund EH et al (2007) Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 25:5203–5209
McGuire KP, Santillan AA, Kaur P et al (2009) Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 16:2682–2690
Guadagnoli E, Weeks JC, Shapiro CL et al (1998) Use of breast-conserving surgery for treatment of stage I and stage II breast cancer. J Clin Oncol 16:101–106
Habermann EB, Abbott A, Parsons HM et al (2010) Are mastectomy rates really increasing in the United States? J Clin Oncol 28:3437–3441
Nattinger AB, Gottlieb MS, Veum J et al (1992) Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med 326:1102–1107
Morrow M, White J, Moughan J et al (2001) Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol 19:2254–2262
Gregorio DI, Kulldorff M, Barry L et al (2001) Geographical differences in primary therapy for early-stage breast cancer. Ann Surg Oncol 8:844–849
Greenberg CC, Lipsitz SR, Hughes ME et al (2011) Institutional variation in the surgical treatment of breast cancer: a study of the NCCN. Ann Surg 254:339–345
Kummerow KL, Du L, Penson DF et al (2015) Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 150:9–16
Lizarraga I, Schroeder MC, Weigel RJ, Thomas A (2015) Surgical management of breast cancer in 2010–2011 SEER registries by hormone and HER2 receptor status. Ann Surg Oncol 22:566–572
Showalter SL, Grover S, Sharma S et al (2013) Factors influencing surgical and adjuvant therapy in stage I breast cancer: a SEER 18 database analysis. Ann Surg Oncol 20:1287–1294
Weeks JC (1997) Outcomes assessment in the NCCN. Oncol (Williston Park) 11:137–140
Punglia RS, Hughes ME, Edge SB et al (2008) Factors associated with guideline-concordant use of radiotherapy after mastectomy in the national comprehensive cancer network. Int J Radiat Oncol Biol Phys 72:1434–1440
Klabunde CN, Potosky AL, Legler JM, Warren JL (2000) Development of a comorbidity index using physician claims data. J Clin Epidemiol 53:1258–1267
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Katz JN, Chang LC, Sangha O et al (1996) Can comorbidity be measured by questionnaire rather than medical record review? Med Care 34:73–84
Morrow M (2005) Limiting breast surgery to the proper minimum. Breast 14:523–526
Farrow DC, Hunt WC, Samet JM (1992) Geographic variation in the treatment of localized breast cancer. N Engl J Med 326:1097–1101
Tuttle TM, Rueth NM, Abbott A, Virnig BA (2012) United States trends in the surgical treatment of primary breast cancer. World J Surg 36:1475–1479
Chagpar AB, Studts JL, Scoggins CR et al (2006) Factors associated with surgical options for breast carcinoma. Cancer 106:1462–1466
Rosenberg SM, Tracy MS, Meyer ME et al (2013) Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med 159:373–381
Vandergrift JL, Niland JC, Theriault RL et al (2013) Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst 105:104–112
Acknowledgments
This work was supported by Fundacao para a Ciencia e Tecnologia-HMSP-ICS/0004/201, National Cancer Institute Specialized Program of Research Excellence in Breast Cancer (NIH P50 CA089393).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hope S. Rugo declared remuneration from Genomic Health and a Consultant/advisory role at Sandoz. No other disclosures were declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vaz-Luis, I., Hughes, M.E., Cronin, A. et al. Trends in the use of mastectomy in women with small node-negative breast cancer treated at US academic centers. Breast Cancer Res Treat 155, 569–578 (2016). https://doi.org/10.1007/s10549-016-3707-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3707-1