Abstract
Introduction
Surgical therapy for newly diagnosed breast cancer has changed over the past decade, but these trends have not been well documented in patients undergoing neoadjuvant therapy (NAC).
Methods
In a retrospective cohort study of the National Cancer Database (NCDB), we selected 285,514 women with clinical stage I–III breast cancer who underwent NAC or adjuvant therapy (AC) from 2006 to 2014. Breast-conserving surgery (BCS), unilateral mastectomy (UM), and bilateral mastectomy (BM) rates were compared between patients undergoing NAC and AC.
Results
Of 285,514 women, 68,850 (24.1%) underwent NAC. Of NAC patients, 18,158 (26.4%) underwent BM and 27,349 (39.7%) BCS compared with 31,886 (14.7%) and 120,626 (55.7%) AC patients, respectively. From 2006 to 2014, BM increased from 16.1 to 28.8% (p < 0.001) for NAC and from 7.4 to 17.5% (p < 0.001) for AC. After adjusting for patient, tumor, and facility factors, NAC patients were 1.50 times [odds ratio (OR) 1.50, confidence interval (CI) 1.42–1.51] more likely to undergo BM then AC patients. The difference in BM rates between patients receiving NAC versus AC varied significantly by cT classification. This difference was the greatest among cT1 tumors between NAC and AC (31.7 vs. 13.0%, p < 0.001), followed by cT2 tumors (24.1 vs. 16.6%, p < 0.001) and cT3 tumors (24.3 vs. 22.3%).
Conclusions and Relevance
More NAC patients are undergoing BM while fewer are undergoing BCS compared with patients undergoing AC. This trend is particularly striking for those patients with smaller tumors who would otherwise be candidates for BCS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Indications for NAC in the current era have expanded to include early-stage cancers.1 Studies have shown that the tumor response to NAC gives clinicians valuable prognostic information; patients who have complete pathologic response (pCR) to NAC have better disease-free and overall survival then patients without pCR.2 , 3 NAC can also limit the extent of axillary surgery.4 The neoadjuvant setting is also an ideal way to examine new novel targeted therapies for tumor response.5
Since the National Institutes of Health published their consensus statement in 1990,6 BCS has been the preferred treatment for early-stage breast cancer. However, over the past decade, we have seen a decrease in BCS and an increase in bilateral mastectomy (BM) for newly diagnosed breast cancer patients7,8,9,10, – 11 even amongst patients who are clear candidates for BCS.8 Early randomized trials of NAC showed that more patients underwent BCS then patients randomized to AC. In the National Surgical Adjuvant Bowel and Breast Project (NSABP) B-18, 68% of NAC patients underwent BCS compared with 60% in the AC arm.12 Nearly a third of patients who were told they needed a mastectomy ended up having BCS. In the NSABP B-27 trial, approximately 61–63% of patients underwent BCS,3 and in the Cancer and Leukemia Group (CALGB) 40603 trial, the overall BCS eligibility rate rose from 54 to 68% with NAC.13 However, recent studies have shown a shift toward more mastectomies. A study of the National Cancer Database (NCDB) from 2006 to 2011 showed that mastectomy rates were 65% for NAC compared with 50% for AC.14 A secondary analysis of the Translational Breast Cancer Research Consortium (TBCRC) 017 trial of 770 patients from 2002 to 2011 who underwent NAC and magnetic resonance imaging (MRI) reported that 55% of patients had mastectomy.15 However, neither of these studies differentiated whether patients were undergoing BM or unilateral mastectomy (UM). Additionally, studies examining patient surgical decision-making have common themes of patient factors such as “peace of mind” and avoiding future recurrences and treatments to be the most important factors in decision-making.16,17, – 18 The experience of neoadjuvant chemotherapy possibly amplifies some of these patient factors.
We hypothesized that BM rates among NAC patients would be higher than patients undergoing adjuvant chemotherapy (AC), including amongst those patients with smaller tumors who would be candidates for BCS. This hypothesis is based on the fact that BM rates have been increasing in the adjuvant setting, so we hypothesized that we would see a similar trend in NAC patients, particularly for small tumors that would otherwise be BCS candidates regardless of NAC. Patients who undergo NAC may feel that BM will allow them to avoid future chemotherapy treatments despite the fact that BM does not eliminate risk of recurrence. Indeed, several studies have shown that up to a third of patients do not understand that BM does not prevent distant recurrence16 , 19 and therefore will not necessarily obviate the need for future systemic treatments. We utilized the National Cancer Database to examine our hypothesis, as it contains information on whether NAC was part of the treatment algorithm. If our hypothesis is true, it would demonstrate the strong influence that NAC has on surgical decision-making for breast cancer patients. These findings are clinically significant since more patients are undergoing NAC and more clinical trials are adopting a NAC trial design.1
Methods
Data Source
The NCDB is a nationwide, facility-based oncology database that is a joint project of the American Cancer Society (ACS) and the American College of Surgeons Commission on Cancer (CoC).20 Data are coded and reported according to national established protocols coordinated under the North American Association of Central Cancer Registries and are compliant with the privacy requirements of the Health Insurance Portability and Accountability Act (HIPAA). Our institutional review board granted a waiver for approval for this study because the collected information was deidentified, no protected health information was reviewed, and the analysis was retrospective. The American Cancer Society and the CoC have not verified and are not responsible for the analytic or statistical methodology employed, nor the conclusions drawn from these data by the investigators.
Study Population
The 2014 breast cancer NCDB participant user file was used to identify women with unilateral, American Joint Committee on Cancer (AJCC) clinical stage I–III breast cancer who underwent surgery and either NAC or AC from 2006 to 2014. Data on clinical stage was missing in 312 (0.4%) NAC patients and 1223 (0.6%) AC patients, and these patients were excluded from analyses. Patients with inflammatory or stage IV breast cancer, and undergoing neoadjuvant hormonal therapy were also excluded. All patients had received all or part of their therapy at the reporting institution. Patient covariates included age, race, Charlson cormorbidity index, insurance, and socioeconomic status. Tumor covariates included the clinical tumor, clinical node status, tumor molecular subtype [hormone receptor (HR)+ human epidermal growth factor receptor (Her)2+, HR+ Her2−, HR− Her2+, and HR− Her2−], histology, and tumor grade. Staging information was in accordance with the AJCC 7th edition.21 Facility covariates included facility type, facility annual volume (low < 100 cases, medium 100–250 cases, and high > 250 cases), and location. Facility location was classified by reported state of residence into four regions as defined by the US Census Bureau.22 NAC was defined as chemotherapy administered prior to surgery, and AC was defined as chemotherapy administered after surgery. Pathologic complete response (pCR) was defined as no invasive or noninvasive tumor in the breast or axillary nodes after NAC.
Statistical Analysis
Patient demographic, tumor, and facility characteristics were compared using chi-square test for categorical variables and independent t-test for continuous variables between NAC and AC cohorts. Trends in BCS, UM, and BM rates from 2006 to 2014 were examined. The proportion of patients undergoing BCS, UM, and BM in the NAC and AC cohorts were stratified by cT classification and molecular subtype. Multivariable logistic regression was used to examine odds of undergoing BM, adjusting for patient factors (age, race, socioeconomic status, insurance, comorbidity), tumor factors (clinical T and N classification, grade, molecular subtype), facility factors (type, volume, location), and year of diagnosis. This analysis was restricted to years 2010–2014, when Her2neu status was available. We first performed a multivariable model in all patients to examine the association of NAC with BM when adjusting for the aforementioned factors. We then performed the similar multivariable analysis to examine independent predictors of BM in the NAC and AC cohorts separately. Odds ratio (OR) > 1 signifies higher odds for BM. All confidence intervals (CI) are reported at 95% significance level. All analysis was performed using SAS 9.4 (SAS Inc., Cary, NC), and p < 0.05 was considered statistically significant.
Results
Cohort Characteristics
We identified 285,514 women diagnosed with breast cancer from 2006 to 2014 who met our inclusion criteria. Demographic, tumor, and facility factors were compared between patients receiving NAC and AC (Table 1). Patients undergoing NAC were on average 4.9 years younger (p < 0.01) and were less likely to be Caucasian (74.5 vs. 79.8%) than those undergoing AC. NAC patients were also more likely to have larger tumors and node-positive tumors than AC patients.
Temporal Trends in Bilateral Mastectomy, Unilateral Mastectomy, and BCS from 2006 to 2014
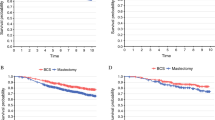
Trends in BCS, UM, and BM varied amongst patients undergoing NAC, AC, and no chemotherapy (Fig. 1a). Overall NAC rates went from 8.4% in 2006 to 15.2% in 2014 for cT1 tumors, 29.2 to 45.0% for cT2 tumors, and 68.6 to 73.0% for cT3 tumors. NAC patients were more likely to undergo BM compared with AC patients (26.4 vs. 14.7%, p < 0.01). From 2006 to 2014, BM increased from 16.1 to 28.8% (p < 0.001) for NAC and 7.4 to 17.5% (p < 0.001) for AC, respectively. Of note, NAC rates overall increased from 22.8% of the entire cohort undergoing NAC in 2006 to 32.8% in 2014. In women < 40 years old, rates of BM were higher amongst NAC, AC, and no chemotherapy groups than in women ≥ 40 years old (Fig. 1b). There was not significant interaction effect between NAC/AC and surgical procedure over time (p = 0.6449). The difference in BM rates between patients receiving NAC versus AC also varied by cT classification. Overall this difference was the greatest among cT1 tumors between NAC and AC (31.7 vs. 13.0%, p < 0.001) followed by a smaller difference among cT2 tumors (24.1 vs. 16.6%, p < 0.001), and a difference that was somewhat insignificant among cT3 tumors (24.3 vs. 22.3%).
Surgical Procedure Type Stratified by Molecular Subtype and Clinical Classification
Surgical procedure type for NAC and AC patients is stratified by cT classifcation in Table 2. Amongst patients with cT1 tumors undergoing NAC, 4413 (31.7%) patients underwent BM compared with 16,595 (13.0%) AC patients. BM rates amongst different molecular subtypes and stratified by cT classification are listed in Table 3. BM rates were higher among NAC patients compared with AC for all molecular subtypes. We examined how BM rates differed according to the pCR rate for cT1, cT2, and cT3 tumors. Overall, BM rates were similar between those patients with and without pCR (26.2 vs. 26.9%, p = 0.09). However, for patients with cT1 tumors, BM rates were 31.8 versus 31.2% (p = 0.57) between pCR and non-pCR, 23.8 versus 25.3% (p = 0.004) for cT2 tumors, and 27.0 versus 27.8% (p = 0.38) for cT3 tumors, respectively.
Independent Predictors of Bilateral Mastectomy
After adjusting for patient, tumor, facility factors, and breast reconstruction, patients undergoing NAC were about 50% more likely to undergo BM than those undergoing AC (OR 1.52, CI 95%: 1.47–1.57) (data not shown). This difference remained significant regardless of reconstruction status. In 2014, 1499 (15.0%) NAC patients who did not undergo reconstruction had BM compared with 1818 (8.2%) AC patients; 2362 (69.0%) NAC patients who had reconstruction underwent BM compared with 2961 (58.0%) AC patients.
Multivariable analysis of predictors of BM was performed separately for NAC and AC patients (Fig. 2). Similar independent predictors of BM were seen between the NAC and AC groups except for clinical tumor classifcation. Patients with cT2/T3 tumors were less likely to undergo BM then cT1 tumors in patients undergoing NAC but more likely to undergo BM then cT1 tumors in patients undergoing AC. The influence of clinical node status was not different between NAC and AC patients. Finally, pCR was not an independent factor associated with BM rates (OR 0.99, CI 95%: 0.93–1.04) among patients undergoing NAC.
Discussion
Our study has shown that patients undergoing NAC are undergoing different surgical procedures then patients undergoing AC. Over roughly 8 years, BM rates have increased and UM rates have decreased amongst NAC patients and AC patients, but these trends are more striking in NAC versus AC patients. Approximately 15% of AC patients are undergoing BM in contrast to nearly 30% of NAC patients. Because this is an observational cohort study, we were not able to control for all the factors that could influence which surgical procedure a patients undergoes. However, on adjusted analysis for patient, tumor, and facility factors available in the NCDB, we still found that NAC patients were just over 50% more likely to undergo BM then AC patients. At the same time, BCS rates have remained relatively stable across all cohorts but only 40% of NAC patients are undergoing BCS in contrast to 60–70% of AC patients and patients who did not reveive chemotherapy. This trend is in sharp contrast to over a decade ago, when greater then 60% of patients undergoing NAC underwent BCS.3 , 12
Interestingly, we saw a large disparity for BM when stratifying by clinical tumor size. Thirty-two percent of cT1 patients undergoing NAC underwent BM compared with 13% of AC patients. Likewise, only 41% of NAC patients with AJCC cT1 tumors underwent BCS compared with 65% of AC patients. This finding is unexpected since most smaller tumors (cT1) are likely candidates for BCS and would even be better candidates for BCS after NAC. Furthermore, independent predictors of BM were essentially the same for both NAC and AC except clinical tumor size. While a patient with a cT1 tumor in the past would have undergone BCS if given AC, they are much more likely to undergo BM if undergoing NAC, likely based on the fact that they had NAC in the first place. Patients may feel that BM will enable them to avoid any future chemotherapy and its toxicities and side effects. It is clear that, while tumor size was a criterion for BCS in the past, it is no longer the sole driver of patient’s surgical decisions today.
We did not see an interaction between BM rates and pCR rates. Presumably, those patients with pCR would more likely undergo BCS and not BM then patients without pCR. Pathologic complete response has also been associated with improved survival outcomes,2 which could prompt patients to be less aggressive with surgery. However, pCR was not associated with lower BM rates in our multivariate analysis of NAC patients, and thus patients were just as likely to undergo BM regardless of the response rate of the tumor to NAC. MRI has been associated with a fairly high predictive capability for pCR,23 but the NCDB does not contain any imaging information or clinical response to NAC so we could not analyze how clinical response to NAC impacted surgical decision-making. Thus, despite the important prognostic information that pCR can give a patient, it is not necessarily impacting surgical decisions.
NAC is known to have higher tumor response rates for certain molecular subtypes of breast cancer.2 , 24 Our study did demonstrate some variability in BM rates across different molecular subtypes. Amongst NAC patients, a greater proportion of patients with triple-negative molecular subtypes underwent BM then other subtypes for cT1 and T2 tumors. The triple-negative molecular subtype has been associated with BRCA mutation carriers,25 , 26 and it is possible that these patients underwent genetic testing while undergoing NAC and were discovered to be BRCA carriers. Because BRCA mutation carriers with breast cancer are known to have higher risk of developing contralateral breast cancer then noncarriers,27 , 28 patients with BRCA genetic mutations may more often choose BM. At the same time, genetic testing has been associated with increased BM rates,29 , 30 and a higher proportion of patients at higher genetic risk undergo BM.16 It is not clear whether NAC patients are more frequently undergoing BM because they have had genetic testing or because they were found to be BRCA carriers, because the NCDB does not contain genetic information or family history information. On multivariate analysis, both triple-negative and HR-HER2neu+ tumors were associated with more BM then HR + HER2neu− tumors. Patients with triple-negative and Her2neu+ tumors often undergo longer chemotherapy regimens that are associated with more toxicities, and these factors could have played a role in patients choosing BM.
Due to the limitations of the NCDB, our study is missing variables that would help us further understand why NAC patients are more often choosing BM. The NCDB only contains pathologic response to NAC, but there is no information on the clinical response to NAC, and therefore we could not evaluate how clinical complete response could affect surgical decision-making. Since the NCDB is an observational database, selection bias for patients to undergo NAC or AC may account for our findings. Lastly, we used clinical T staging instead of tumor size. Tumor size for NAC cases is recorded prior to NAC in the NCDB. We felt there can be many inaccuracies in the preoperative size measurement prior to NAC and therefore used clinical T classification instead.
There are no universally accepted indications for BM. Most guidelines encourage individualized decision-making.31 , 32 Physician recommendations strongly influence patient decision-making,16 and it is important for surgeons to understand which factors influence patient surgical decision-making. In the past, surgical decisions were often based on anatomic factors and whether a tumor was amenable to BCS.33 Our data demonstrate that these factors have lost relevancy for patients. Avoiding future cancers, possible future treatments, maximizing their cosmetic result, and getting “peace of mind” have become more important factors that drive patient surgical decision-making.16,17, – 18 While BM has not been shown to have a survival benefit over mastectomy or breast-conserving sugery in patients with unilateral cancer,29 , 31 , 32 patients continue to choose BM in both the adjuvant and neoadjuvant setting.7,8,9,10, – 11 Survey studies show that patients often choose BM for “peace of mind.”16 , 18 However it is not always clear what “peace of mind” means to each patient, particularly in the neoadjuvant versus adjuvant setting. In the neoadjuvant setting it may mean to avoid future chemotherapy regimens, while in the adjuvant setting it may mean avoiding future recurrences. It is also not entirely clear whether BM gives patients better “peace of mind” then other surgeries. Future prospective studies will need to study the impact of different surgical procedures on “peace of mind” and whether this impact is sustainable over the long term.
References
Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I–III breast cancer in the United States. Cancer. 2015;121(15):2544–2552.
von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–756.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–1461.
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). 2014; http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. Accessed February 13, 2017.
NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265(3):391–395.
King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–2164.
Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16.
Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209.
Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367.
Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol. 2010;17(10):2554–2562.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685.
Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II–III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. 2015;262(3):434-439. discussion 438-439.
Killelea BK, Long JB, Chagpar AB, et al. Trends and clinical implications of preoperative breast MRI in Medicare beneficiaries with breast cancer. Breast Cancer Res Treatment. 2013;141(1):155–163.
McGuire KP, Hwang ES, Cantor A, et al. Surgical patterns of care in patients with invasive breast cancer treated with neoadjuvant systemic therapy and breast magnetic resonance imaging: results of a secondary analysis of TBCRC 017. Ann Surg Oncol. 2015;22(1):75–81.
Jagsi R, Hawley ST, Griffith KA, et al. Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg. 2017;152(3):274–282.
Covelli AM, Baxter NN, Fitch MI, McCready DR, Wright FC. ‘Taking control of cancer’: understanding women’s choice for mastectomy. Ann Surg Oncol. 2015;22(2):383–391.
Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159(6):373–381.
Yao K, Belkora J, Bedrosian I, et al. Impact of an in-visit decision aid on patient knowledge about contralateral prophylactic mastectomy: a pilot study. Ann Surg Oncol. 2017;24(1):91–99.
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and –nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–81.
American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. Chicago, IL: Springer; 2010.
US Census. Statistical Groupings of States and Counties. 2010; http://www.census.gov/geo/reference/pdfs/GARM/Ch6GARM.pdf. Accessed February 13, 2017.
De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119(10):1776–1783.
Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24(12):1940–1949.
Krammer J, Pinker-Domenig K, Robson ME, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treatment. 2017;163(3):565–571.
Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26(26):4282–4288.
van der Kolk DM, de Bock GH, Leegte BK, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treatment. 2010;124(3):643–651.
Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22(12):2328–2335.
Wang F, Amara D, Peled AW, et al. Negative genetic testing does not deter contralateral prophylactic mastectomy in younger patients with greater family histories of breast cancer. Ann Surg Oncol. 2015;22(10):3338–3345.
Hawley ST JR, Morrow M, Katz SJ. Correlates of contralateral prophylactic mastectomy in a population based sample. J Clin Oncol. 2011;29:6010.
Hunt KK, Euhus DM, Boughey JC, et al. Society of surgical oncology breast disease working group statement on prophylactic (risk-reducing) mastectomy. Ann Surg Oncol. 2017;24(2):375–397.
Boughey JC, Attai DJ, Chen SL, et al. Contralateral prophylactic mastectomy (CPM) consensus statement from the American Society of Breast Surgeons: data on CPM outcomes and risks. Ann Surg Oncol. 2016;23(10):3100-3105.
NIH Consensus Development Conference statement on the treatment of early-stage breast cancer. Oncology (Williston Park). 1991;5(2):120–124.
Disclosure
The author have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kantor, O., Ajmani, G., Wang, CH. et al. The Shifting Paradigm for Breast Cancer Surgery in Patients Undergoing Neoadjuvant Chemotherapy. Ann Surg Oncol 25, 164–172 (2018). https://doi.org/10.1245/s10434-017-6217-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6217-4