Abstract
Addition of carboplatin to neoadjuvant chemotherapy in HER2-negative breast cancer may improve pathological complete response (pCR) rates. We evaluated the efficacy and safety of carboplatin and weekly paclitaxel (wPTX) followed by cyclophosphamide, epirubicin, and 5-fluorouracil (CEF) as neoadjuvant chemotherapy for HER2-negative breast cancer. Patients with stage II/IIIA HER2-negative breast cancer were randomly assigned to preoperatively receive CP-CEF (four 3-week cycles of carboplatin [area under the curve 5 mg/mL/min, day 1] and wPTX [80 mg/m2, day 1, 8, 15] followed by four 3-week cycles of CEF [500/100/500 mg/m2] or P-CEF (four cycles of wPTX followed by four cycles of CEF). The primary objective was pCR rate. Of 181 eligible patients, 89 were randomly assigned to the CP-CEF and 92 to the P-CEF. Two patients in each arm refused to receive neoadjuvant chemotherapy. Overall 88 patients in the CP-CEF and 91 patients in the P-CEF were assessable for efficacy and safety. The pCR rate in the CP-CEF was significantly higher than that in the P-CEF (31.8 vs. 17.6 %, one-sided P = 0.01). Among patients with triple-negative breast cancer, the pCR rate in the CP-CEF was significantly higher than that in the P-CEF [61.2 (23/37) vs. 26.3 % (10/38), P = 0.003]. Grade 3–4 neutropenia was observed in the CP-CEF more frequently than in the P-CEF (65.9 vs. 38.5 %). Adding carboplatin to neoadjuvant wPTX followed by CEF for HER2-negative breast cancer improved the pCR rate and exacerbated hematotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy is a widely accepted treatment option for patients with operable breast cancer [1, 2]. Currently, anthracyclines and taxanes in sequence or in combination are recommended for patients with HER2-negative disease, and anthracyclines followed by combinations of taxanes and trastuzumab are recommended for patients with HER2-positive disease [3–5]. Pathological complete response (pCR), which is defined as disappearance of all invasive carcinomas in primary and axillary nodes and is associated with long-term survival, occurs in about 15–20 % of patients with HER2-negative disease treated with anthracyclines and taxanes [3, 4].
Several new chemotherapeutic regimens have been evaluated in patients with HER2-negative disease. Adding capecitabine or gemcitabine to epirubicin and cyclophosphamide followed by taxane therapy did not improve pCR rates in the neoadjuvant setting [6, 7]. Carboplatin, a platinum compound, has yielded response rates of 20–35 % in phase II studies of previously untreated patients with metastatic breast cancer (MBC) [8–10]. In patients with HER2-positive disease, combinations of carboplatin, taxanes, and trastuzumab are active in both the adjuvant and metastatic settings [11, 12]. In a phase III study of MBC patients who previously received anthracycline-based adjuvant chemotherapy, ~70 % of whom had HER2-negative disease, first-line therapy consisting of triweekly carboplatin and paclitaxel resulted in similar progression-free survival as gemcitabine plus docetaxel [13]. Weekly paclitaxel (wPTX) followed by cyclophosphamide, epirubicin, and 5-fluorouracil (CEF) is a commonly used neoadjuvant chemotherapy regimen for patients with HER2-negative breast cancer [14]. Recently, triple-negative breast cancers (TNBC) were classified into six subtypes depending on gene profiles, and basal-like 1–2 subtypes were suggested as highly sensitive to cisplatin in the vitro study [15]. The previous randomized phase II study suggested a potential benefit of platinum for metastatic TNBC [16].
We hypothesized that carboplatin would enhance the anti-tumor activity of wPTX and that this combination would improve pCR rates over the conventional regimens of wPTX followed by CEF. We conducted this randomized phase II trial to assess the efficacy and safety of adding carboplatin to wPTX followed by CEF in the neoadjuvant setting for patients with HER2-negative breast cancer.
Patients and methods
Patient eligibility
Eligible patients had previously untreated, unilateral, histologically confirmed, invasive, non-inflammatory, breast carcinoma. Histologic confirmation of invasive cancer was performed by core needle biopsy (CNB). HER2-negative disease was defined as a score of 0 or 1 + by immunohistochemistry (IHC) or HER2 gene copy: chromosome 17 ratio of <2.0 by fluorescence in situ hybridization (FISH). Patients with a tumor >2.0 cm at the largest dimension by ultrasonography, or ≤2.0 cm with axillary lymph node metastasis clinically diagnosed as positive, were eligible (clinical stage II and IIIA). Patients with axillary nodes enlarged by >1 cm at the largest dimension according to ultrasonography were considered to be clinically node positive. Patients with T4, N3, (supraclavicular lymph node), or distant metastatic disease (M1) were excluded from this study.
Other requirements included age 18–70 years, ECOG performance status 0–2, adequate bone marrow function (absolute granulocyte count ≥1,500/mm3 and platelet count ≥100,000/mm3), liver function (total bilirubin ≤1.5 mg/dL and liver transaminase [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] ≤60 IU/L), and renal function (serum creatinine ≤1.5 mg/dL), and written informed consent. Patients with a history of ischemic cardiac disease were excluded. Patients with clinically negative axillary lymph nodes had the option of undergoing pretreatment sentinel lymph node biopsy (SLNB).
Study design and neoadjuvant chemotherapy
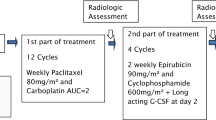
This was a randomized, multicenter (10 institutions), non-blinded phase II study. The study design is shown in Fig. 1. Enrolled patients were randomly assigned to receive either wPTX (P) followed by CEF (P-CEF arm) or combination carboplatin and wPTX (CP) followed by CEF (CP-CEF arm) by the minimization method, with balancing of the treatment arms according to disease status (stage II vs. IIIA), hormone receptor (HR) status, and institution. Paclitaxel was administered at 80 mg/m2 IV over 1 h on days 1, 8, and 15 every 3 weeks for four cycles. Carboplatin and wPTX were administered at area under blood concentration time curve (AUC) 5 mg/mL/min IV over 1 h on day 1 and at 80 mg/m2 IV over 1 h on days 1, 8, and 15, respectively, every 3 weeks for four cycles. CEF consisted of CEF (500/100/500 mg/m2) IV on day 1 every 3 weeks for four cycles. Carboplatin was provided by Bristol-Myers Squibb K.K., Tokyo, Japan as an investigational drug.
If a patient developed grade ≥3 febrile neutropenia, thrombocytopenia <25,000/mm3, or grade ≥3 non-hematologic toxicity while receiving CP or CEF, the doses of carboplatin and epirubicin were reduced by 20 and 25 %, respectively, in subsequent cycles. The doses of paclitaxel during CP and P were reduced by 25 % in subsequent cycles if a patient developed grade 3 neurotoxicity. Before administration of the following cycle of CP, P, or CEF, patients were required to have a granulocyte count ≥1,500/mm3, platelet count ≥75,000/mm3, and no non-hematologic toxicity of grade ≤2 (excluding alopecia). Before administration of CP on day 8 and 15, patients were required to have a granulocyte count ≥500/mm3, platelet count ≥75,000/mm3, and peripheral neuropathy of grade ≤2. If toxicity did not improve within 2 weeks on the P or CP regimen, chemotherapy was discontinued and initiation of CEF was recommended. If toxicity did not improve within 2 weeks on CEF, chemotherapy was discontinued and surgery was recommended.
Therapy after neoadjuvant chemotherapy
Patients who were considered candidates for breast-conserving therapy (BCT) were offered lumpectomy. Axillary lymph node dissection (AxLND) was mandatory, except in patients diagnosed as having no metastases by SLNB before neoadjuvant chemotherapy. Surgery was performed within 8 weeks after completion of preoperative chemotherapy. All patients who underwent BCT received whole-breast irradiation. After completion of neoadjuvant chemotherapy and surgery, patients with HR-positive disease received adjuvant endocrine therapy.
Study evaluation and criteria
The HER2 status of CNB specimens was determined by IHC and/or FISH performed at each institution before study enrollment, and was not subject to central review. HR status [estrogen receptor (ER) and progesterone receptor (PgR)] of CNB specimens was assessed by IHC, for which ≥10 % staining of cancer cell nuclei was diagnosed as positive. HR positivity was defined as ER-positive and/or PgR-positive disease. Histological grade was scored according to the modified Scarff–Bloom–Richardson classification [17]. After completion of neoadjuvant chemotherapy, resected specimens and CNB specimens were evaluated centrally by 3 breast pathologists. A pCR was defined as the absence of viable invasive tumor in both the breast and axillary nodes. Patients with residual ductal carcinoma in situ (DCIS) in the breast and no viable invasive tumor in the axillary nodes were also classified as having a pCR. Clinical response was evaluated by palpation and caliper after each cycle according to the Response Evaluation Criteria In Solid Tumors version 1.1. All adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 4.03.
Endpoints and statistical analysis
The primary endpoint was the pCR rate. Secondary endpoints included disease-free survival, clinical response rate, breast conservation rate, and safety. Efficacy and safety analysis were performed in the intent-to-treat (ITT) population, which consisted of subjects fulfilling the study inclusion criteria who had received at least one dose of study chemotherapy. The per-protocol population consisted of subjects who had completed chemotherapy and underwent surgery in this study without serious violations of the inclusion criteria.
Based on previous studies of neoadjuvant anthracyclines and taxanes, patients with HER2-positive disease account for 6–30 % of the treatment population, and pCR rates (defined in the same manner as the present study) ranged from 16 to 26 % [4, 6, 13]. The present study was designed for patients with HER2-negative disease, and P-CEF was expected to produce a pCR rate of 15 %. The study was originally planned to enroll 110 patients in each treatment arm in order to detect a 30 % increase in pCR in the CP-CEF arm with 90 % power using the Pearson’s chi squared test and one-sided 10 % significance level. Due to an administrative reason (the termination of financial support due to the end of a government-sponsored clinical trial program), the revised sample size with 87 % power was a total of 180 patients. Study accrual was not stopped on the basis of an interim analysis. An exploratory logistic regression analysis was conducted to examine the influence of clinical stage (II, IIIA), clinical nodal status (positive, negative), histological grade (grade 1, 2, 3), HR status (positive, negative), and age (<50, ≥50 years) on pCR. The primary test of the pCR rate was reported as one-sided and other reported P values were two-sided tests. Analyses were conducted using JMP® software version 8.0.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Between March 2010 and September 2011, 181 patients entered into this study. Of these, 88 patients treated with CP-CEF and 91 treated with P-CEF were evaluable in the ITT population. Two patients in each arm refused to receive neoadjuvant chemotherapy. Furthermore 38 patients in the CP-CEF arm and 29 patients in the P-CEF arm were excluded from the per-protocol population (Fig. 2). According to central review, 9 patients were considered ineligible [HER2 score 2 + by IHC and FISH not done (n = 6), CNB specimen not evaluable for invasive component (n = 1), and CNB specimen not evaluable (n = 2)]. Two patients had proven stage IIIC disease after enrollment.
CONSORT flow diagram. Disposition of study participants. + 6 of the 61 patients who discontinued neoadjuvant chemotherapy or showed disease progression after the completion of chemotherapy were diagnosed as ineligible by pathological central review (3 patients in the CP-CEF and 3 patients in the P-CEF arm), ++ patients who were diagnosed as ineligible by pathological central review, and +++ patients who were determined to have Stage IIIC disease after enrollment. ITT intent to treat, PCT preoperative chemotherapy by study protocol, and PPP per-protocol population
Characteristics of the ITT population are shown in Table 1. The median age was 47 years old. Distributions of tumor size, nuclear grade, and clinical axillary node status were similar; and more than 95 % of patients were diagnosed with invasive ductal carcinoma in the two arms. In the both arms, 42 % of patients had HR-negative (and thus triple-negative) tumors and 41 % had ER- and PgR-positive disease.
Treatment exposure
In the CP-CEF and P-CEF arms, 55 of 88 patients (62.5 %) and 67 of 91 patients (73.6 %), respectively, received all of the planned treatment cycles. In the CP-CEF arm, 64 patients (72.7 %) completed four cycles of CP; while in the P-CEF arm, 82 patients (90.1 %) completed four cycles of P (Table 2). In the CP-CEF arm, 33 patients did not complete chemotherapy due to adverse events (n = 29) or disease progression (n = 4). In the P-CEF arm, 24 patients did not complete chemotherapy due to adverse events (n = 6), refusal (n = 6), ineligibility (n = 2), or disease progression (n = 10).
Of 88 patients treated with CP, 65 (73.9 %) required delayed administration or at least one dose reduction of paclitaxel, 18 of whom required one dose reduction of carboplatin. Of 91 patients treated with P, 28 (30.8 %) required delayed administration or at least one dose reduction of paclitaxel. Sixteen patients in each treatment arm required at least one dose reduction of CEF.
Efficacy
After chemotherapy, 88 patients in the CP-CEF arm and 89 patients in the P-CEF arm underwent breast surgery. Two patients in the P-CEF arm did not undergo surgery due to proven stage IIIC disease after enrollment (n = 1), and patient refusal to continue treatment due to adverse events experienced during CEF (n = 1). The breast conservation rates were 61.4 % in the CP-CEF arm and 64.8 % in the P-CEF arm. Fifty-nine patients (67.0 %) in the CP-CEF arm and fifty-nine patients (67.0 %) in the P-CEF arm underwent AxLND (Table 2).
The overall clinical response rate to CP-CEF was significantly higher than that to P-CEF (84.1 vs. 70.3 %, P = 0.03). Disease progression was observed in 4 patients who received CP-CEF (3 during CP and 1 during CEF) and 10 patients who received P-CEF (8 during P and 2 during CEF). After completion of neoadjuvant chemotherapy, 1 patient in the CP-CEF arm and 3 patients in the P-CEF arm experienced disease progression. All 3 patients in the CP-CEF arm and 10 of 13 patients in the P-CEF arm who experienced disease progression had HR-negative disease.
The pCR rate in the CP-CEF arm was significantly higher than that in the P-CEF arm (31.8 vs. 17.6 %, one-sided P = 0.01). Among these pCR patients, 9 of 28 patients in the CP-CEF arm and 4 of 16 patients in the P-CEF arm had DCIS. In the per-protocol population, the difference in pCR rates between the two arms was not significant [28.0 % (14/50) in the CP-CEF arm vs. 24.2 % (15/62) in the P-CEF arm, one-sided P = 0.179]. By univariate analysis, treatment arm, clinical tumor size, and HR status were significantly associated with pCR (Table 3), and these were all shown to be independent factors by multivariate analysis. Among HR-negative patients, 23 of 37 patients (61.2 %) in the CP-CEF arm achieved a pCR; this rate was significantly higher than that in the P-CEF arm [26.3 % (10/38), P = 0.003, Fig. 3]. Among patients with HR-positive and histological grade 1 disease, 0 of 12 patients in the CP-CEF arm and 1 of 11 patients in the P-CEF arm experienced a pCR. In contrast, among patients with HR-positive and histological grade 2–3 disease, 5 of 39 patients (12.8 %) in the CP-CEF arm and 5 of 42 patients (11.9 %) in the P-CEF arm experienced a pCR. Other factors associated with significantly higher pCR rates in the CP-CEF arm included age (≥50 years), clinical tumor size (T1–2), and histological grade (grade 2–3). After a median follow-up of 12.0 months, 4 and three patients experienced disease recurrence in the CP-CEF and P-CEF arms, respectively.
Odds ratios for pCR rates between the two treatment arms by subgroup. pCR pathological complete response, T tumor size, T1 (≤2.0 cm), T2 (2.1–5.0 cm), and T3 (≥5.1 cm). Asterisk including 3 patients in the P-CEF arm (1 patient with stage IIIC disease and 2 patients who did not undergo breast surgery)
Safety
Grade 3–4 hematologic toxicities were more common in patients treated with CP than in those treated with P (neutropenia 58.0 vs. 9.9 %, anemia 15.9 vs. 0 %, and thrombocytopenia 1.1 vs. 0 %, respectively, Table 4). Non-hematologic toxicities were similar between the two treatment arms. In the CP-CEF arm, 26 patients discontinued CP due to adverse events, which were predominantly hematologic toxicities [prolonged neutropenia (n = 19), febrile neutropenia (n = 1), thrombocytopenia (n = 2), peripheral sensory neuropathy (n = 2), infection (n = 1), and elevation of liver transaminase (n = 1)], and 3 patients discontinued CEF due to adverse events. Five and six patients in the P-CEF arm discontinued P and CEF, respectively, due to adverse events. One patient in the CP-CEF arm developed acute monocytic leukemia 1.5 years after completion of neoadjuvant chemotherapy.
Discussion
The addition of carboplatin to wPTX followed by CEF significantly improved the pCR rates in the ITT population in the present study. No difference in pCR rates was observed in the per-protocol population, although this could be due to the high rate of discontinuation of neoadjuvant chemotherapy in the CP-CEF arm (37.5 %) and the small sample size.
A meta-analysis of 12 randomized neoadjuvant trials for breast cancer (12,993 patients total) suggested that pCR rates differed by tumor subtype [18]. In patients with HER2-negative and HR-positive disease, the pCR rates of patients with grade 1–2 and 3 were 7 and 16 %, respectively. The pCR rate of patients with TNBC was 34 %. Furthermore, the association between pCR and event-free survival in patients with HR-positive and grade 3 disease or TNBC was significant. In the present study, the difference in pCR rates between the two arms was not significant in patients with HR-positive disease. However, in patients with TNBC, the pCR rate in the CP-CEF arm was significantly higher than that in the P-CEF arm (Fig. 3). In the randomized studies of the addition of carboplatin to anthracycline and taxane for TNBC in neoadjuvant settings, one study showed no improvement of the pCR rate by addition of carboplatin (GEICAM/2006-03: n = 93, 29.8 vs. 3.48 %, P = 0.606) and the other two studies suggested any improvement of the pCR rates (GeparSixto: n = 315, 58.7 vs. 37.9 %, P < 0.05; CALGB40603: n = 233, 60 vs. 46 %, P < 0.0018) [19–21]. The present results combined with those of previous studies suggested an advantage associated with the addition of platinum compounds to anthracyclines and taxanes as neoadjuvant therapy for TNBC.
The dosage and schedule of carboplatin and wPTX in the experimental arm of our study were chosen on the basis of the results of a previous study in advanced ovarian cancer, in which improved survival was observed in patients who received wPTX compared with the conventional triweekly schedule. In that study, 312 patients were treated with carboplatin (AUC of 6 on day 1) plus wPTX (80 mg/m2 on day 1, 8, and 15) every 3 weeks, and carboplatin doses were reduced for hematologic toxicities in 48 % of patients. Therefore, the AUC of carboplatin in the present study was reduced to 5 [22]. In the present study, hematologic toxicities were more common in the CP-CEF arm, and they resulted in delayed administration or at least one dose reduction of paclitaxel (73.9 %) and dose reduction of carboplatin (20.5 %). In the CALGB 40603 trial, 4 cycles of triweekly administration of carboplatin (AUC6) with wPTX increased grade 3/4 neutropenia and thrombocytopenia [21]. In the 18 weekly administrations of liposomal doxorubicin, paclitaxel, and carboplatin (AUC2) of the GeparSixto study, all treatments were completed by 52.2 % and discontinuations due to adverse events occurred in 37.7 % [20]. The optimum dosage and schedule of carboplatin and wPTX have not yet been established. The frequency of neutropenia in patients who received paclitaxel and carboplatin, which were given every week, was lower than that reported in the present study. A weekly carboplatin and paclitaxel may be an alternative regimen with mild hematologic toxicities. A randomized trial of sequential taxane and anthracycline neoadjuvant regimens showed no significant difference in pCR rates between the two sequences, although the regimen of a taxane followed by an anthracycline was associated with milder hematologic toxicity [23]. In the present study, due to concerns about hematologic toxicities associated with the combination of carboplatin and wPTX, a sequence of a taxane followed by an anthracycline was chosen.
The present study has a number of limitations, and was stopped early before full accrual keeping with 87 % power and one-sided 10 % significance level. In the present study, the definition of HR negativity was <10 % staining of cancer cell nuclei by IHC. Out of concerns about false negative or positive, The ER- and PgR-negativities are recommended <1 % staining of cancer nuclei irrespective of staining intensity with the objectives of clinical trial eligibility for TNBC [24]. In the vitro study, basal-like subtypes of TNBC depending on gene profiles were suggested a highly sensitive to cisplatin, and pragmatic selection method of basal-like subtypes is an issue in the future [15]. The primary endpoint was a pCR rate rather than indicative of long-term outcome. A meta-analysis of neoadjuvant breast cancer trials showed that the magnitude of improvement in pCR did not predict long-term outcomes. However, in patients with TNBC, improvement of pCR was significantly associated with improvement of event-free and overall survival [18]. Therefore, the improvement of pCR associated with the addition of carboplatin in patients with TNBC in the present study may contribute to improved long-term outcomes.
In conclusion, the addition of carboplatin to wPTX followed by CEF for HER2-negative breast cancer improved the pCR rate but resulted in more hematologic toxicity.
References
Mauri D, Pavlidis N, Ioannidis HPA (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer. J Natl Cancer Inst 97:188–194
Kaufmann M, von Minckwitz G, Mamounas EP et al (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19:1508–1516
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 21:4165–4174
von Minckwitz G, Kummel S, Vogel P et al (2008) Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized Gepar Trio Study. J Natl Cancer Inst 100:552–562
Gianni L, Eiermann W, Semiglazov V et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomized controlled superiority trial with a parallel HER-negative cohort. Lancet 375:377–384
von Minckwitz G, Rezai M, Loibl S et al (2010) Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III Gepar Quattro study. J Clin Oncol 28:2015–2023
Earl HM, Vallier AL, Hiller L et al (2014) Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for woman with high-risk early breast cancer (Neo-tAnGo): an open-label, 2 × 2 factorial randomized phase 3 trial. Lancet Oncol 15:201–212
Kolaric K, Vukas D (1991) Carboplatin activity in untreated metastatic breast cancer patients—results of a phase II study. Cancer Chemother Pharmacol 27:409–412
Martin M, Diaz-Rubio E, Casado A et al (1992) Carboplatin: an active drug in metastatic breast cancer. J Clin Oncol 10:433–437
O’Brien ME, Talbot DC, Smith IE (1993) Carboplatin in the treatment of advanced breast cancer: a phase II study using a pharmacokinetically guided dose schedule. J Clin Oncol 11:2112–2117
Slamon D, Eiermann W, Robert N et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283
Robert N, Leyland-Jones B, Asmar L et al (2006) Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2 overexpressing metastatic breast cancer. J Clin Oncol 24:2786–2792
Fountzilas G, Dafni U, Dimopoulos MA et al (2009) A randomized phase III study comparing three anthracycline-free taxane-based regimens, as first-line chemotherapy, in metastatic breast cancer: a Hellenic Cooperative Oncology Group study. Breast Cancer Res Treat 115:87–99
Kelly CM, Green MC, Broglio K et al (2012) Phase III trial evaluating weekly paclitaxel versus docetaxel in combination with capecitabine in operable breast cancer. J Clin Oncol 30:930–935
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767
Fan Y, Xu BH, Yuan P et al (2013) Docetaxel-cisplatin might be superior to docetaxel–capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann Oncol 24:1219–1225
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. doi:10.1016/S0140-6736(13)62422-8
Alba E, Chacon JI, Llush A et al (2012) A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant seeting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat 136:487–493
Sikow WM, Berry DA, Perou CM et al (2013) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant weekly paclitaxel followed by dose-dense AC on pathologic complete response rates in triple-negative breast cancer: CALGB/Alliance 40603. In: 36th annual San Antonio Breast Cancer Symposium abstract S5-01
von Minckwitz G, Schneeweiss A, Salat C et al (2013) A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). J Clin Oncol 31:860–867
Katsumata N, Yasuda M, Takahashi F et al (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:1331–1338
Thiery-Vuillemin A, Llombart-Cussac A, Chaiqneau L et al (2011) Sequential taxane and anthracycline-containing neoadjuvant regimens: the sequential order impact. Breast 20:46–49
Eiermann W, Bergh J, Cardoso F et al (2012) Triple negative breast cancer: proposals for a pragmatic definition and implications for patient management and trial design. Breast 21:20–26
Acknowledgments
We thank the women who participated in this trial; Hitoshi Tsuda, Futoshi Akiyama, and Shinobu Masuda for central review of pathological diagnoses; Hiroi Kasai for preparing for this study; and Midori Tanaka for writing the study report. This study had been performed as a registration-directed trial in accordance with the Good Clinical Practice guideline [Enforcement Regulation No. 24 of the MHLW (revised GCP) dated on February 29, 2008], which is published by the revised Pharmaceutical Affairs Act in Japan (No. 84 dated on June 21, 2006). This study was supported by the Health and Labour Sciences Research Grants (Clinical Cancer Research), Ministry of Health, Labour and Welfare (Grant Number: MHLW, 2009 Clinical Cancer Research General-020) and the Cancer Research and Development grants, and National Cancer Center (Grant Number: 2011-A-42).
Conflicts of interest
MA has declared conflicts related to lecture fees form Kyowa Hakko Kirin Co., Ltd. SO has declared conflicts related to lecture fees from Astra Zeneca K. K., Novartis Pharma K. K., and Chugai Pharmaceutical Co., Ltd. YF has declared conflicts related to conducting research sponsored by Kyowa Hakko Kirin Co., Ltd., Glaxo Smith Kline K. K., Sanofi-Aventis K. K., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Bio Development Center Limited, Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K. K., Pfizer Japan Inc., Janssen Pharmaceutical K. K., and Kissei Pharmaceutical Co., Ltd., and remunerations from Astra Zeneca K. K., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Glaxo Smith Kline K. K., Sanofi-Aventis K. K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K. K., Nippon Kayaku Co., Ltd., Novartis Pharma K. K., and Bristol-Myers Squibb K. K. All remaining authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ando, M., Yamauchi, H., Aogi, K. et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res Treat 145, 401–409 (2014). https://doi.org/10.1007/s10549-014-2947-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2947-1