Abstract
Aim
To evaluate the pCR rate and toxicity of the addition of weekly carboplatin (Cp) to paclitaxel (wP) and dose-dense (dd) epirubicin/cyclophosphamide (EC) in an open-label phase II study in TNBC patients.
Methods
Patients were included if they had stage II and III TNBC and received wP (80 mg/m2/week) concurrent with weekly Cp (AUC = 2) for 12 weeks, followed by bi-weekly epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2) plus granulocyte colony-stimulating factor (G-CSF) for four cycles, followed by surgery. The primary endpoint was the rate of pCR [(ypT0/isypN0)]. Secondary endpoints included safety and drug delivery.
Results
Sixty-three eligible patients were included. Median age was 51 years (range 29–74); 88.9% had stage II disease, 46% were clinically node positive, and 77.8% had grade 3 tumors. Fifty-four percent achieved a pCR. Twelve percent missed two or more doses of wP, whereas at least two cycles of EC were missed in 9.5%. The rate of tolerance without delays or dose reductions is very low (16%). Sixty-two percent had G3/4 neutropenia. Febrile neutropenia occurred in 18 patients of which more than eighty percent occurred during EC despite primary prophylaxis with G-CSF. Thrombocytopenia grade 3/4 was noticed in 11 pts. Three patients developed grade 3 peripheral neuropathy.
Conclusion
The addition of weekly carboplatin to neoadjuvant paclitaxel and dd EC leads to a pCR rate comparable to prior studies (54%). However, hematological toxicity and febrile neutropenia rate was unexpectedly high. Future investigations could focus on reversing the sequence, which may lead to better hematological tolerability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) consists of four major subtypes based upon microarray expression profile: the luminal subtypes A and B, which express hormone receptor-related genes, basal-like BC, and HER2-amplified BC [1, 2]. Triple-negative breast cancer (TNBC), which is defined by the absence of expression of the estrogen and progesterone receptors as well as the absence of Her 2 overexpression or gene amplification, accounts for approximately 15% of all newly diagnosed early breast cancers [3]. Unfortunately, TNBC has in most cases a dismal prognosis due to the high pathologic grade of the tumor, young age of onset, the presence of c-Myc amplification and p53 mutation. Despite the high acute efficacy of combination chemotherapy, a significant proportion will develop metastatic disease, with a median survival of 12–18 months [4]. This highlights the need for more efficacious systemic therapies for this subtype of BC [5]. From a molecular point of view, TNBC is a heterogeneous disease based on transcriptional and mutational heterogeneity as well as numerous copy number variations. TNBC biology is characterized by an increased immunological infiltrate, a basal-like and a mesenchymal phenotype, and a deficiency in homologous recombination [6]. The prevalence of BRCA1, BRCA2 or other germline mutations and phenotypic “BRCAness” in TNBC is estimated to be approximately 20–30%. Genomic instability in the homologous recombination repair genes provides specific therapeutic opportunities for the use of DNA double-strand break-inducing agents, including platinum salts, anthracyclines, cyclophosphamide and poly-ADP-ribose polymerase (PARP) inhibitors [7,8,9]. Two large neoadjuvant trials in TNBC, the GeparSixto and CALGB (Cancer and Leukemia Group B) 40603 trial, have reported that the addition of platinum to an anthracycline/taxane sequential regimen could significantly increase the pathologic complete response (pCR) rate from respectively 36.9–53.2% and from 41 to 54% in unselected triple-negative breast cancers [10]. Dose-dense therapy approaches are particularly effective in the hormone receptor-negative subgroup as shown in a meta-analysis of 3337 patients from ten clinical studies [11]. Two meta-analyses of platinum-based neoadjuvant chemotherapy in TNBC could demonstrate a significant improvement in pCR for patients treated with cisplatin or carboplatin combination (p = 0.019), but this was associated with a higher risk of hematological toxicities and no significant difference in overall survival (OS) (p = 0.09) [12, 13]. For BRCA-mutant patients, there was a non-significant benefit for the addition of carboplatin (p = 0.61), compared to patients without a BRCA mutation (p < 0.001) [13]. However, the routine clinical use of carboplatin remains controversial because of the prospect of increased toxicity and unclear long-term benefits regarding survival. Since pCR is a surrogate marker for prediction of long-term clinical disease-free survival and OS in TNBC, it can be used as an endpoint in the exploration of new therapeutic options [14, 15].
We performed a multicenter Belgian phase II trial with the hypothesis that the addition of weekly platinum to the anthracycline/taxane chemotherapy backbone could lead to a similar high pCR rate compared to a schedule wherein carboplatin is given every 3 weeks, but with more manageable hematological and non-hematological toxicities.
Patients and methods
Study design
This is a prospective multicenter phase II study performed in Belgium under the umbrella of the Belgian Society of Medical Oncology (BSMO) Breast Cancer Task Force. Patients older than 18 years, with an operable stage II or III (excluding inflammatory breast cancer) previously untreated TNBC were included after signing informed consent. The study was approved by the ethics committee of each participating center.
Triple-negative was defined as estrogen and progesterone receptor expression < 10% and no Her2 amplification as defined by Her2 IHC 0-1 or FISH ratio less than 2. (ASCO/CAP guideline recommendations for HER2 testing) [16]. Other previous or concomitant invasive cancers, except for a localized squamous cell cancer or basal cell cancer of the skin or an in situ squamous cell cancer of the cervix, were not allowed. Adequate bone marrow, hepatic function and normal left ventricular ejection function (> 55% by ultrasound or MUGA scan) were required. A creatinine clearance above 40 ml/min according to the local laboratory standard was needed to enter the study. For patients aged 65 or older a G8 geriatric screening test above 14 on a total of 17 was warranted [17]. Patients with a pre-existing grade ≥ 2 polyneuropathy were not eligible for the study.
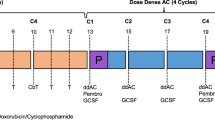
In the first part of the neoadjuvant scheme, all patients received weekly paclitaxel (wP) 80 mg/m2 concurrent with weekly carboplatin (Cp) at an area under the curve (AUC) dose of 2 for 12 weeks followed by epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 (EC) bi-weekly with myeloid growth factor support on day 2 for four cycles (Fig. 1).
If progression occurred after the first part, the treating physician could decide to go immediately for part two of the neoadjuvant regimen or discuss with the surgeon the indication for immediate surgery. Dose adjustments according to the following guidelines were allowed. Weekly P and Cp were postponed one week if absolute neutrophil count (ANC) < 1000/µl or platelet count < 75.000/µl and were definitively reduced by 25% after one or two weeks’ recovery in case of neutropenia less than 500/µl or febrile neutropenia. Delayed cycles longer than 2 weeks could not be recovered. In case of peripheral neuropathy (PNP) grade 2, only wP was reduced by 25% for all subsequent cycles, and when a grade 3 or 4 PNP occurred paclitaxel was suspended until resolution to grade 1 and restarted at a dose of 60 mg/m2. Dose-dense EC was delayed without dose reduction for ANC < 1000/µl and platelet count < 75.000/µl and was reduced at a dose of 500 mg/m2 for C and 75 mg/m2 for E, in case of prolonged ANC < 500/µl or febrile neutropenia or platelet count < 50.000/µl. All four cycles were to be administered unless patient consent withdrawal, toxicity or disease progression prohibits the completion of the EC part. Tumour assessment was planned after part one and two of the neoadjuvant systemic therapy. The extent of surgery and subsequent irradiation was according to the local guidelines of the participating physicians, and no further adjuvant chemotherapy was foreseen in the study although this was at the discretion of the investigator.
Study objectives
The main objectives of the study were clinical activity, drug delivery, and safety. The primary endpoint was the total pCR rate, which was defined as no invasive cancer in the breast and resected axillary lymph nodes (ypT0/isypN0). Toxicity, drug delivery, clinical response, event-free survival (EFS) and OS were the secondary endpoints. EFS was defined as the time between the initiation of the neoadjuvant chemotherapy and occurrence of an event (local or distant recurrence or new primary BC), while OS was defined as the time between treatment initiation and death from any cause and censored at the last visit or patient loss to follow-up.
Assessment of response and safety
Baseline mammography and ultrasound were done in every patient, whereas magnetic resonance imaging was as per clinical indication. Fine needle aspiration was indicated in case of a clinically suspicious axillary lymph node, and a pre-treatment sentinel biopsy was done in patients with a clinically or cytological negative axilla. Additional staging exams, such as CT scan of the body and bone scan, were performed per local practice.
At the end of part one and two radiologic tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) preferentially by mammography and sonography of the breast and MR if clinically indicated. If progression occurred after part one, the treating physician could decide to go for surgery or to continue with part two.
Histopathologic evaluation of response after neoadjuvant chemotherapy was done in accordance to the Pinder tumour response system [18]. Pathologic response was evaluated locally without a central pathologic review. The principal investigator centrally reviewed all surgical pathology reports. Germline testing for BRCA1/2 was performed utilizing commercially available tests according to routine local practice.
To assess treatment safety, we classified the expected adverse events (AEs), including hematological, non-hematological and peripheral neurologic toxicities according to the NCI-CTCAE criteria v 4.03. We also calculated the missed doses of part one and two and the rate of treatment discontinuation.
Statistical analysis
The study sample size was calculated according to the optimal Simon’s two-stage design method. The target sample size was 63 patients with an 80% power to detect a pCR rate of ≥ 47% (α = 0.05). The optimal Simon two-stage design was used to test the null hypothesis (H0) that the weekly regimen of paclitaxel and carboplatin followed by dose-dense EC elicit a pCR (ypT0/is, ypN0) rate in a cohort of triple-negative patients of ≤ 30% versus the alternative hypothesis (H1) that the observed pCR rate is ≥ 47% at a 0.05 level of significance and 80% power.
This design specified that 21 patients could be enrolled in the first stage and 42 in the second stage, with a requirement of seven or more patients with a pCR rate in stage one to continue in stage two. If 30 or more achieved a pCR rate of a total of 63 patients than the regimen should be accepted. If seven or less of the initial 21 patients enrolled during the first stage achieved a pCR rate, then the trial would be terminated early and the regimen considered insufficiently active.
The data were analyzed using descriptive statistics. Continuous variables were presented as means, standard deviation, median, minimum and maximum. Discrete variables were presented as frequencies and percentages. IBM SPSS Statistics (version 25.0) was used for statistical analysis. Missing data were not replaced nor extrapolated.
Results
Patient characteristics
Between June 2015 and May 2016, 65 patients were included in the study. (CONSORT diagram is shown in Fig. 2). Two patients were excluded from the analysis: one received doxorubicin instead of epirubicin and one refused surgery and therefore both were not assessable for the primary endpoint. After the accrual of the prospectively intended 63 patients, centers continued to use the same regimen in another 20 patients, which were registered but not included in the current analysis. These patients will be in the subject of a later report on the correlation between whole exome sequencing and treatment outcome. Table 1 describes the demographic and baseline clinical characteristics of the intention-to-treat (ITT) population. Most of the patients were between 40 and 60 years old, with a median age of 51 years. Eighty-nine percent of the patients were stage IIA or IIB, with the majority T2 tumors and clinically node-negative disease. Ninety-seven percent of the patients were diagnosed with invasive ductal carcinoma and in particular with a grade 3 tumor in a vast majority. Other histologies were lobular carcinoma and mixed ductal/lobular carcinoma. Breast-conserving surgery was performed in 44 patients and axillary dissection in 36 patients. Routine germline BRCA1/2 testing results were available for 81% of the patients of which 17.5% carried a deleterious BRCA1/2 mutation (nine patients).
Clinical efficacy
Primary endpoint
All eligible patients received surgery after neoadjuvant chemotherapy. A pCR (ypT0/is ypN0) was obtained in 34 of 63 patients (54%) (Table 2). The correlation of the BRCA1/2 germline status with the response is shown in Fig. 3. (Figure 3) The highest pCR rate was obtained in patients with a BRCA1/2 germline mutation. Of the nine patients with a BRCA 1 or 2 germline mutation, seven (77.8%) reached a pCR after neoadjuvant chemotherapy (Table 2). There is a trend but no significant correlation between pCR and BRCA 1/2 status (p = 0.27).
Secondary endpoints
Radiologic complete and partial responses were observed in 25 and 29 patients, respectively. Five patients had stable disease, four patients had no radiologic evaluation after the end of the preoperative chemotherapy, and none of the patients had progressive disease while on neoadjuvant therapy.
Drug delivery and safety
Eight patients missed two or more doses of weekly carboplatin, and six patients skipped two or more doses of EC. More patients required a dose reduction of EC (32%) compared to weekly Cp and wP (27%). Because of treatment delays and dose reductions, only 19 (30%) patients received all planned doses of Cp and wP over 12 weeks, while 24 (38%) patients received all planned doses of EC. Only ten patients could tolerate the whole treatment plan without any delay or dose adaption. The mean relative dose intensity for paclitaxel for the entire group was 84.5% (SD 14) and for carboplatin 83.2% (SD 16.9).
There was no significant difference between the mean relative dose intensity for paclitaxel (85.5% vs. 83.5%), or for carboplatin (85.8% vs. 80.1%) between patients achieving a pCR and those who did not (p = 0.6; p = 0.2 respectively). There was also no difference in the mean relative dose intensity for paclitaxel in BRCA1/2 mutant patients versus BRCA1/2 wild-type patients (83.4% vs. 84.5%; p = 0.8), and neither for carboplatin (84.3% vs. 82.4%; p = 0.8).
Table 3 gives an overview of the grade 3 and 4 hematologic and non-hematological toxicities. Grade 3 and 4 neutropenia and febrile neutropenia were observed in 62% and 29% of the patients, respectively.
The total number of serious AEs involving significant toxicity or toxicity requiring hospitalization or surgical intervention was 27. They comprised febrile neutropenia, neutropenia G4, suicide attempt, anemia, central catheter infection, cystitis, depression, diverticulitis, septicemia and pulmonary embolism.
Event-free survival and overall survival
The median follow-up was 22 months (SD 4 months). EFS and OS Kaplan–Meier curves are shown in Figs. 4 and 5. Nine out of 63 patients (14.3%) relapsed; two locoregional relapses only and seven with distant metastases only. Eight of these patients did not reach a pCR after neoadjuvant chemotherapy. Five succumbed due to metastatic disease and four patients are still alive.
Discussion
TNBC has a dismal prognosis especially if the primary treatment fails to induce a pCR [14, 15]. In 2010, Byrski et al. published the results of neoadjuvant chemotherapy in 102 women with a germline BRCA 1 mutation: In the subgroup of patients treated with neoadjuvant cisplatin,10 out of the 12 patients achieved a pCR [19]. These data generated a resurgence of interest in the use of platinum-based chemotherapy especially in patient populations possibly enriched for a defective DNA repair capacity such as in TNBC. In particular, homologous recombination deficiency (HRD) could lead to increased sensitivity to cisplatin as the HR pathway is also involved in the repair of platinum-induced DNA damage [20].
In the current study, we have observed a high pCR rate after neoadjuvant chemotherapy with Cp and wP followed by EC combination, particularly in the BRCA mutant population.
In addition to the study reported here, four larger and randomized trials have interrogated the efficacy of the platinum in combination with a taxane/anthracyclines backbone as neoadjuvant therapy for early TNBC patients. The largest study was the BrighTNess trial which was designed to assess the activity of combining Cp AUC 6 with or without veliparib, with the standard chemotherapy sequence of wP followed by doxorubicin and cyclophosphamide. The pCR rate in the breast/axilla in the patients treated with Cp increased from 31 to 57.5%. Veliparib did not significantly affect these results. Neutropenia, thrombocytopenia, anemia, nausea, and vomiting were increased with the addition of Cp, whereas veliparib did not affect toxicity [21].
The CALGB 40603 trial randomized 443 patients between wP with or without Cp AUC 6 every 3 weeks for four cycles ± bevacizumab followed by doxorubicin and cyclophosphamide every 2 weeks for four cycles. The addition of Cp did significantly increase the pCR rate in the breast/axilla from 41 to 54% (p = 0.0029) with an EFS and OS far superior to those left with residual disease. Patients assigned to the Cp or bevacizumab arm were less likely to complete wP and dose-dense anthracycline/cyclophosphamide without missed doses, dose modification or early discontinuation due to treatment-related toxicities. Grade ≥ 3 neutropenia, febrile neutropenia, and thrombocytopenia occurred more often with Cp while hypertension, infection, thromboembolic events, bleeding and postoperative complications with bevacizumab [22].
In the sixth German Preoperative trial, 315 TNBC patients received wP 80 mg/m2, pegylated liposomal doxorubicin 20 mg/m2 once per week, and bevacizumab 15 mg/kg every 3 weeks with or without weekly Cp AUC 2 for 18 weeks. Hematological side effects were more common in the arm with Cp and included significantly more grade 3 neutropenia 65% versus 27%, more grade 3 anemia 15% versus < 1% and more grade 3 thrombocytopenia 14% versus 1%, but no significant difference in G3 febrile neutropenia (p = 0.14) were observed. The use of Cp was associated with dose discontinuation in 48% compared to 39% without Cp (p = 0.031). Despite a high rate of grade ≥ 3 hematologic toxicities and early treatment discontinuation, pCR rate breast/axilla rose from 43 to 57% with the addition of Cp (p = 0.015). The 3-year disease-free survival was 85% in the Cp arm compared to 76.1% in the control group (HR 0.56, 95% CI 0.33–0.96, p = 0.035) [23]. In the GEICAM 2006-03 study, a randomized phase II, 94 patients received four cycles of EC followed either by docetaxel (100 mg/m2) or docetaxel (75 mg/m2) plus Cp AUC 6 for four cycles. A pCR breast/axilla rate of 30% was reported in both arms as well as similar G3/4 toxicity in both arms [24].
The pCR rate breast/axilla of the present BSMO study is in line with the three previously mentioned trials in which Cp was administered q3–4 weeks. In an attempt to mitigate the added toxicity of Cp we introduced a weekly fractionated dosing of Cp at an AUC of 2 in the current study. Despite this fractionation, we still observed a quite high rate of febrile neutropenia and a dose reduction for the wP and platinum combination in 27% of the patients. Dose reductions were even more frequent in the sequential EC cycles.
The current schedule with weekly Cp and wP followed by EC is thus associated with a high pCR rate similar to Cp given every 3 weeks but does not seem to have lower hematological toxicity rates (mainly during the EC part) [22]. There may be several solutions to this. A first hypothesis could be that the hematological tolerability of the EC part after weekly Cp and wP could be better after 1–2 weeks additional break after the last Cp and wP (in the current regimen, EC started 1 week after the last Cp and wP).
Second, the chemotherapy could start with EC, followed by weekly Cp and wP. In this sequence, the weekly Cp and wP administration may allow more flexibility compared to 3-weekly Cp for dose and schedule modifications. Moreover, after 8 weeks of EC, the decision of adding Cp or not can also be taken with more detailed information on the response to EC, and with available genetic information by that time.
Third, further research should evaluate how vital the EC part (including the dose reductions) is in these patients and in particular in patients with a BRCA1/2 mutation.
In patients who were not fit enough to go for an anthracycline combination, Cp/docetaxel combination alone was examined in a study of Sharma P et al. [25]. The Cp/docetaxel regimen, applied in 190 patients was well tolerated with only 28% of the patients experiencing G3–4 adverse events. A pCR rate of 59% was obtained in the BRCA-associated TNBC patients and 56% in the wild type. Patients with stage III disease in this study were, however, less likely to respond to this combination compared to stage I–II disease (pCR rate: 37% vs. 63%, respectively; p = 0.002)). The ongoing NCT02413320 trial is the first randomized study to assess whether similar pCR rates can be achieved with or without an anthracycline combination in TNBC patients.
The optimal dose, sequence and chemotherapy backbone for efficient incorporation of platinum in the treatment of early-stage TNBC are not yet well known. Several ongoing randomized phase III trials are evaluating various schedules and combinations of platinum salts in early TNBC, such as the EA1131 trial comparing the efficacy of adjuvant carboplatin vs capecitabine in patients with residual disease post neoadjuvant chemotherapy [25].
In the current study, we have observed a trend towards a high pCR rate (80%) in patients with a BRCA1/2 germline mutation. The TNT trial in metastatic TNBC also strongly supports the use of platinum in patients with a germline BRCA1/2 mutation [26]. We are currently investigating the BRCA wild type patients with exome analysis to potentially identify other HRD deficiencies which were not examined so far. Of course the small number of patients and the non-randomized design will make it difficult to draw final conclusions.
To conclude, the current study is the first phase II trial to demonstrate a high pCR rate of 54% with weekly platinum and wP combination followed by dd EC as neoadjuvant treatment in unselected triple-negative breast cancer patients and a pCR rate of almost 80% in the small BRCA-mutated subpopulation. Hematological toxicity, however, was not decreased by this fractionation compared to the 3-weekly Cp arm in the CALGB 40603 trial [22]. Further research is needed to optimize carboplatin containing neoadjuvant chemotherapy regimens with high efficacy but toxicity as low as possible.
References
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumors. Nature 406:747–752
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Castrellon AB, Pidhorecky I, Valero V, Reaz LE (2017) The role of carboplatin in the neoadjuvant chemotherapy treatment of triple negative breast cancer. Oncol Rev 11:324
Kennecke H, Yerushalmi R, Woods R et al (2010) Metastatic behaviour of breast cancer subtypes. J Clin Oncol 28:3271–3277
Szekely B, Silber AL, Pusztai L et al (2017) New therapeutic strategies for triple negative breast cancer. Oncology (Williston park) 31:130–137
Denkert C, Liedtke C, Tutt A, von Minckwitz G (2017) Molecular alterations in triple-negative breast cancer: the road to new treatment strategies. Lancet 389:2430–2442
Stockmans G, Deraedt K, Wildiers H, Moerman P, Paridaens R (2008) Triple-negative breast cancer. Curr Opin Oncol 20:614–620
Collignon J, Lousberg L, Schroeder H, Jerusalem G (2016) Triple-negative breast cancer and solutions. Breast Cancer 8:93–107
Yang F, Kemp CJ, Henikoff S (2015) Anthracyclines induce double-strand DNA breaks at active gene promotors. Mutat Res 773:9–15
Oualla K, El-zawahry HM, Arun B et al (2017) Novel therapeutic strategies in the treatment of triple-negative breast cancer. Ther Adv Med Oncol 9:493–511
Bonilla L, Ben-Aharon I, Vidal L et al (2010) Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 102:1845–1854
Poggio F, Bruzzone M, Ceppi M et al (2018) Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systemic review and meta-analysis. Ann of Oncol 7:1497–1508
Petrelli F, CoinuA Borgonovo K et al (2014) The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 144:223–232
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Cortazar P, Zhang L, Untch M et al (2014) The CTNeoBC pooled analysis. Lancet 384:164–172
Tchrakian N, Flanagan L, Harford J (2016) New ASCO/CAP guideline recommendations for Her2 testing increase the proportion of reflex in situ hybridization tests and of her2 positive breast cancers. Virchows Arch 468:207–211
Decoster L, Kenis C, Van Puyvelde K et al (2013) The influence of clinical assessment (including age) and geriatric assessment on treatment decisions in older patients with cancer. J Geriatr Oncol 4:235–241
Pinder SE, Provenzano E, Earl H et al (2007) Laboratory handling and histology reporting of breast specimensfrom patients who received neoadjuvant chemotherapy. Histopathology 50:409–417
Byrski T, Gronwald J, Huzarski T et al (2010) Pathologic complete response rates in young women with BRCA-1 positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28:375–379
D’Andrea AD (2010) Susceptibility pathways in Fanconi’s anemia and breast cancer. N Engl J Med 362:1909–1919
Loibl S, o’Shaughnessy J, Untch M et al (2018) Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomized, phase 3 trial. Lancet Oncol 19:497–509
Sikov WM, Berry DA, Perou CM et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple negative breast cancer. CALGB 40603 (Alliance). J Clin Oncol 33:13–21
von Minckwitz G, Schneeweiss A, Loibl S et al (2014) Neoadjuvant carboplatin in patients with triple negative and Her2-positive early breast cancer (GeparSixto; GBG 66): a randomized phase 2 trial. Lancet Oncol 15:747–756
Alba E, Chacon JI, Anton A et al (2012) A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting: results from the GEICAM/2006-03 multicenter study. Breast Cancer Res Treat 136:487–493
Sharam P, Lopez-Tarruella S et al (2017) Efficacy of neoadjuvant carboplatin plus docetaxel in triple negative breast cancer: combined analysis of two cohorts. Clin Cancer Res 23(3):649–657
Tutt A, Tovey H, Cheang MCU et al (2018) Carboplatin in BRCA 1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med 24:628–637
Acknowledgements
We want to acknowledge all the co-investigators of the BSMO, the pathology departments, data nurses of the participant sites, and the patients.
Funding
The study was financially supported by Amgen and Teva.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fontaine, C., Renard, V., Van den Bulk, H. et al. Weekly carboplatin plus neoadjuvant anthracycline-taxane-based regimen in early triple-negative breast cancer: a prospective phase II trial by the Breast Cancer Task Force of the Belgian Society of Medical Oncology (BSMO). Breast Cancer Res Treat 176, 607–615 (2019). https://doi.org/10.1007/s10549-019-05259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05259-z