Abstract
Chemotherapy remains as the only systemic treatment option available for basal-like breast cancer (BC) patients. Preclinical models and several phase II studies suggested that platinum salts are active drugs in this BC subtype though there is no randomized study supporting this hypothesis. This study investigates if the addition of carboplatin to a combination of an alkylating agent together with anthracyclines and taxanes is able to increase the efficacy in the neoadjuvant treatment context. Patients with operable breast cancer and immunophenotypically defined basal-like disease (ER-/PR-/HER2- and cytokeratin 5/6+ or EGFR+) were recruited. Patients were randomized to receive EC (epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 for 4 cycles) followed either by D (docetaxel 100 mg/m2 × 4 cycles; EC–D) or DCb (docetaxel 75 mg/m2 plus carboplatin AUC 6 × 4 cycles; EC–DCb). The primary end point was pathological complete response (pCR) in the breast following the Miller and Payne criteria. Ninety-four patients were randomized (46 EC–D, 48 EC–DCb). pCR rate in the breast was seen in 16 patients (35 %) with EC–D and 14 patients (30 %) with EC–DCb (P value = 0.61). pCR in the breast and axilla was seen in 30 % of patients in both arms. The overall clinical response rate was 70 % (95 % CI 56–83) in the EC–D arm and 77 % (95 % CI 65–87) in the EC–DCb arm. Grade 3/4 toxicity was similar in both arms. The addition of carboplatin to conventional chemotherapy with EC–D in basal-like breast cancer patients did not improve the efficacy probably because they had already received an alkylating agent. These findings should be taken into consideration when developing new agents for this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is a heterogeneous disease, comprised of distinct biological subtypes with different natural history, presenting a varied spectrum of clinical presentation, pathologic and molecular features with different prognostic and therapeutic implications.
One of the most widely studied classifications of BC was proposed by the Perou group, which discriminates at least five intrinsic subtypes: luminal A, luminal B, HER2-enriched, basal-like (BL), and normal-like [1–5]. The BL subtype accounts for 10–25 % of all new cases of breast cancer (depending on the population demographic characteristics) and represents 50–75 % of the triple negative tumors [6]. From a biological point of view, these are tumors with a high proliferation rate as most of them are deficient in Rb1 y p53. In addition, they are associated with BRCA1 mutations. The deficiency in Rb1, p53, and BRCA1 is producing a high rate of aneuploidy and chromosomal translocations and deletions in these tumors [7].
The BL subtype is characterized by having a RE-/RP-/HER2- and CK 5/6+ or EGFR+ profile by means of immunohistochemistry [8]. From a clinical point of view, those patients have an early recurrence pattern and shorter DFS and OS than the other subtypes [9, 10].
BL patients treated with anthracycline and taxanes-based neoadjuvant chemotherapy (CT) generally achieve high pCR rates [10–12]; however, their prognosis is the worst when compared to patients with other BC subtypes. There have been attempts to treat these patients with CT in combination with drugs inhibiting the EGFR pathway like cetuximab [13–15] or the DNA repair [16]. A high proportion of the triple negative patients have a BRCA1 functional alteration; these tumors are sensitive to the interstrand cross-linking agents like the platinum salts. Based on that, these drugs have been tested in monotherapy in phase II trials in the neoadjuvant setting in triple negative patients, achieving pCR rates of 22–83 %, being the higher ones in patients with BRCA1 mutations [17, 18]. However, there is no randomized study exploring if the addition of a platinum salt to a standard regimen with alkylating agents, anthracycline, and taxanes is able to improve the efficacy in these patients.
This phase II randomized study investigates for the first time if the addition of carboplatin to one of the most used standard CT combinations is able to increase the pCR rate in basal-like BC patients.
Patients and methods
Eligibility criteria
Patients >18-years old and with histologically confirmed (by surgical or core biopsy) basal-like breast cancer, defined as ER negative, PgR negative, HER2 negative, and cytokeratin 5/6 or epidermal growth factor receptor (EGFR) positive by immunohistochemistry (IHC), were included. Tumor size had to be >2 cm or less if there was axillary involvement (pathologically confirmed). Patients were required to have ECOG performance status ≤1, normal cardiac function, and adequate bone marrow reserve and liver and renal functions. Patients were excluded if they had received previous treatment for the present disease, previous anthracycline and/or taxane administration, have concurrent treatment with corticosteroids, selective estrogen-receptor modulators or hormonal replacement therapy, had inflammatory, bilateral invasive, or metastatic breast cancer, or if they had any other severe or uncontrolled systemic disease. Adequate contraception and a negative pregnancy test were required for women with child-bearing potential. Patients with a previous history of cancer other than skin (no-melanoma), or cervix tumors adequately treated and other cancers treated more than 10 years before the study entry, were also excluded.
Study design and treatment plan
This was a multicenter, open label, randomized phase II trial. All eligible patients were randomly assigned, in a 1:1 ratio, to receive neoadjuvant CT without carboplatin (standard arm) or with carboplatin (experimental arm) in blocks of four. Randomization was centralized at the GEICAM headquarters. Patients were stratified according to institution, tumor size (<1 vs 1–2 vs 2–5 vs >5 cm), histological tumor grade (I vs II vs III), and axillary status (N0 vs N1/2).
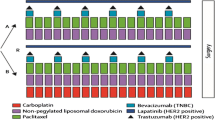
Standard CT (EC–D) consisted of epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 both administered intravenously (iv) on day 1 every 21 days, for four cycles (first sequence) followed by docetaxel 100 mg/m2 administered iv on day 1 every 21 days for four cycles (second sequence). Experimental CT (EC–DCb) consisted of the same first sequence followed by a second sequence with docetaxel 75 mg/m2 plus carboplatin AUC 6 administered iv on day 1 every 21 days for four cycles (Fig. 1). After the neoadjuvant therapy, patients underwent mastectomy or conservative surgery plus axillary lymph node dissection (unless previous negative sentinel lymph node biopsy). Postoperative treatment was left under the investigator’s criteria.
This trial was approved by the local Ethical Review Boards and the Spanish Ministry of Health, and registered in Clinical Trials. Gov with the number NCT00432172. The trial was conducted in compliance with Good Clinical Practices and the tenets of the Declaration of Helsinki. All patients provided written informed consent before entering the study.
Assessments and endpoints
The primary endpoint of the study was pathological complete response (pCR) rate in the breast. Secondary endpoints included safety, clinical response, breast conservative surgery rate, and axillary node status at the time of surgery.
Before study entry, all patients underwent a breast and axillary disease assessment by physical examination, bilateral mammography, or magnetic resonance imaging (MRI). In addition, patients had ECOG performance status evaluation, surgical or core biopsy, complete blood cell count, serum chemistry, electrocardiogram (ECG), and left ventricular ejection fraction measurement (if clinically indicated).
pCR was assessed at surgery following the Miller and Payne criteria [19]. Clinical response was evaluated according to the RECIST criteria after the first sequence and before surgery [20]. Adverse events were graded following the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0 [21]; the worst grade for each patient was reported.
Tumor tissue assessment
ER, PgR, cytokeratin 5/6, and EGFR status was assessed by immunohistochemistry (IHC) in a central laboratory on formalin-fixed paraffin-embedded (FFPE) tumor sections using Clone SP1 (ER), Clone PGR Y85 (PR), Clone D5/16B4 (CK5/6) (Master Diagnostica, Sevilla, Spain), and Clone 2-18C9 (EGFR) (DakoCytomation, Glostrup, Denmark). ER and PR status were considered as positive if >1 % of tumor cells showed nuclear staining. CK5/6 and EGFR tumor immunoreactivity was scored into two categories: negative (IHQ 0) and positive (IHQ 1+, 2+, 3+). HER2 was evaluated by IHQ using HercepTest™ (DakoCytomation) and FISH (in case of IHC 2+) using PathVysion kit (Abbott Park, IL, USA).
Sixty-nine patient samples were also gene expression profiled using the PAM50 classifier performed by Nanostring nCounter technology and analyzed by means of the clinical algorithm for subtype prediction [22, 23]. Samples were assigned into the following intrinsic subtype categories: luminal A, luminal B, HER2-enriched, basal-like, and normal-like.
Statistical methods
A randomized phase II design was chosen not to enable comparison of the efficacy of the two arms, but to explore whether the EC–DCb efficacy precluded a comparison with EC–D in a formal randomized phase III trial.
The sample size was calculated for the experimental arm by the 2-stage Simon method based on the primary study objective, pathological complete response. Sample size was based on a null hypothesis (H0) of pCR of 25 % and an alternative hypothesis (H1) of an assumed pCR of 45 %. Assuming an alpha error of 0.05 and a test power of 80 %, forty-three evaluable patients were required to be recruited in the EC–DCb arm. No minimum response criterion was proposed for the EC–D arm because it was considered the gold standard.
The analyses of the primary endpoint and safety were performed on the evaluable population defined as the randomized and treated patients. The number and proportion of pCRs in each treatment arm and their two-sided 95 % confidence intervals were analyzed for the overall response analysis.
Results
Patient characteristics
Between April-2007 and January-2010, 94 patients from 21 participating centers were included and randomized (46 patients to EC–D and 48 to EC–DCb). Patient characteristics were well balanced between treatment arms. Median age was 47 years (range 27–75 years). Sixty-six per cent of the patients were premenopausal, 85 % had a histological diagnosis of ductal carcinoma, and 3, 23, and 73 % had histological grade I, II, and III, respectively. Sixty-nine per cent of patients have T2 tumors and 52 % positive axillary lymph-nodes (Table 1).
All patients were ER/PgR/HER2 negative, 80 patients (85 %) were positive for CK 5/6, 78 patients (83 %) were positive for EGFR, and 64 patients (68 %) were positive for both.
Sixty-nine patient samples were analyzed by PAM50 and 61 of the tumors (88 %) were classified as basal-like by this test.
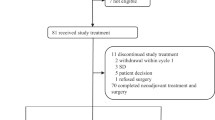
Figure 2 shows the consort study flowchart. One patient never received treatment, thus was not considered evaluable for efficacy or safety. Seventeen patients, eleven and six in the EC–D and EC–DCb arms, respectively, discontinued the treatment early. Reasons for discontinuation were toxicity in 7 patients, disease progression in 6 patients, and other reasons in 4 patients. Thus, 76 patients, 35 in the standard arm and 41 in the experimental arm, completed the treatment as planned.
Efficacy
The efficacy data are shown in Table 2. The pCR rate was 35 % (95 % CI 21–49) in the EC–D arm and 30 % (95 % CI 17–43) in the EC–DCb arm (not meeting the pre-specified goal of 45 %) (P value = 0.606).
A pathological complete response in the breast and axilla was achieved in 30 % of the patients in both treatment arms.
The overall clinical response rate was 70 % (95 % CI 56–83) in the EC–D arm (33 % CR and 37 % PR) and 77 % (95 % CI 65–87) in the EC–DCb arm (34 % CR and 43 % PR) (P value = 0.445). Nine patients, 7 in the EC–D arm and 2 in the EC–DCb arm progressed during treatment.
Mastectomy rate was 33 % (95 % CI 19–46) in the EC–D arm and 28 % (95 % CI 15–40) in the EC–DCb arm (P value = 0.603).
Safety
Adverse events were similar in both groups (Table 3). Adverse events of grade 3–4 were observed in 54 % of patients receiving EC–D and 55 % of those who received EC–DCb (P value = 1).
Patients treated with EC–D experienced more neutropenia, febrile neutropenia, fatigue, and infection (without a statistical difference); patients in the EC–DCb arm have more anemia and thrombocytopenia.
Discussion
Triple negative (TN) breast cancer is biologically characterized by having a high proliferation rate. From a clinical point of view, TN patients have a high recurrence rate after primary treatment [7, 9, 10]. The BL subtype, defined as ER-/PR-/HER2- and EGFR or CK 5/6 positivity by IHC [8], accounts for 50–75 % of the TN tumors and have the highest proliferation rate (6). BLBC patients are associated with high pCR rates when treated with neoadjuvant chemotherapy [11, 12]. In addition, it has been shown that pCR in these patients is associated with a better DFS and OS, suggesting that pCR is a good surrogate marker of long-term outcome in this subtype [24].
In contrast to the other subtypes, BLBC does not have any therapeutic target to block to achieve a consistent therapeutic benefit. Till now, the use of EGFR or PARP inhibitors has not demonstrated any clinically meaningful result in spite of previous robust data in preclinical models [13, 14, 25]. The biological similarity between BL tumors and those with BRCA1 mutation, as well as the fact that, in preclinical models, they show high sensitivity to DNA damaging drugs (like alkylating agents), led the scientific community to the hypothesis that these drugs could be potentially useful in patients with this histological subtype [26, 27]. Several phase II studies have been performed with platinum salts in monotherapy in the neoadjuvant treatment of triple negative patients showing pCR rates of 22–83 % [17, 18]. Of note, the higher efficacy was seen in the BRCA1-mutated patients in whom pCR rates were up to 75 % [17, 18]. In the same way, there are also available data of the combination of taxanes and platinum salts in the treatment of triple negative breast cancer patients with interesting results [28–30]. From these data, it has been speculated that platinum salts are especially active in the basal-like subtype and they are now the CT backbone to which new targeted drugs have been associated with [13, 16, 25]. However, their actual value has never been demonstrated in a randomized study.
The GEICAM/2006-03 study (basal subtype) is the first trial exploring this issue. In this particular study, the addition of carboplatin AUC 6 to a combination of epirubicin, cyclophosphamide, and docetaxel did not demonstrate an increase in the activity. In fact, the pCR rates in the breast in both combinations were the same; 35 % in the EC–D arm and 30 % in the EC–DCb arm (P value = 0.606). The pCR rates in both the breast and axilla were 30 % in both treatment arms. The adverse event rate was also not different between the schedules.
The absence of an increase in the pCR rate makes the addition of carboplatin to a combination of alkylating agents plus anthracycline and taxanes will result in an increase in DFS with a longer follow-up very improbable.
This lack of improvement in efficacy with the carboplatin addition may be explained by a real lack of activity of platinum agents in these patients. Perhaps, the most probable explanation is that the platinum salts mechanism of action is biologically equivalent to the use of a classic alkylating agent like cyclophosphamide. In fact, combinations containing cyclophosphamide plus anthracyclines and taxanes have demonstrated high pCR rates in patients with triple negative tumors [11, 12, 24], even in BRCA1 mutation carriers [31].
Whether platinum salts, either in monotherapy or included in standard chemotherapy regimens, are more effective than alkylating agents in combination with anthracyclines and taxanes (like the one used in this study) in patients with BRCA1 mutation, is a question to be elucidated. Comparative studies in triple negative breast cancer patients using platinum salts in combination with taxanes against standard chemotherapy regimens are also warranted. So, in our opinion, until it is not demonstrated that new CT schedules with platinum salts are superior to ECD, the design of new therapeutic strategies including new targeted agents in this patient population should include standard chemotherapy regimens as a backbone.
References
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Børresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423
Perreard L, Fan C, Quackenbush JF, Mullins M, Gauthier NP, Nelson E, Mone M, Hansen H, Buys SS, Rasmussen K, Orrico AR, Dreher D, Walters R, Parker J, Hu Z, He X, Palazzo JP, Olopade OI, Szabo A, Perou CM, Bernard PS (2006) Classification and risk stratification of invasive breast carcinomas using a real-time quantitative RT-PCR assay. Breast Cancer Res 8:R23
Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L, Nobel A, Parker J, Ewend MG, Sawyer LR, Wu J, Liu Y, Nanda R, Tretiakova M, Ruiz Orrico A, Dreher D, Palazzo JP, Perreard L, Nelson E, Mone M, Hansen H, Mullins M, Quackenbush JF, Ellis MJ, Olopade OI, Bernard PS, Perou CM (2006) The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 7:96
Prat A, Perou CM (2011) Deconstructing the molecular portraits of breast cancer. Mol Oncol 5(1):5–23
Perou CM (2010) Molecular stratification of triple-negative breast cancer. Oncologist 15(5s):39–48
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10:5367–5374
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11:5678–5685
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–2334
Carey LA, Rugo HS, Marcom PK, Irvin W, Ferraro M, Burrows E, He X, Perou CM, Winer EP, on behalf of the Translational Breast Cancer Research Consortium (2008) TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer. J Clin Oncol 26 (15s):abstr 1009
O’Shaughnessy J, Weckstein DJ, Vukelja SJ, McIntyre K, Krekow L, Holmes FA, Asmar L, Blum JL (2007) Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. Breast Cancer Res Treat 106(1s):S32: abstr 308
Baselga J, Stemmer S, Pego A, Chan A, Goeminne J-C, Graas M-P, Kennedy J, Ciruelos Gil EM, Zubel A, Groos J, Kia U, Schneeweiss A (2010) Cetuximab + cisplatin in estrogen receptor-negative, progesterone receptor-negative, HER2-negative (triple-negative) metastatic breast cancer: results of the randomized phase ii BALI-1 tr. Cancer Res 70(24s):95s
O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364:205–214
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M, Dent R, Lubinski J, Narod S (2010) Pathologic complete response rates in young women with BRCA 1 positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28:375–379
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE (2010) Efficacy of neoadjuvant cisplatin in triple negative breast cancer. J Clin Oncol 28:1145–1153
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
National Cancer Institute (2006) Common terminology criteria for adverse events (NCI-CTCAE) version 3.0. http://ctep.cancer.gov/. Accessed 3 Apr 2012
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27:1160–1167
Arup Laboratories (2012) PAM50 Breast Cancer Intrinsic Classifier Information. http://www.aruplab.com/Lab-Tests/General-Oncology/PAM50/index.jsp. Accessed 3 April 2012
Loibl S, von Minckwitz G, Blohmer JU, Costa S, Eidtmann H, Fasching PA, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny G, Denkert C, Nekljudova V, Mehta K, Untch M (2011) pCR as surrogate in HER2-positive patients treated with trastuzumab. Cancer Res 71(24); supp:111s. S5–S4
O’Shaughnessy J, Schwartzberg LS, Danso MA, Rugo HS, Miller K, Yardley DA, Carlson RW, Finn RS, Charpentier E, Freese M, Gupta S, Blackwood-Chirchir A, Winer EP (2011) A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol 29(81s):abstr 1007
Scully R (2011) Interactions between BRCA proteins and DNA structure. Exp Cell Res 264(1):67–73
Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, Goel A, Barbieri V, Costanzo F, Boland CR, Venuta S (2003) BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer 88(8):1285–1291
Roy V, Pockaj BA, Northfelt DW, Allred JB, Liu H, Nikcevich DA, Mattar BI, Perez EA (2008) N0338 phase ll trial of docetaxel and carboplatin administered every two weeks as induction therapy for stage ll or lll breast cancer. J Clin Oncol 26(15S, pt1):21s Abstract 563
Chang HR, Glaspy J, Allison MA, Kass FC, Elashoff R, Chung DU, Gornbein J (2010) Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer 116(18):4227–4237
Kern P, Kimmig R, Kolberg HC, Pott D, Kalisch A, Otterbach F (2010) Neoadjuvant carboplatin and docetaxel for triple-negative breast cancer. Am Soc Clin Oncol Breast Cancer Symp. Abstract 279
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, Litton JK, Valero V, Meric-Bernstam F, Hortobagyi GN, Albarracin C (2011) Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and non carriers: a single institution experience. J Clin Oncol 29:3739–3746
Acknowledgments
Authors thank Dr. A. Plazaola from the Onkologikoa, Dr. S. Gonzalez from the H. Mutua de Terrassa, Dr. B. Munarriz from the Hospital la Fe, Dr. J. R. de la Haba from the H. U. Reina Sofia, Dr. A. Miguel from the Hospital Althaia Xarxa Assistencial Universitaria de Manresa, Dr. A. Arcusa from the Hospital Sanitari de Terrassa, Dr. M. Muñoz from the Hospital Clinic i Provincial, Dr. M. Gil from the Instituto Catalan de Oncologia de Hospitalet, Dr. R. Andres from the Hospital Universitario Lozano Blesa, Dr. A. Velasco from the Hospital universitario de la Princesa, Dr. J. M. Baena from the Hospital Puerta del Mar, Dr. P. Sanchez-Rovira from the Complejo Hospitalario de Jaen, and the respective pathology departments of the involved sites for their valuable support in the inclusion of patients in this study. This trial was partially supported by Pfizer S.L.U.
Disclosure
All authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of GEICAM.
Rights and permissions
About this article
Cite this article
Alba, E., Chacon, J.I., Lluch, A. et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat 136, 487–493 (2012). https://doi.org/10.1007/s10549-012-2100-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2100-y