Abstract
Objective
The main goal of the present research is to explore the potential link of body mass index (BMI) with different survival metrics in breast cancer patients. Our aim is to offer the latest and most thorough meta-analysis, assessing the strength and reliability of the connection that BMI has with prognostic indicators in this disease.
Patients and methods
As of January 2024, we conducted a systematic literature search across PubMed, Embase, Web of Science, and the Cochrane Library databases. Our search aimed to identify studies examining BMI as an exposure factor, with breast cancer patients constituting the study population, and utilizing adjusted hazard ratio (HR) as the data type of interest.
Results
The evidence synthesis incorporated a total of 61 eligible articles involving 201,006 patients. Being underweight posed a risk factor for overall survival (OS) in breast cancer patients compared to normal weight (HR 1.15, 95% CI 0.98–1.35; P = 0.08). Overweight or obesity, in comparison to normal weight, was a risk factor for OS (HR 1.18, 95% CI 1.14–1.23; P < 0.00001), disease-free survival (DFS) (HR 1.11, 95% CI 1.08–1.13; P < 0.00001), relapse-free survival (RFS) (HR 1.14, 95% CI 1.06–1.22; P = 0.03), and breast cancer-specific survival (BCSS) (HR 1.18, 95% CI 1.11–1.26; P < 0.00001), but not for progression-free survival (PFS) (HR 0.91, 95% CI 0.76–1.10; P = 0.33). Notably, in subgroup analyses, overweight patients achieved prolonged PFS (HR 0.80, 95% CI 0.64–0.99; P = 0.04), and compared to the obese population, the overweight cohort exhibited a significant difference in OS (HR 1.11, 95% CI 1.05–1.16; P < 0.00001) and DFS (HR 1.06, 95% CI 1.03–1.10; P = 0.0004), with a considerably stronger association. Furthermore, compared to HER- patients, HER + patients exhibited a greater predictive value for OS (HR 1.23, 95% CI 1.10–1.37; P = 0.0004), RFS (HR 1.30, 95% CI 1.03–1.64; P < 0.00001), and DFS (HR 1.10, 95% CI 1.03–1.17; P = 0.003).
Conclusions
The results of our meta-analysis reveal a notable association between BMI and various survival measures in breast cancer prognosis. These findings provide a solid basis for predicting breast cancer outcomes and implementing more effective therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer remains the most prevalent cancer type and the primary cause of cancer-related deaths among women worldwide [1]. The latest global cancer statistics from 2022 reveal a shift where female breast cancer has surpassed lung cancer, becoming the most common form of cancer [2]. Projections suggest that the global incidence of breast cancer in women is likely to continue rising by 2035 [3]. Epidemiological research has highlighted a notable increase in breast cancer incidence rates among women under 50 who balance professional careers and family obligations [4]. Identifying risk factors associated with the incidence and prognosis of breast cancer is, therefore, a pressing issue. Recent research has explored various risk factors, including age [5], dietary habits [6], smoking [7], alcohol consumption [8], among others, that may influence the development and outcomes of this disease. Alcohol consumption, obesity, physical inactivity, and excess weight play crucial roles in about 21% of breast cancer fatalities worldwide [9]. Numerous investigations have examined the influence of body mass index (BMI) on survival outcomes in breast cancer patients.
BMI, a globally recognized metric for assessing physical obesity and overall health status, has been identified as a prognostic risk factor for breast cancer in postmenopausal women [10]. Numerous investigations have demonstrated that interventions, such as exercise, weight loss through dietary changes, increased physical activity, and psychosocial support, can significantly enhance the quality of life for breast cancer survivors [11].
In a retrospective analysis of a research involving 3891 primary breast cancer cases (stages I to IV), Chen et al. [12] concluded that a low BMI was an independent prognostic factor for overall survival (OS) among younger patients. However, in a retrospective analysis of 418 triple-negative breast cancer (TNBC) patients, the relationship between BMI and OS or relapse-free survival (RFS) was not statistically significant [13]. A separate retrospective analysis involving 501 TNBC patients corroborated this finding, concluding that the presence of diabetes and BMI did not significantly impact the survival outcomes of TNBC patients [14]. Additionally, Pezo et al. determined through a large retrospective cohort analysis of 11,601 breast cancer patients that BMI had no significant effect on OS [15].
The relationship between baseline BMI and patient survival is complex, as its prognostic significance can be influenced by factors, such as hormone receptor status [16], menopausal status [17], and different treatment modalities. In the non-docetaxel-based chemotherapy group, no significant correlation was observed between BMI and disease-free survival (DFS) or OS [18]. Conversely, within the docetaxel-based adjuvant chemotherapy group, an increase in BMI category was associated with a decrease in both DFS and OS. Similarly, overweight or obese premenopausal patients did not exhibit a statistically significant benefit from anastrozole treatment in the ABCSG-12 trial [19]. However, in the large international trial BIG 1-98, BMI was found to have no impact on the efficacy of trozole compared to tamoxifen over a 5-year period [20]. Thus, the association between BMI and breast cancer prognostic risk remains a topic of ongoing debate. To address this, we present a pooled analysis involving the retrieval and extraction of current data, categorizing patients into underweight, normal weight, overweight, and obese groups based on their BMI values. Our objective is to enhance the accuracy and reliability of our findings through a more comprehensive synthesis and analysis of the data.
Materials and methods
Data sources and search
We conducted the study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) [21] guidelines, and it was prospectively registered with PROSPERO (CRD42023492002). We performed a systematic literature search in PubMed, Embase, Web of Science, and Cochrane Library up to January 2024 for English language studies that compared the effect of BMI on breast cancer patients. The search terms used were “breast neoplasms”, “Body Mass Index”, “obesity”, “prognosis”, “randomized controlled trial”, “controlled clinical study” and “clinical study”. Detailed search strategies are provided in Supplementary Table S1. Additionally, we carefully reviewed the reference lists of all eligible studies. Two researchers independently searched and evaluated the literature, and all discrepancies during the literature search were resolved by consensus.
Identification of eligible studies
The studies included research meeting specific criteria: (1) studies involving women diagnosed with breast cancer through histopathology, aged 18 or older; (2) incorporation of BMI as an exposure factor with clear definitions and stratification criteria; (3) availability of adequate data to report hazard ratios (HR); (4) evaluation of at least one survival indicator, such as overall survival (OS), recurrence-free survival (RFS), progression-free survival (PFS), disease-free survival (DFS), or breast cancer-specific survival (BCSS); (5) adoption of randomized controlled, cohort, or case-control study designs. Studies were excluded primarily for: (1) patients with severe illnesses impacting survival outcomes other than breast cancer; (2) inability to extract BMI-related data; (3) data unattainability; (4) lack of full-text access; (5) inclusion of reviews, letters, editorial comments, case reports, conference abstracts, pediatric articles, unpublished, or non-English articles.
Data extraction
Two researchers independently extracted data, resolving discrepancies through discussion or consultation with a third reviewer (Qiu-Shuang Li). The extracted information from studies encompassed the first author, publication year, study duration, country, design, sample size, age, BMI classification, breast cancer type, overall prognosis-related data, and subgroup data. Studies related to BMI data included OS PFS, DFS, RFS, and BCSS. When data were missing or unreported, corresponding authors were contacted for complete information. Pre-diagnostic HR data took precedence over post-diagnostic HR data. Furthermore, when studies presented both univariate and multivariate analyses, the multivariate analysis data were selected.
Quality assessment
Data extraction was independently carried out by two researchers, Yu-huan Kong and Jing-yi Huang, utilizing predefined tables to assess quality. The Newcastle–Ottawa Scale (NOS) [22] evaluated the included cohort studies' quality. For randomized controlled trials (RCTs) inclusion, the Cochrane Collaboration tool assessed six bias domains: selection, performance, detection, attrition, reporting, and other biases [23]. Studies were graded low, moderate, or high quality based on NOS or Cochrane scores. NOS scores ranged 0–3 (low), 4–6 (moderate), 7–9 (high), while RCTs were categorized as low, unclear or high risk across the aforementioned domains.
Exposure definition
BMI was computed using the formula: weight (kg) divided by the square of height (m2). Following the World Health Organization (WHO) classification criteria, BMI categories were defined as: underweight (BMI < 18.5 kg/m2), normal range (18.5 kg/m2 ≤ BMI ≤ 24.9 kg/m2), overweight (25.0 kg/m2 ≤ BMI ≤ 29.9 kg/m2), and obese (BMI ≥ 30 kg/m2).
Statistical analysis
In the meta-analysis of BMI-related data, the HR value for any survival indicator was utilized. The study categorized participants into four groups: underweight, normal weight, overweight, and obese. If there are HRs for multiple comparison groups in an article, all of them will be extracted and included in the meta-analysis. Data synthesis was conducted using Review Manager version 5.4 (Cochrane Collaboration, Oxford, UK), with all metrics reported along with a 95% confidence interval (CI). The I2 value and Cochran's Q statistic were utilized to evaluate the heterogeneity across studies and quantify the extent of inconsistency. Substantial heterogeneity was defined as an I2 > 0.5 or P < 0.1 in the Q test [24]. In instances where substantial heterogeneity was present, the fixed effects model was utilized. Conversely, the random effects model was employed in other circumstances [25]. Sensitivity analyses were also performed to assess the influence of individual studies on outcomes with significant heterogeneity. Funnel plots were generated using Review Manager version 5.4 (Cochrane Collaboration, Oxford, UK) to evaluate publication bias, while Egger's regression test [26] was conducted using Stata version 17.0 (Stata Corp, LLP, College Station, TX, authorized by School of Public Health, Zhejiang Chinese Medicine University). P < 0.05 indicated statistically significant publication bias.
Results
Study selection and characteristics
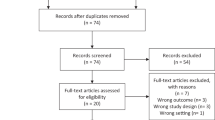
The process of researching and screening results is illustrated in Figure 1. 8764 records were identified from PubMed (n = 2118), Embase (n = 2370), Web of Science (n = 3136), and Cochrane Library (n = 1133). After removing duplicates, 5759 titles and abstracts underwent screening. Subsequently, 61 full-text articles were selected for meta-analysis, excluding non-original studies and those lacking HR values for relevant outcome indicators. Among these, 3 were randomized controlled trials [18, 27, 28] and 58 were cohort studies, comprising 48 retrospective cohort studies [12,15, 20, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] and 10 prospective cohort studies [74,75,76,77,78,79,80,81,82,83].
The characteristics and quality scores of each study are detailed in Supplementary Table S2. The median quality score for cohort studies was 8 (range 6–9), with 2 studies scoring 9, 15 scoring 8, and 15 scoring 7, indicating a moderate overall quality level. Regarding the randomized controlled trials, one was classified as low-risk, and two as medium-risk. Supplementary Table S3 provides further information on the quality assessment of cohort studies and Supplementary Figure S1 shows details of the quality assessment of RCTs.
Impact of BMI on OS
The data presented in Figure 2 demonstrate a significant association for patients with higher BMI compared to those with normal BMI. A total of 50 studies [12, 15, 18, 20, 27, 28, 30,31,32,33,34,35,36,37, 39,40,41,42, 45,46,47,48,49,50,51,52,53, 55, 56, 59,60,61,62,63,64,65,66, 68,69,70, 72, 74,75,76,77,78,79,80, 82, 83] (including 96 data points) reported HR for OS. The combined analysis revealed an HR of 1.18 (95% CI 1.14–1.23; P <0.00001), indicating a statistically significant difference between the two groups, with BMI and OS being negatively correlated (Figure 2A). Considerable heterogeneity was observed between these groups (I2 = 59%, P <0.00001). Visual assessment of the funnel plot did not reveal substantial publication bias (Supplementary Figure S2A), whereas the Egger test yielded statistical significance (P = 0.001).
Figure 2B illustrates the analysis outcomes comparing underweight and normal weight patients. The inclusion of 8 studies [12, 37, 42, 46, 53, 55, 69, 82] (including 10 data points) with an I2 value of 0% revealed a HR of 1.21 (95% CI: 1.11–1.31; P <0.00001), indicating a statistically significant difference between these two groups at a P threshold of 0.05. Visual inspection of the funnel plot did not suggest substantial publication bias (Supplementary Figure S2B), and the Egger test result was not statistically significant (P = 0.068).
Impact of BMI on RFS
A total of 6 studies [41, 45, 55, 71, 81, 84] (including 10 data points) reported the RFS data comparing high BMI to normal BMI patients. The synthesized evidence demonstrated that high BMI was significantly associated with shortened RFS (HR: 1.14; 95% CI: 1.06–1.22; P = 0.0002), without substantial heterogeneity (I2 = 11%, P = 0.35) (Figure 2C). Furthermore, no statistical (Egger's test, P = 0.584) or visual (Supplementary Figure S2C) evidence of publication bias was observed.
Impact of BMI on PFS
Merely 7 studies [29, 54, 57, 65, 74, 78, 80] (comprising 9 data points) reported PFS data. The pooled analysis demonstrated a hazard ratio of 0.91 (95% CI 0.76–1.10; P = 0.33), suggesting no significant association between PFS and high BMI. However, substantial heterogeneity existed between the two groups (I2 = 73%, P = 0.0003) (Figure 2D). Visual examination of the funnel plot did not indicate significant publication bias (Supplementary Figure S2D), corroborated by the non-significant Egger's test result (P = 0.628).
Impact of BMI on DFS
An analysis of 31 studies [12, 18, 20, 27, 30,31,32, 34,35,36, 38, 41,42,43,44, 48, 51,52,53, 59, 60, 62,63,64, 67, 68, 70, 72, 76, 79, 83] (encompassing 58 data points) investigated the impact of high BMI versus normal BMI on DFS. The synthesized evidence revealed a hazard ratio of 1.11 (95% CI 1.08–1.13; P <0.00001), suggesting that high BMI was associated with shortened DFS. Significant heterogeneity existed between the two groups (I2 = 34%, P =0.008) (Figure 2E). While visual inspection of the funnel plot did not indicate substantial publication bias (Supplementary Figure S2E), the Egger's test detected the presence of publication bias (P = 0.032).
Impact of BMI on BCSS
A total of 6 studies [37, 46, 56, 69, 82, 84] (encompassing 13 data points) were included for analyzing the outcome indicator of BCSS. The combined analysis yielded a HR of 1.18 (95% CI 1.11–1.26; P <0.00001), suggesting that higher BMI was associated with shorter BCSS (I2 = 43%, P =0.05) (Figure 2F). Visual assessment using the funnel plot (Supplementary Figure S2F) and the Egger's test did not indicate significant publication bias (P = 0.118).
Subgroup analysis
The investigation into the source of heterogeneity involved analyzing seven factors: BMI categorization, study design, study population, treatment discrepancies across regions, age distribution, and menopausal status. These precise details are documented in Table 1. In the BMI categorization subgroups, depicted in Table 1, all studies were categorized into three groups according to the World Health Organization (WHO) guidelines for elevated BMI, utilizing the specific BMI thresholds from each study. Since there was a lack of data for BMI ≥ 40 kg/m2 in the included studies, it was combined with the BMI ≥ 35 kg/m2 category.
The initial segment investigated the comparison between high BMI and normal BMI. In the examination of OS, irrespective of study type, population demographics, geographical region, treatment, and age, the subgroup analysis consistently indicated a negative correlation between high BMI and OS, with statistically significant differences. Nevertheless, the statistical significance diminished within the BMI range of 25 to 29.9 kg/m2 and across different menopausal statuses. Regarding RFS, subgroup data from the HER2-negative subgroup, Asian region, standard treatment group, and all menopausal status categories contradicted the overarching conclusion that "high BMI is not significantly linked to RFS." Conversely, the subgroup analysis of PFS did not reveal a significant association between PFS and BMI in hazard ratios from both prospective and retrospective studies, the HER2-positive population, all classified regions (Africa, Asia, Europe, and America), and all age groups. Notably, within the BMI range of 25 to 29.9 kg/m2 and the group with BMI ≥ 50, higher BMI corresponded to a prolonged PFS. In the examination of DFS data, the findings were largely consistent, except for discrepancies in the HER2-negative subgroup and standard therapy group; all other cohorts corroborated the general observation that high BMI inversely correlated with DFS. Lastly, across analyses of BCSS, all subgroup assessments aligned with the overarching conclusion that high BMI was associated with reduced BCSS.
The second segment involves comparing lower BMI with normal BMI in subgroup analysis, considering factors, such as study type, treatment, and age. The consistent finding was that low BMI was associated with a shortened OS. However, within the HER2-positive subgroup in the American region and among premenopausal individuals, the data did not demonstrate an inverse relationship between low BMI and OS.
Sensitivity analysis
We conducted sensitivity analyses for OS and PFS to evaluate the impact of individual studies on the overall combined effect size by excluding them one by one. The results remained stable even after excluding any OS/PFS data indicating that our findings were robust and stable, as illustrated in Supplementary Figure S3A and Supplementary Figure S3B.
Discussion
The prevalence of obesity has seen a marked rise in recent decades [85], with projections signaling that obesity rates will touch 20% by 2025 [86]. BMI is widely accepted as a significant risk factor for the development and progression of breast cancer [87]. Despite recent studies indicating a potential link, the relationship between BMI and breast cancer prognosis continues to be extensively debated. Research by Tan X et al. demonstrated that BMI does not notably impact the prognosis of breast cancer patients in Asian populations [88]. However, recent meta-analyses involving breast cancer patients [89] and 22,362 Asian premenopausal women [90] both underscored that elevated BMI significantly influences prognosis. Within this specific context, we undertook an updated and comprehensive systematic review and meta-analysis of 60 studies, encompassing a total of 201,006 patients. Our findings have yielded several significant insights.
The current meta-analysis investigated the correlation between BMI and the prognosis of breast cancer patients. Our results indicate that being underweight, rather than having a normal weight, significantly reduces OS in breast cancer patients. Furthermore, the forest plot analysis illustrated that a high BMI poses a risk for OS, DFS, RFS, and BCSS. However, no significant link was found between high BMI and PFS. Sensitivity analyses evaluating publication bias indicated a low risk for the outcome measures of OS and PFS. Hence, we suggest that BMI status could be considered as one of the prognostic factors for breast cancer.
To further evaluate the predictive value of BMI for outcomes in breast cancer patients with different characteristics, we conducted subgroup analyses. Compared to other survival outcome measures, notable differences were observed across most subgroups of OS, DFS, and BCSS, indicating that BMI may hold greater relevance for these particular measures. The heterogeneity within the obese patient group results in varying effects on patient prognosis, may depend on the degree of obesity [30]. We performed analyses of different BMI ranges and found that, in terms of OS and DFS, high BMI had a more significant impact on the overweight group (25 ≤ BMI ≤ 29.9 kg/m2) compared to the other two groups: 30≤BMI≤34.9 kg/m2 and BMI ≥ 35 kg/m2. This finding contradicts previous studies suggesting a U-shaped association between BMI and OS [91]/DFS [92]. The inconsistent conclusion may be partially attributed to variations in the inclusion of studies. Moreover, the subgroup analysis revealed a noteworthy finding that overweight patients demonstrated significantly prolonged PFS (HR 0.80, 95% CI 0.64–0.99; P = 0.04), which is similar to the results of previous meta-analyses [93]. Further subgroup analysis showed that, across different breast cancer subtypes, high BMI was significantly associated with OS HR 1.23, 95% CI 1.10–1.37; P = 0.0004), RFS (HR 1.30, 95% CI 1.03–1.64; P < 0.00001), and DFS (HR 1.10, 95% CI 1.03–1.17; P= 0.003) in HER2+ patients. In HER2- patients, DFS was non-significant (HR 1.03, 95% CI 0.92–1.15; P = 0.58), so it is speculated that BMI may have a higher predictive value in HER2+ patients. Our research findings also suggest that the impact of obesity varies across different age groups. Specifically, patients aged ≥50 demonstrated a more pronounced correlation in terms of RFS (HR 1.15, 95% CI 1.04–1.27; P = 0.005). The correlation may stem from the exacerbation of overweight issues as individuals age [12].
Elevated BMI poses a risk factor for OS, DFS, RFS, and BCSS in this meta-analysis, potentially due to delayed diagnosis linked with advanced cancer stages or larger tumor sizes resulting from obesity [94]. The research findings have consistently revealed a positive association between high BMI and advanced breast cancer stages, alongside an amplified tendency for lymph node metastasis among patients [56]. The study results demonstrate a consistent positive relationship between elevated BMI, advanced breast cancer stages, and an increased likelihood of lymph node metastasis in patients. According to mechanistic study findings [42], the escalation in lymph node metastases among obese patients is associated with heightened aromatase activity in hypertrophic adipose tissue and augmented peripheral transforming androgen precursors of estradiol, owing to diminished sex hormone-binding globulin levels. Additionally, obesity contributes to an upsurge in insulin concentrations, as well as insulin growth factor and obesity-related regulatory proteins, thereby prompting the development of insulin resistance [95, 96]. Furthermore, reports indicate that obesity can induce a phenotypic conversion of PD-1-CD8+ non-depleting T cells to PD-1+ CD8+ depleting T cells, thus facilitating the progression of breast cancer [97]. In HER+ patients, OS and DFS exhibited a more pronounced predictive capacity, which may be related to the fact that crosstalk between leptin and IGF-1 can lead to overactivation of the HER2 pathway, regulate the phosphorylation of HER2, diminish the sensitivity of targeted therapy, and heighten the risk of disease recurrence [98,99,100]. It is important to discuss that patients with a higher BMI show prolonged PFS compared to RFS and DFS. Obesity generally has a detrimental effect on cancer progression by altering adipokines, insulin metabolism, sex hormone levels, and causing inflammation[101, 102]. The "obesity paradox" might be attributed to less aggressive tumor biology and enhanced tumor reactivity [103]. Related studies have indicated that higher levels of adiponectin and estrogen can sustain chronic inflammation [104, 105]. This chronic inflammatory state can activate the immune system to attack tumor cells and temporarily control cancer progression [106]. Moreover, research has indicated that certain inflammatory molecules can have anti-tumor effects [107]. Furthermore, taking into account the scarcity of literature and the definitions of the three outcome measures, it is clear that PFS, in comparison to RFS and DFS, highlights the phase during which breast cancer does not show significant progression, but this does not necessarily reflect overall disease control [108]. When the disease progresses or treatment ends, factors that extend PFS may not prevent relapse, leading to shorter RFS and DFS. The findings of this study concur with other scholars' conclusions that methods to improve PFS might not effectively prevent tumor recurrence and should not be considered definitive indicators of cancer control [108]. Although PFS is widely used in clinical practice, researchers must assess whether extending PFS results in better long-term disease outcomes [109].
The research also uncovered a noteworthy association between low BMI and diminished overall survival rates. At the outset, individuals categorized as underweight exhibit reduced physiological reserves, rendering them more susceptible to adverse events [110]. Moreover, underweight patients face an elevated likelihood of experiencing severe complications stemming from medications or surgical interventions [111, 112]. Being underweight hinders the healing process [113] and compromises the immune system's functionality, thereby weakening the body's capability to combat cancer [114]. Nevertheless, it is crucial to analyze each case individually, as the underweight category encompasses genetically slender yet healthy individuals, which may not entirely reflect their nutritional status accurately [12, 46].
It is important to recognize certain constraints associated with our present investigation. Despite analyzing nearly 200,000 women, our statistical capacity was constrained when stratifying the study population by BMI, breast cancer type, age, menstrual status, and other variables. This constraint is notably prominent in the subgroup analysis exploring diverse treatments and their impacts on breast cancer prognosis. The unavailability of data in the report hindered our ability to conduct a detailed treatment survival analysis. Additionally, the insufficiency of data for studies involving low BMI could potentially introduce bias into our conclusions. Additionally, the meta-analysis did not consider confounding factors like dietary habits and physical activity in relation to BMI. It is crucial to highlight that BMI may not precisely reflect muscle mass or the distribution of relative fat mass.
Therefore, upcoming studies should contemplate integrating additional obesity metrics in conjunction with BMI. The primary robustness of this study emanates from its basis in a large-scale, top-notch, contemporary, multi-regional meta-analysis of breast cancer patients—the most extensive to date—enabling result extrapolation. Furthermore, we have conducted a thorough analysis by simultaneously exploring the correlation between various survival metrics and breast cancer prognosis, surpassing the limitation of assessing only one metric. Moreover, we performed a multifactor subgroup analysis to evaluate the robustness of conclusions across diverse populations.
Conclusion
In summary, our research findings unveil a correlation between BMI and outcome measures for breast cancer patients. First, a significant association exists between elevated BMI (overweight and obesity) and diminished OS, DFS, RFS, and BCSS. Second, a low BMI poses a risk factor for reduced OS in breast cancer patients when compared to those with a normal BMI. Owing to restricted access to study data and potential bias, further investigations are necessary to substantiate the associations between outcome measures and varying BMI levels.
Data availability
The manuscript and its supplementary files provide access to all the data that support the findings of the present study.
References
Xu M, Huang S, Dong X, Chen Y, Li M, Shi W, Wang G, Huang C, Wang Q, Liu Y, et al. A novel isoform of ATOH8 promotes the metastasis of breast cancer by regulating RhoC. J Mol Cell Biol. 2021;13(1):59–71.
Cai YW, Shao ZM, Yu KD. De-escalation of five-year adjuvant endocrine therapy in patients with estrogen receptor-low positive (immunohistochemistry staining 1%-10%) breast cancer: Propensity-matched analysis from a prospectively maintained cohort. Cancer. 2022;128(9):1748–56.
Li Y, Zheng J, Deng Y, Deng X, Lou W, Wei B, Xiang D, Hu J, Zheng Y, Xu P, et al. Global Burden of Female Breast Cancer: Age-Period-Cohort Analysis of Incidence Trends From 1990 to 2019 and Forecasts for 2035. Front Oncol. 2022;12:891824.
Ośmiałowska E, Staś J, Chabowski M, Jankowska-Polańska B. Illness Perception and Quality of Life in Patients with Breast Cancer. Cancers. 2022;14:5.
Liu W, Xiong XF, Mo YZ, Chen WG, Li M, Liang R, Zhang ZB, Zhang Z. Young age at diagnosis is associated with better prognosis in stage IV breast cancer. Aging. 2019;11(23):11382–90.
Marzbani B, Nazari J, Najafi F, Marzbani B, Shahabadi S, Amini M, Moradinazar M, Pasdar Y, Shakiba E, Amini S. Dietary patterns, nutrition, and risk of breast cancer: a case-control study in the west of Iran. Epidemiol Health. 2019;41:e2019003.
Gram IT, Wiik AB, Lund E, Licaj I, Braaten T. Never-smokers and the fraction of breast cancer attributable to second-hand smoke from parents during childhood: the Norwegian Women and Cancer Study 1991–2018. Int J Epidemiol. 2022;50(6):1927–35.
Rainey L, Eriksson M, Trinh T, Czene K, Broeders MJM, van der Waal D, Hall P. The impact of alcohol consumption and physical activity on breast cancer: The role of breast cancer risk. Int J Cancer. 2020;147(4):931–9.
Tzenios N, Tazanios ME, Chahine M. The impact of BMI on breast cancer - an updated systematic review and meta-analysis. Medicine. 2024;103(5):e36831.
Cui Y, Deming-Halverson SL, Beeghly-Fadiel A, Lipworth L, Shrubsole MJ, Fair AM, Shu XO, Zheng W. Interactions of hormone replacement therapy, body weight, and bilateral oophorectomy in breast cancer risk. Clin Cancer Res. 2014;20(5):1169–78.
Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I. The effect of aerobic exercise on quality of life among breast cancer survivors: a randomized controlled trial. J Cancer Res Ther. 2014;10(3):658–64.
Chen B, Lai J, Guo L, Dai D, Chen R, Wei G, Liao N. Adverse effects of being underweight on young female breast cancer patients with lymph node metastases. J Cancer. 2020;11(7):1976–84.
Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117(18):4132–40.
Tait S, Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat. 2014;146(1):189–97.
Pezo RC, Chan KKW, Mata DGMM, Menjak I, Eisen A, Trudeau M. Impact of body mass index on survival in women receiving chemotherapy for early breast cancer. Breast Cancer Res Treat. 2022;196(2):329–39.
Luo SP, Zhang J, Wu QS, Lin YX, Song CG. Association of Axillary Lymph Node Evaluation With Survival in Women Aged 70 Years or Older With Breast Cancer. Front Oncol. 2020;10: 596545.
Brooks JD, Christensen RAG, Sung JS, Pike MC, Orlow I, Bernstein JL, Morris EA. MRI background parenchymal enhancement, breast density and breast cancer risk factors: A cross-sectional study in pre- and post-menopausal women. NPJ Breast Cancer. 2022;8(1):97.
Desmedt C, Fornili M, Clatot F, Demicheli R, de Bortoli D, Di Leo A, Viale G, de Azambuja E, Crown J, Francis PA, et al. Differential benefit of adjuvant docetaxel-based chemotherapy in patients with early breast cancer according to baseline body mass index. J Clin Oncol. 2020;38(25):2883–91.
Gnant M, Pfeiler G, Stöger H, Mlineritsch B, Fitzal F, Balic M, Kwasny W, Seifert M, Stierer M, Dubsky P, et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: An analysis of the randomised ABCSG-6a trial. Br J Cancer. 2013;109(3):589–96.
Ewertz M, Gray KP, Regan MM, Ejlertsen B, Price KN, Thürlimann B, Bonnefoi H, Forbes JF, Paridaens RJ, Rabaglio M, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1–98 trial. J Clin Oncol. 2012;30(32):3967–75.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372.
Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Our Res. 2011;2(1):1–12.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAJB. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;2011:343.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Borenstein M, Hedges LV, Higgins JP. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111.
Egger M, Smith GD, Schneider M, Minder CJB. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Gnant M, Pfeiler G, Stoeger H, Mlineritsch B, Fitzal F, Balic M, Kwasny W, Seifert M, Stierer M, Dubsky P, et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG-6a trial. Br J Cancer. 2013;109(3):589–96.
Pfeiler G, Königsberg R, Mlineritsch B, Stoger H, Singer CF, Poestlberger S, Steger GG, Seifert M, Dubsky PC, Jakesz R, et al. Effect of change of body mass index (BMI) during therapy on the efficacy of endocrine therapy in premenopausal patients with breast cancer: An analysis of the ABCSG-12 trial. J Clin Oncol. 2011;29:15.
Barba M, Pizzuti L, Sperduti I, Natoli C, Gamucci T, Sergi D, Di Lauro L, Moscetti L, Izzo F, Rinaldi M, et al. Body Mass Index and Treatment Outcomes in Metastatic Breast Cancer Patients Treated With Eribulin. J Cell Physiol. 2016;231(5):986–91.
Chen H, Qian X, Tao Y et al. Impact of body mass index and its change on survival outcomes in patients with early breast cancer: A pooled analysis of individual-level data from BCIRG-001 and BCIRG-005 trials . J Breast. 2023;71:1–12.
Abdelwarith AM, Alsaleh K, Abdelaziz N, Ali A, Elsamany S, Rasmy A, et al. A multicenter study of the impact of body mass index (BMI) on the incidence of pathologic complete response (pCR) among Saudi patients with locally advanced breast cancer (LABC) post neoadjuvant chemotherapy (NCth). Ann Oncol. 2016;27.
Al Jarroudi O, Abda N, Seddik Y, Brahmi SA, Afqir S. Overweight: Is it a prognostic factor in women with triple negative breast cancer? Asian Pac J Cancer Prev. 2017;18(6):1519–23.
Ballinger T J JG. Impact of African ancestry on the relationship between body mass index and survival in an early-stage breast cancer trial (ECOG-ACRIN E5103). J Cancer. 2022(11):2174–81.
Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam C-M, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15(6):875–84.
Chen HL, Ding A, Wang ML. Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. J SpringerPlus. 2016;5.
Cho WK, Choi DH, Park W et al. Effect of Body Mass Index on Survival in Breast Cancer Patients According to Subtype, Metabolic Syndrome, and Treatment. Clinical Breast Cancer. 2018;18(5):e1141–7.
Chung IY, Lee JW, Lee JS et al. Interaction between body mass index and hormone-receptor status as a prognostic factor in lymph-node-positive breast cancer J. PLoS One, 2017:12(3).
Crozier JA, Moreno-Aspitia A, Ballman KV et al. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831 [J]. Cancer 2013;119(13): 2447-54.
D’Aiuto M, Chirico A, De Riggi MA, Frasci G, De Laurentiis M, Di Bonito M, Vici P, Pizzuti L, Sergi D, Maugeri-Sacca M, et al. Body mass index and treatment outcomes following neoadjuvant therapy in women aged 45 y or younger: Evidence from a historic cohort. Cancer Biol Ther. 2016;17(5):470–6.
Dawood S, Broglio K, Gonzalez-Angulo AM, Kau SW, Islam R, Hortobagyi GN, Cristofanilli M. Prognostic value of body mass index in locally advanced breast cancer. Clin Cancer Res. 2008;14(6):1718–25.
Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of Body Mass Index on Survival Outcome Among Women With Early Stage Triple-Negative Breast Cancer. Clin Breast Cancer. 2012;12(5):364–72.
De Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjöld B, Gutiérez J, Andersson M, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02–98 trial. Breast Cancer Res Treat. 2010;119(1):145–53.
Dignam JJ, Wieand K, Johnson KA, Raich P, Anderson SJ, Somkin C, Wickerham DL. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–54.
Fadelu T, Damuse R, Lormil J, Pecan E, Greenberg L, Dubuisson C, Pierre V, Triedman SA, Shulman LN, Rebbeck TR. Body Mass Index, Chemotherapy-Related Weight Changes, and Disease-Free Survival in Haitian Women With Nonmetastatic Breast Cancer. JCO Glob Oncol. 2020;6:1656–65.
Fan Y, Ding X, Wang J, Ma F, Yuan P, Li Q, Zhang P, Xu B. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers. 2015;30(2):e200–7.
Gondo N, Sawaki M, Hattori M, Yoshimura A, Kotani H, Adachi Y, Kataoka A, Sugino K, Mori M, Horisawa N, et al. Impact of BMI for clinical outcomes in Japanese breast cancer patients. Jpn J Clin Oncol. 2020;50(3):230–40.
Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506–14.
Lammers SWM, Geurts SME, van Hellemond IEG, Swinkels ACP, Smorenburg CH, van der Sangen MJC, Kroep JR, de Graaf H, Honkoop AH, Erdkamp FLG, et al. The prognostic and predictive effect of body mass index in hormone receptor-positive breast cancer. JNCI cancer spectrum. 2023;7:6.
Lee K-H, Keam B, Im S-A, Kim T-Y, Han S-W, Oh D-Y, Kim JH, Lee S-H, Han W, Kim D-W, et al. Body Mass Index Is Not Associated with Treatment Outcomes of Breast Cancer Patients Receiving Neoadjuvant Chemotherapy: Korean Data. J Breast Cancer. 2012;15(4):427–33.
Ligibel JA, Cirrincione CT, Liu M, Citron M, Ingle JN, Gradishar W, Martino S, Sikov W, Michaelson R, Mardis E, et al. Body Mass Index, PAM50 Subtype, and Outcomes in Node-Positive Breast Cancer: CALGB 9741 (Alliance). J Natl Cancer Inst. 2015;107:9.
Lin Y-C, Cheng H-H, Chen S-C, Shen W-C, Huang Y-T. Pre-treatment high body mass index is associated with poor survival in Asian premenopausal women with localized breast cancer. J Cancer. 2021;12(15):4488–96.
Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111(2):329–42.
Martel S, Lamertini M, Agbor-Tarh D et al. Body Mass Index and Weight Change in Patients With HER2-Positive Early Breast Cancer: Exploratory Analysis of the ALTTO BIG 2-06 Trial. J Natl Compr Cancer Netw. 2021;19(2):181.
Martel S, Poletto E, Ferreira AR et al. Impact of body mass index on the clinical outcomes of patients with HER2-positive metastatic breast cancer. 2018;37:142-7.
Morrison VA, Mccall L, Muss HB et al. The impact of actual body weight-based chemotherapy dosing and body size on adverse events and outcome in older patients with breast cancer: Results from Cancer and Leukemia Group B (CALGB) trial 49907. J Geriatr Oncol. 2018;9(3):228-34.
Ozaki Y, Masuda J, Kataoka A et al. The impact of obesity and endocrine therapy on the prognosis of premenopausal women with hormone receptor-positive breast cancer: A single-institute retrospective study. Cancer reports. 2023;6(2):1695.
Patel R, Li Z, Zimmerman BS et al. Impact of body mass index on the efficacy of aromatase inhibitors in patients with metastatic breast cancer. J Cancer Res. 2022;82(4 SUPPL).
Saleh K, Carton M, Dieras V et al. Impact of body mass index on overall survival in patients with metastatic breast cancer. J Breast 2021;55: 16-24.
Scholz C, Andergassen U, Hepp P et al. Obesity as an independent risk factor for decreased survival in node-positive high-risk breast cancer. J Breast Cancer Res Treat. 2015;151(3): 569-76.
Sparano JA, Wang M, Zhao F et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor positive operable breast cancer. 2012;118(23): 5937-46.
Sun L, Zhu Y, Qian Q et al. Body mass index and prognosis of breast cancer An analysis by menstruation status when breast cancer diagnosis Medicine 2018;97(26).
Tait S, Pacheco J M, Gao F et al. Body mass index, diabetes, and triple-negative breast cancer prognosis. J Breast Cancer Res Treat. 2014;146(1): 189-97.
Tao MH, Shu XO, Ruan ZX et al. Association of overweight with breast cancer survival. Am J Epidemiol. 2006;163(2): 101-7.
Turkoz FP, Solak M, Petekkaya I et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON 2013;8(2): 335-41.
Von Drygalski A, Tran TB, MESSER K et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Cancer 2011;523276.
Wang H, Yee D, Potter D et al. Impact of body mass index onpathological complete response after neoadjuvantchemotherapy: Results from the I-SPY 2 trial. J Cancer Res. 2021;81(4).
Wang K, Wu YT, Zhang X et al. Clinicopathologic and Prognostic Significance of Body Mass Index (BMI) among Breast Cancer Patients in Western China: A Retrospective Multicenter Cohort Based on Western China Clinical Cooperation Group (WCCCG). J BioMed Res Int 2019: 3692093.
Wang X, Hui TL, Wang MQ et al. Body Mass Index at Diagnosis as a Prognostic Factor for Early-Stage Invasive Breast Cancer after Surgical Resection. J Oncol Res Treat. 2019;42(4): 190-6.
Wei W, Wei S, Huang Z et al. The relationship between women's body mass index and breast cancer outcomes was U shaped. J Frontiers in Oncology. 2023.
Widschwendter P, Friedl TWP, Schwenter L et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17(1): 129.
Wolters R, Schwentner L, Regierer A et al. Endocrine therapy in obese patients with primary breast cancer: Another piece of evidence in an unfinished puzzle. J Breast Cancer Res Treat. 2012;131(3): 925-31.
Xing P, Li JG, Jin F et al. Prognostic significance of body mass index in breast cancer patients with hormone receptor-positive tumours after curative surgery. J Clin Investig Med. 2013;36(6): E297-E305.
Zheng Z, Cao H, Qu S et al. Clinical features and prognosis of obese breast cancer patients: A retrospective study Chinese-German. Am J Clin Oncol. 2013;12(9): 411-5.
Alarfi H, Salamoon M, Kadri M et al. The impact of baseline body mass index on clinical outcomes in metastatic breast cancer: a prospective study. BMC research notes. 2017;10(1): 550.
Arce salinas C, Aguilar ponce JL, Villarreal Garza C et al. Overweight and obesity as poor prognostic factors in locally advanced breast cancer patients. J Breast Cancer Res Treat. 2014;146(1): 183-8.
Buono G, Crispo A, Giuliano M et al. Combined effect of obesity and diabetes on early breast cancer outcome: A prospective observational study. Oncotarget. 2017;8(70): 115709-17.
Copson ER, Cutress RI, Maishman T et al. Obesity and the outcome of young breast cancer patients in the UK: the POSH study. J Ann Oncol : official journal of the European Society for Medical Oncology. 2015;26(1): 101-12.
Eriseld K, Laura P, Giacomo B et al. Impact of BMI on HER2+metastatic breast cancer patients treated with pertuzumab and/or trastuzumab emtansine. Real-world evidence. J Cell Physiol. 2020;235(11): 7900-10.
Gennari A, Amadori D, Scarpi E et al. Impact of body mass index (BMI) on the prognosis of high-risk early breast cancer (EBC) patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2016;159(1): 79–86.
Gennari A, Nanni O, Puntoni M et al. Body Mass Index and Prognosis of Metastatic Breast Cancer Patients Receiving First-Line Chemotherapy. Cancer Epidemiol Biomarkers Prev. 2013;22(10): 1862-7.
Imkampe AK, Bates T. Impact of a raised body mass index on breast cancer survival in relation to age and disease extent at diagnosis. Breast J. 2010;16(2): 156-61.
Jeon YW, Kang SH, Park MH et al. Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC Cancer. 2015;15(1):865.
Ladoire S, Dalbon C, Roche H et al. Effect of obesity on disease-free and overall survival in node-positive breast cancer patients in a large French population: A pooled analysis of two randomised trials. Eur J Cancer. 2014;50(3): 506-16.
Jiralerspong S, Kim ES, DONG W et al. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10): 2506-14.
Dougan MM, Willett WC, Michels KB. Prenatal vitamin intake during pregnancy and offspring obesity. Int J Obes. 2015;39(1): 69-74.
Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026): 1377-96.
Wasmann R E, Smit C, Van donselaar M H et al. Implications for IV posaconazole dosing in the era of obesity. The J Antimicrob Chemother. 2020;75(4): 1006-13.
Tan X, Huang D, Zhang F et al. Evaluation of the body mass index in breast cancer prognosis in a cohort of small-stature overweight patients: multi-center study in China. Gland surgery. 2021;10(1): 23-34.
Wen H, Deng G, Shi X et al. Body mass index, weight change, and cancer prognosis: a meta-analysis and systematic review of 73 cohort studies. ESMO Open. 2024;9(3): 102241.
Nindrea RD, Aryandono T, Lazuardi L et al. Association of Overweight and Obesity with Breast Cancer During Premenopausal Period in Asia: A Meta-Analysis. Int J Prev Med. 2019;10(1):192.
Wei W, Wei S, Huang Z et al. The relationship between women's body mass index and breast cancer outcomes was U shaped. Front Oncol. 2023;13: 1191093.
Wang K, WU YT, Zhang X et al. Clinicopathologic and Prognostic Significance of Body Mass Index (BMI) among Breast Cancer Patients in Western China: A Retrospective Multicenter Cohort Based on Western China Clinical Cooperation Group (WCCCG). Biomed Res Int. 2019: 3692093.
Zhang T, Li S, Chang J et al. Impact of BMI on the survival outcomes of non-small cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. BMC Cancer. 2023;23(1): 1023.
Biglia N, Peano E, Sgandurra P et al. Body mass index (BMI) and breast cancer: impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecological endocrinology : the official J Gynaecol Endocrinol. 2013;29(3): 263-7.
Papa V, Belfiore A. Insulin receptors in breast cancer: biological and clinical role. J Gynaecol Endocrinol. 1996;19(5): 324-33.
Peres LC, Bandera EV, Qin B et al. Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. Int J Cancer. 2017;140(3): 535-43.
Kado T, Nawaz A, Takikawa A et al. Linkage of CD8(+) T cell exhaustion with high-fat diet-induced tumourigenesis [J]. Scientific reports. 2019;9(1): 12284.
Fiorio E, Mercanti A, Terrasi M et al. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8(1):305.
Klil Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing?. Nature reviews Clinical oncology. 2017;14(2): 85-99.
Saxena NK, Taliaferro Smith L, Knight BB et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. J Cancer Res. 2008;68(23): 9712-22.
Arcidiacono D, Dedja A, Giacometti C et al. Hyperinsulinemia Promotes Esophageal Cancer Development in a Surgically-Induced Duodeno-Esophageal Reflux Murine Model. Int J Mol Sci. 2018;19(4).
Cao Z, Zheng X, Yang H et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123(8): 1336-44.
LENNON H, SPERRIN M, BADRICK E, et al. The Obesity Paradox in Cancer: a Review [J]. Curr Oncol Rep, 2016, 18(9): 56.
Chen K, Zhang J, Beeraka NM et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front Oncol. 2022;12: 820968.
Glassman I, Le N, Asif A et al. The Role of Obesity in Breast Cancer Pathogenesis. Cells, 2023;12(16).
Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nature reviews Molecular cell biology. 2008;9(8): 628-38.
Moore KW, De Waal Malefyt R, Coffman R L et al. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19: 683-765.
Korn RL, Crowley JJ. Overview: progression-free survival as an endpoint in clinical trials with solid tumors. Clin Cancer Res. 2013;19(10):2607–12
Zhu J, Tao J, Dai Z et al. Progression-Free Survival as Early Efficacy Endpoint in Resectable Esophageal Cancer Treated With Neoadjuvant Therapy: A Systematic Review. Front Oncol. 2021;11: 771546.
Brouwers B, Dalmasso B, Hatse S et al. Biological ageing and frailty markers in breast cancer patients. 2015;7(5): 319-33.
Correction: Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries. PLoS Med, 2016;13(6): e1002061.
Santa Mina D, Sellers D, Au D et al. A Pragmatic Non-Randomized Trial of Prehabilitation Prior to Cancer Surgery: Study Protocol and COVID-19-Related Adaptations. Front Oncol. 2021;11: 629207.
Nguyen GC, Du L, Chong RY et al. Hypoalbuminaemia and Postoperative Outcomes in Inflammatory Bowel Disease: the NSQIP Surgical Cohort. J Crohns Colitis. 2019;13(11): 1433-8.
Roxburgh CS, Mcmillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future oncology (London, England). 2010;6(1): 149-63.
Funding
This study was supported by China National University Student Innovation & Entrepreneurship Development Program (grant number 202310344051), the National Natural Science Foundation of Zhejiang Province (grant number LZ22H290001), the National Natural Science Foundation of China (82174095) and the Zhejiang Province Traditional Chinese Medicine Science and Technology Project (2023ZL362).
Author information
Authors and Affiliations
Contributions
Yu-Huan Kong: Conceptualization, methodology, data collection and analysis, illustration, project administration, review writing, and editing. Jing-Yi Huang: Methodology, data collection, and analysis, review writing. Ye Ding and Shu-Hua Chen: Review writing and editing. Qiu-Shuang Li and Yang Xiong: Conceptualization, methodology, data analysis, project administration, review writing and editing, and supervision. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the author(s).
Ethics statement
This study involving human participants did not necessitate an ethical review or approval, as it adhered to local legislative norms and institutional mandates.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, Yh., Huang, Jy., Ding, Y. et al. The effect of BMI on survival outcome of breast cancer patients: a systematic review and meta-analysis. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03563-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03563-9