Abstract
The spectrum of germline mutations among Jewish non Ashkenazi high risk breast/ovarian cancer families includes a few predominant mutations in BRCA1 (185delAG and Tyr978X) and BRCA2 (8765delAG). A few additional recurring mutations [A1708E, 981delAT, C61G (BRCA1) R2336P, and IVS2 + 1G > A (BRCA2)] have been reported in Jewish non Ashkenazi families. The 4153delA*BRCA1 C61G*BRCA1 and the 4075delGT*BRCA2 has been reported to recur in Russian/Polish non Jews and Ashkenazim, respectively. The rate of these recurring mutations has not been reported in Israeli high risk families. Genotyping for these recurring mutations by restriction enzyme digest and sequencing method was applied to high risk, predominantly cancer affected, unrelated Israeli individuals of Ashkenazi (n = 827), non Ashkenazi (n = 2,777), non Jewish Caucasians (n = 193), and 395 of mixed ethnicity. Jewish participants included 827 Ashkenazi, 804 Balkans, 847 North Africans, 234 Yemenites, and 892 Asians (Iraq and Iran). Age at diagnosis of breast cancer (median ± SD) (n = 2,484) was 47.2 ± 9.6 for all women participants. Males (n = 236) were also included, of whom 24 had breast cancer and 35 had pancreatic cancer. Overall, 8/282 (2.8%) of the Balkan cases carried the BRCA1*A1708E mutation, 4/180 (2.2%) the R2336P mutation, and 0/270 the IVS2 + 1G > A BRCA2 mutations, respectively. Of North Africans, 7/264 (2.65%) carried the BRCA1*981delAT mutation. The BRCA1*C61G mutation was detected in 3/269 Ashkenazi, non Ashkenazi, and non Jewish Russians; the BRCA1*Tyr978X mutation was detected in 23/3220 individuals of non Ashkenazi origin, exclusively of Asian ethnicity (23/892, 2.6% of the Asians tested). The BRCA1*4153delA mutation was noted in 2/285 non Jewish Caucasians, and none of the Ashkenazim (n = 500) carried the BRCA2*4075delGT mutation. Jewish high risk families of North African, Asian, and Balkan descent should be screened for the 981delAT, Tyr978X, A1708E BRCA1, and the R2336P BRCA2 mutations, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germline mutations in the BRCA1 (MIM# 113705) and BRCA2 (MIM# 600185) genes can be detected in high risk breast/ovarian cancer families, and serve to estimate the lifetime risk for developing these neoplasms in mutation carriers and the consequential recommendations for early detection and risk reducing surgeries [1]. More than 3,000 pathogenic mutations and sequence alterations have been reported within both genes since they were identified in the mid 1990s [2]. Among Ashkenazi Jews (i.e., Jews of east European ancestry) three mutations in BRCA1 (185delAG and 5382InsC) and BRCA2 (6174delT) occur frequently. These three mutations can be detected in the overwhelming majority of high risk Jewish Ashkenazi families, in about 35% of consecutive ovarian cancer cases and even in 2.5% of the general Jewish Ashkenazi population [3–6]. There are only a handful of other, family specific, mutations in Jewish Ashkenazi high risk families [7, 8]. Notably, one of these seemingly private mutations (8075delAT*BRCA2) was reported in more than one Jewish Ashkenazi high risk family [9]. Among the non Ashkenazim (i.e., Jews from diverse ethnicities such as the Balkans, Iraq, Iran, North Africa, and Yemen) there are also a handful of recurring mutations in both genes: 185delAG Tyr978X (BRCA1) and 8765delAG (BRCA2) [10, 11]. Over the past few years, the mutational spectrum of BRCA1 and BRCA2 in high risk non Ashkenazi families has been reported [12] and few mutations have been reported in more than two families: A1708E*BRCA1 IVS2+ 1G > A*BRCA2 in Jews of Balkan origin [13]. In addition, anecdotal cases of additional mutations were noted in the course of genetic testing: 981delAT*BRCA1 (BIC; 1100delAT accession number 1006) in Jews of North African origin, the R2336P*BRCA2 (BIC; R2336P accession number 2111) in families of Balkan and Mediterranean origin.
Since the early 1990s, about one million immigrants came to Israel from the ex Soviet Union. A sizeable portion of these immigrants (estimated at 50%) are of Russian non Jewish origin. Two recurring mutations in BRCA1 in Russian and Baltic region high risk families have been reported: 5382InsC and 4153delA in BRCA1 [14–18]. In addition, the C61G is a mutation frequently detected in non Jewish Polish high risk families [14, 15] and rarely in non Ashkenazi Jews [19].
The aim of the study was to define the rate of these recurring germline mutations in both the BRCA1 BRCA2 genes in high risk Israeli individuals of Ashkenazi, non Ashkenazi, and Russian non Jewish origin.
Patients and methods
Participant identification and recruitment
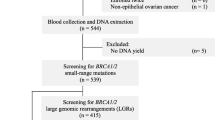
The study population was recruited from among individuals counseled and tested at one of the two Oncogenetics services located at the Sheba Medical Center, Tel-Hashomer, or the Rivkah Ziv Medical center in Zefat, since January 1, 2000. Participants recruited were diagnosed with either breast or ovarian cancer [in the minority of cases (n = 664)] or tested individuals were asymptomatic women from “high risk breast/ovarian cancer families” based on well accepted criteria [20]. All study participants were unrelated to each other (i.e., only one patient per family was included). The study was approved by the IRB, and each patient signed an informed consent.
DNA extraction
Peripheral blood leukocyte DNA was extracted using the PureGene kit (Gentra Inc., Minneapolis, MN), following the manufacturer’s recommended protocol.
Analysis for the predominant Jewish mutations in BRCA1 BRCA2 genes
Analysis for the predominant Jewish mutations in BRCA1 (185delAG 5382InsC) and BRCA2 (6174delT) was carried out by a PCR directed mutagenesis assay to introduce a restriction site that distinguishes between the wild type and the mutant allele, as previously described [3, 10, 11, 21].
Mutation genotyping
BRCA1 mutation genotyping was performed for the A1708E, Tyr978X the C61G, and the 4153delA using a restriction enzyme digest of PCR amplified fragments targeting the specific mutation bearing DNA region, with subsequent analysis on agarose gels, as previously described [10, 13, 15, 19]. The 981delAT BRCA1 mutation was detected by PCR in the relevant genomic region using the following primers: Forward primer 5′-AGCCAGTTGGTTGATTTCCACCTC-3′ and reverse primer 5′-TTCAGTTACATGGCTTAAGTTGGG-3′. The amplified 867-bp fragment was subsequently digested by the XceI restriction enzyme (Fermentas, Kaunas, Lithuania) and analyzed on an agarose gel. The mutation resulted in a different fragment pattern than the wild type allele. All fragments displaying an abnormal digest pattern were sequenced in an independent PCR. In every experiment, a known mutation was included (= positive control). The 4075delAT, R2336P, and IVS2+ 1G > A mutations were genotyped by direct sequencing of the amplified PCR fragment.
Results
Participants’ characteristics
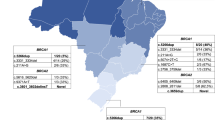
Overall, there were 4,192 participants (5,270 lab tests) in the study: 827 Ashkenazim, 2,777 non Ashkenazi Jews, and 193 non Jewish Caucasians. The ethnic origin of the non Ashkenazim was as follows: Balkans (i.e., Greece, Turkey, Bulgaria, and ex Yugoslavia) 804; North Africans (Moroccans, Tunisians, Libyans, and Algiers) 847, Asians (Iraqi, Iranian, and Afghans) 892. Of the participants, 2,484 women were diagnosed with breast cancer (mean age at diagnosis 47.2 ± 9.6 years), 324 women diagnosed with ovarian cancer (mean age at diagnosis 56.7 ± 12.2 years). In addition, 664 cancer-free women with a significant family history of breast/ovarian cancer participated, and the affected individual could not be tested. The mean age at genotyping of these women was 44.1 ± 11.4 years. Males (n = 236) were also included in the study, of whom 24 had breast cancer and 35 were diagnosed with pancreatic cancer. The other tested women participants (n = 484) had other types of cancer (mostly pancreas (n = 61), endometrial (n = 112), and gastric cancers (n = 137). The relevant features of the study participants are shown in table 1.
BRCA1 and BRCA2 genotyping results
Overall, 47 of the total of 4,192 genotyped individuals carried one of the eight pathogenic mutations (1.1% rate for mutations detected). The types and rates of mutations by ethnicity are shown in table 2.
Discussion
In the present study, the rate of some seemingly recurring germline mutations in both the BRCA1 and BRCA2 genes in breast/ovarian high risk Israeli women of diverse ethnic origin were determined. For the mostly Ashkenazi Jewish high risk families, none of the 500 individuals from seemingly independent families was found to harbor the 4075delAG*BRCA2 mutation. These data are consistent with data reported by us [12] and by the Memorial Sloan Kettering Cancer Center in New York [7], where the rate of any “private mutation” in both BRCA1 and BRCA2 in Jewish Ashkenazi high risk families is 4–5% if there are ovarian cancer cases, and less than 2% in families with site specific breast cancer. Thus, it seems that specific genotyping for the 4075delAG*BRCA2 mutation is of no added value as a routine test for Ashkenazi Jewish high risk families.
Among non Ashkenazi Jewish high risk cases, three mutations were detected at rates that may justify adding these mutations to the panel of tested mutations in BRCA1 and BRCA2 in the Israeli population: the Tyr978X mutation was detected in 2.6% of individuals, and was exclusively confined to Jews of Asian (i.e., Iraqi, Iranian, and Afghan) origin. The inability to detect this mutation in a large number of non Ashkenazi high risk families of non Asian origin, confirms the prevailing notion being practiced in oncogenetic clinics in Israel, to offer this test only to Jews of Iraqi, Iranian, and Afghan origin.
In Jewish high risk families of North African origin, there is a paucity of mutations in high risk families: our own data [12] show that none of 27 high risk North African women carried either a BRCA1 or a BRCA2 mutations when full analysis of both genes is applied. Noteworthy, in the present study, 7/264 individuals of North African ancestry carried the same mutation—a rate of 2.65%. The mutational spectrum of BRCA1 and BRCA2 mutations in non Jewish families of North African origin is also limited, and in none of these reports the 981delAT*BRCA1 mutation is noted [22, 23]. Noteworthy, this mutation was reported six times in the BIC database [2], with three of these reported cases is of Italian origin, one of British origin, one of American–German origin, and one from Australia, with no assignment of ethnicity. Based on the Jewish way of life in North Africa and the recurring nature of this mutation in the Jewish North African population, it seems plausible that the 981delAT*BRCA1 mutation is seemingly a founder mutation in North African Jewish families.
The origin of Jews from the Balkan is attributed for the most part to Jews expelled by the inquisition from Spain in 1492 and Portugal in 1497 [24]. However, these Jewish populations also exhibit admixtures with the local communities, as well as some influence of the original Ashkenazim originating from Italy [25, 26]. In that ethnically mixed population, two of the three genotyped mutations (A1708E*BRCA1 and R2336P*BRCA2) were noted at rates of 2.8 and 2.2%, respectively. These mutations were detected in other ethnically diverse populations [9, 27], and therefore in all likelihood do not represent founder Jewish mutations but rather mutations that are prevalent in the geographical region where these Jewish communities resided. Regardless of the origin of these mutations, the rates of these recurring mutations in the present study justify adding at least the A1708E*BRCA1 and R2336P*BRCA2 to the panel of germline mutations routinely tested in Israeli high risk families of the relevant ethnic origin.
In the 1990s, Israel experienced a wave of migration that added about 1,000,000 people to a country of six million. A sizeable portion of these immigrants form the ex Soviet Union are non Jews from Russia and east Europe. For high risk families of this origin, the rates of the predominant mutations in BRCA1 in the present study are 1.1% (C61G) and 0.7% (4153delA). These data are in line with data reported on non Jewish high risk families from Russia [16] Poland [14, 15] and the Baltic republics [17, 18]. Thus, for these unique non Jewish high risk families, these two mutations should be offered in the course of oncogenetics counseling and testing in Israel, prior to offering full mutational analysis of both genes.
This is a study that encompassed high risk families from two medical centers in Israel, and thus the representation of the high risk families and the non Jewish populations may have been suboptimal, as these may under represent some sects in Israel. Hence, the study may have been underpowered to detect some of the mutations, e.g., the 4153delA*BRCA1) due to the small number of tested individuals. We have not formally tested for the “founder origin” of the 981delAT*BRCA1, primarily as a result of testing only one affected individual from a family, thus limiting the ability to accurately phase mutations carriers. So, this attribution-founder mutation should be used cautiously.
In conclusion, there are few recurring mutations in the BRCA1 (981delAT, A1708E, and 4153delA) and BRCA2 (R2336P) genes among high risk non Ashkenazi Jews and non Jewish Caucasians in Israel. These mutations should be added to the panel of germline mutations offered during oncogenetic testing in high risk individuals of the relevant ethnic origin in Israel.
References
Petrucelli N, Daly MB, Feldman GL (2010) Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 12(5):245–259
Abeliovich D, Kaduri L, Lerer I, Weinberg N, Amir G, Sagi M, Zlotogora J, Heching N, Peretz T (1997) The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet 60(3):505–514
Tobias DH, Eng C, McCurdy LD, Kalir T, Mandelli J, Dottino PR, Cohen CJ (2000) Founder BRCA 1 and 2 mutations among a consecutive series of Ashkenazi Jewish ovarian cancer patients. Gynecol Oncol 78(2):148–151
Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham-Hawkins D, Hamel N, Di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet JS, Narod S (1999) Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91(14):1241–1247
Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA (1999) The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 64(4):963–970
Kauff ND, Perez-Segura P, Robson ME, Scheuer L, Siegel B, Schluger A, Rapaport B, Frank TS, Nafa K, Ellis NA, Parmigiani G, Offit K (2002) Incidence of non-founder BRCA1 and BRCA2 mutations in high risk Ashkenazi breast and ovarian cancer families. J Med Genet 39(8):611–614
Palma MD, Domchek SM, Stopfer J, Erlichman J, Siegfried JD, Tigges-Cardwell J, Mason BA, Rebbeck TR, Nathanson KL (2008) The relative contribution of point mutations and genomic rearrangements in BRCA1 and BRCA2 in high-risk breast cancer families. Cancer Res 68(17):7006–7014
Neuhausen SL, Ozcelik H, Southey MC, John EM, Godwin AK, Chung W, Iriondo-Perez J, Miron A, Santella RM, Whittemore A, Andrulis IL, Buys SS, Daly MB, Hopper JL, Seminara D, Senie RT, Terry MB, Breast Cancer Family Registry (2009) BRCA1 and BRCA2 mutation carriers in the Breast Cancer Family Registry: an open resource for collaborative research. Breast Cancer Res Treat 116(2):379–386
Shiri-Sverdlov R, Gershoni-Baruch R, Ichezkel-Hirsch G, Gotlieb WH, Bruchim Bar-Sade R, Chetrit A, Rizel S, Modan B, Friedman E (2001) The Tyr978X BRCA1 mutation in non-Ashkenazi Jews: occurrence in high-risk families, general population and unselected ovarian cancer patients. Community Genet 4(1):50–55
Lerer I, Wang T, Peretz T, Sagi M, Kaduri L, Orr-Urtreger A, Stadler J, Gutman H, Abeliovich D (1998) The 8765delAG mutation in BRCA2 is common among Jews of Yemenite extraction. Am J Hum Genet 63(1):272–274
Laitman Y, Borsthein RT, Stoppa-Lyonnet D, Dagan E, Castera L, Goislard M, Gershoni-Baruch R, Goldberg H, Kaufman B, Ben-Baruch N, Zidan J, Maray T, Soussan-Gutman L, Friedman E (2011) Germline mutations in BRCA1 and BRCA2 genes in ethnically diverse high risk families in Israel. Breast Cancer Res Treat 127(2):489–495
Sagi M, Eilat A, Ben Avi L, Goldberg Y, Bercovich D, Hamburger T, Peretz T, Lerer I (2011) Two BRCA1/2 founder mutations in Jews of Sephardic origin. Fam Cancer 10(1):59–63
Górski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Pluzańska A, Bebenek M, Fischer-Maliszewska L, Grzybowska E, Narod SA, Lubiński J (2000) Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet 66(6):1963–1968
Górski B, Cybulski C, Huzarski T, Byrski T, Gronwald J, Jakubowska A, Stawicka M, Gozdecka-Grodecka S, Szwiec M, Urbański K, Mituś J, Marczyk E, Dziuba J, Wandzel P, Surdyka D, Haus O, Janiszewska H, Debniak T, Tołoczko-Grabarek A, Medrek K, Masojć B, Mierzejewski M, Kowalska E, Narod SA, Lubiński J (2005) Breast cancer predisposing alleles in Poland. Breast Cancer Res Treat 92(1):19–24
Krylova NY, Lobeiko OS, Sokolenko AP, Iyevleva AG, Rozanov ME, Mitiushkina NV, Gergova MM, Porhanova TV, Urmancheyeva AF, Maximov SY, Togo AV, Imyanitov EN (2006) BRCA1 4153delA founder mutation in Russian ovarian cancer patients. Hered Cancer Clin Pract 4(4):193–196
Uglanitsa N, Oszurek O, Uglanitsa K, Savonievich E, Lubiński J, Cybulski C, Debniak T, Narod SA, Gronwald J (2010) The contribution of founder mutations in BRCA1 to breast cancer in Belarus. Clin Genet 78(4):377–380
Elsakov P, Kurtinaitis J, Petraitis S, Ostapenko V, Razumas M, Razumas T, Meskauskas R, Petrulis K, Luksite A, Lubiński J, Górski B, Narod SA, Gronwald J (2010) The contribution of founder mutations in BRCA1 to breast and ovarian cancer in Lithuania. Clin Genet 78(4):373–376
Kaufman B, Laitman Y, Gronwald J, Lubinski J, Friedman E (2009) Haplotype of the C61G BRCA1 mutation in Polish and Jewish individuals. Genet Test Mol Biomarkers 13(4):465–469
Lynch HT, Watson P, Tinley S, Snyder C, Durham C, Lynch J, Kirnarsky Y, Serova O, Lenoir G, Lerman C, Narod SA (1999) An update on DNA-based BRCA1/BRCA2 genetic counseling in hereditary breast cancer. Cancer Genet Cytogenet 109:91–98
Shiri-Sverdlov R, Oefner P, Green L, Baruch RG, Wagner T, Kruglikova A, Haitchick S, Hofstra RM, Papa MZ, Mulder I, Rizel S, Bar Sade RB, Dagan E, Abdeen Z, Goldman B, Friedman E (2000) Mutational analyses of BRCA1 and BRCA2 in Ashkenazi and non-Ashkenazi Jewish women with familial breast and ovarian cancer. Hum Mutat 16(6):491–501
Troudi W, Uhrhammer N, Sibille C, Dahan C, Mahfoudh W, Bouchlaka Souissi C, Jalabert T, Chouchane L, Bignon YJ, Ben Ayed F, Ben Ammar Elgaaied A (2007) Contribution of the BRCA1 and BRCA2 mutations to breast cancer in Tunisia. J Hum Genet 52(11):915–920
Uhrhammer N, Abdelouahab A, Lafarge L, Feillel V, Ben Dib A, Bignon YJ (2008) BRCA1 mutations in Algerian breast cancer patients: high frequency in young, sporadic cases. Int J Med Sci 5(4):197–202
Ostrer H (2001) A genetic profile of contemporary Jewish populations. Nat Rev Genet 2(11):891–898
Behar DM, Yunusbayev B, Metspalu M, Metspalu E, Rosset S, Parik J, Rootsi S, Chaubey G, Kutuev I, Yudkovsky G, Khusnutdinova EK, Balanovsky O, Semino O, Pereira L, Comas D, Gurwitz D, Bonne-Tamir B, Parfitt T, Hammer MF, Skorecki K, Villems R (2010) The genome-wide structure of the Jewish people. Nature 466(7303):238–242
Atzmon G, Hao L, Pe’er I, Velez C, Pearlman A, Palamara PF, Morrow B, Friedman E, Oddoux C, Burns E, Ostrer H (2010) Abraham’s children in the genome era: major Jewish diaspora populations comprise distinct genetic clusters with shared middle eastern ancestry. Am J Hum Genet 86(6):850–859
Wang F, Fang Q, Ge Z, Yu N, Xu S, Fan X (2011) Common BRCA1 and BRCA2 mutations in breast cancer families: a meta-analysis from systematic review. Mol Biol Rep [Epub ahead of print]
Acknowledgments
This study was partially funded by a grant to the Israeli consortium for hereditary breast cancer by the Israel Cancer Association to Eitan Friedman. This work was done in partial fulfillment of the requirements for the Masters degree for Anya Kushnir at the Department of Human Genetics and Molecular Biology at the Tel-Aviv University, Tel-Aviv, Israel.
Conflict of interest
All authors declare that they have no conflict of interest regarding the data published herein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laitman, Y., Simeonov, M., Herskovitz, L. et al. Recurrent germline mutations in BRCA1 and BRCA2 genes in high risk families in Israel. Breast Cancer Res Treat 133, 1153–1157 (2012). https://doi.org/10.1007/s10549-012-2006-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2006-8