Abstract
Vascular endothelial growth factor (VEGF) is a potent regulator of angiogenesis and thereby involved in the development and progression of solid tumours. The association between polymorphisms of angiogenesis pathway genes and risk of breast cancer (BC) has been widely studied, but the results are not conclusive. This information is especially limited in Spanish women, so we decided to conduct a case–control study. Here, we selected four commonly studied polymorphisms in VEGF, rs3025039 (known as +936 C/T), rs1109324, rs154765 and rs833052, one polymorphism at the promoter of the VEGFR1 (−710 C/T) and another in the FGF2, rs1449683, gene to explore their association with BC susceptibility. Genotyping was performed by TaqMan SNP assays and polymerase chain reaction–restriction fragment length polymorphis (PCR-RFLP) on 453 patients and 461 controls in a population from Valencia (Spain). We observed that women carriers of +936 CT + TT VEGF genotypes have a protective effect concerning this disease (p = 0.014; OR 0.67, 95% CI 0.48–0.92) in the global group of patients. The haplotype TGAC of VEGF (rs3025039, rs1109324, rs154764 and rs833052) shows a reduction of the risk to develop BC (p = 3e−04; OR 0.48, 95% CI 0.32–0.72). Furthermore, we found that carriers of −710 CT + TT VEGFR1 genotypes have also a protective effect (p = 0.039; OR 0.55, 95% CI 0.31–0.98). When we stratified by groups of ages these associations were maintained. Our data report for the first time the association of the polymorphism −710 C/T VEGFR1 with BC. Additional experiments focused on VEGF-A, VEGFR1 and sVEGFR1 gene expression demonstrated that carriers of T allele at −710 C/T VEGFR1 genotype have higher levels of sVEGFR1/VEGF-A than the C/C genotype carriers. This was consistent with the hypothesis that this polymorphism may act as low penetrance risk factor. The data provided suggest that +936 C/T VEGF and −710 C/T VEGFR1 genotypes are likely important genetic markers of susceptibility to BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is currently the most frequently occurring cancer and one of the leading causes of cancer-related death in the world [1], which has become a major public health challenge, accounting for 23% of all cancers in women [2, 3]. On the Spanish mainland, increased mortality was observed along the east coast. In the Autonomous Region of Valencia, high risks were concentrated along the coast, particularly in Valencia Province [4].
BC is a disease caused by a complex combination of genetic and environmental factors. Two genes were identified as the major susceptibility genes in high-risk families, namely BC 1 and BC 2 (BRCA1 and BRCA2, respectively) [5, 6]. However, these genes account for only a minority of BC and the exact molecular mechanisms underlying genetic susceptibility to this disease are still under intensive investigation. In this way, it has been suggested that low to moderate penetrance susceptibility genes combining with environmental factors may be important in the development of cancer [7].
Otherwise, angiogenesis plays a critical role in the development of cancer [8], being essential in the formation of new vessel, invasiveness, and distant metastasis [9]. This is a multistep process requiring integrated actions of a number of angiogenic growth factors. Among them, the fibroblast growth factor (FGF) and the vascular endothelial growth factor (VEGF) families are the most potent promoters of angiogenesis [10]. A noteworthy recent discovery has shown an essential role played by endothelial FGF signalling in the maintenance of blood vessels [11]. At the same time, VEGF and its receptors VEGFR1, VEGFR2 and VEGFR3 are essential in vascular development and maintenance of the adult vasculature [12].
A pro-angiogenic pattern with a high microvascular density or high levels of VEGF and others angiogenic growth factors had been associated with a worse survival and the recurrence in BC [13, 14]. The relevant factor, VEGF, which is located at 6p21.1, contains eight exons and seven introns [15]. Its mRNA expression is increased in malignant tumours compared with adjacent normal breast tissue [16], and high tissue VEGF levels are correlated with poor prognosis and decreased overall survival for BC patients. Several polymorphisms of VEGF such as +936 C/T, −2578 C/A, −406 C/T and −1154 G/A have been identified [17], of them the VEGF +936 C/T polymorphism (rs3025039) was the most widely studied [18]. The role of VEGF +936 C/T polymorphism in BC has been reported, but the results remain inconsistent among studies, partially because of the possible small effect of the polymorphism on BC risk or the relatively small sample size [19–25].
Moreover, VEGFR1 is one of the important receptors of VEGF angiogenesis signalling and has a relevant role in process of normal vessels formation [26]. VEGFR1 can be up-regulated in several tumour types, including prostate, breast, colon and non-small cell lung cancer [27] being associated with the cell growth and the development of the tumour. A soluble extracellular form of VEGFR1 (sVEGFR1) can be generated by alternative splicing. This sVEGFR1 binds VEGF with very high affinity, acting as an endogenous inhibitor of angiogenesis by sequestering VEGF [28]. Furthermore, it has been described that BC patients with high sVEGFR1/VEGF-A ratio have a markedly favourable prognosis compared with patients with low ratio [29].
The FGF family is critical in early embryonic development and precedes the appearance of VEGF signalling. In adults, FGFs play key roles in neovascularization, wound healing and cancer [12]. Three hundred and fifty FGF2 single nucleotide polymorphisms (SNPs) are known so far, 182 have been validated [30]. One of these, rs1449683 C/T, located in exon 1, has been found to be associated with FGF2 expression on both a transcriptional and translational level. In particular, the C/C genotype of the rs1449683 C/T polymorphism could be a predictor for both elevated mRNA and protein level independently from possible confounding factors [31].
In our work, we hypothesized that SNPs in genes of the angiogenesis pathway could modify BC risk. A population-based case–control study was used to investigate the possible modifying effect of four polymorphisms in VEGF, one in VEGFR1 and another in FGF. To our knowledge, there are no epidemiological studies that investigate the effect of −710 C/T VEGFR1 in relation to BC or other neoplasia.
Subjects and methods
Study population and data collection
We conducted a case–control study analysing a total of 914 women. This study includes 453 Caucasian BC female patients from the University Clinical Hospital of Valencia, with a mean age of 50.48 years (Table 1). The patients included in the study were diagnosed between 2001 and 2010 and the collection of the blood samples was conducted between 2010 and 2011. The clinical pathological parameters were obtained from hospital clinical records. The control population (n = 461) matched for sex and ethnicity, with no previous or concurrent malignant disease and living in the same area, was recruited between the same time period as the cases (±6 months). Their samples are collected at the blood donor’s bank of the same Hospital. The mean age of this control group was 39.25 years. The anonymity of the patients and control population was guaranteed. All samples of the participants in the study, both patients and healthy controls, were obtained after informed consent, according to the Declaration of Helsinki. Ethical approval was obtained from ethical committee in the institution.
DNA extraction
Genomic DNA from patient blood samples was isolated from buffy coat using QIAcube protocol by QIAGEN® using minor modifications. Genomic DNA from control population was extracted from peripheral blood leucocytes using the Ultra Clean Tissue DNA Isolation Kit by MOBIO® with minor modifications. DNA quantity was measured by NanoDrop spectrophotometer. DNA samples were stored at −20°C.
SNPs selection and genotyping
The six polymorphisms we tested were selected via bioinformatics approach and according to the bibliography date. We selected one 3′UTR polymorphism and three polymorphisms in the promoter region of the VEGF gene, one polymorphism in the promoter of the VEGFR1 gene and one polymorphism at the cds-synon region of the FGF gene (Table 2).
Genotyping analyses of the six SNPs were performed by real-time PCR, using the TaqMan® SNP Genotyping Assays (Applied Biosystems) according to the manufacturer instructions. Thermal cycling and detection was performed on the ABI Prism 7900 using the Sequence Detection Software (Applied Biosystems). In order to validate this methodology, 10% of the samples were carried out twice in independent experiments and we found a 100% concordance. The results were analysed on a 7900 real-time PCR system using the allelic discrimination assay program of Sequence Detection Software version 2.4 (Applied Biosystems).
We analysed additionally by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) the −710 C/T VEGFR1 polymorphism due to the low frequency of the minority allele. We carried out on all samples the PCR–RFLP technique for comparison with the results obtained from the Taqman assay and we obtained a 100% of concordance. VEGFR1 polymorphism was detected by amplifying genomic DNA with the forward primer 5′-GTGGCAACTTTGGGTTACCCA-3′ and the reverse primer 5′- CCTGACCCCTTCAGACTGTCC-3′. The PCR amplification parameters were 5 min at 94°C, 35 cycles each of 30 s at 94°C, 1 min at 60°C and 45 s at 72°C, and a final elongation of 6 min at 72°C. The 665 bp PCR product was digested with NlaIII at 37°C overnight. Digested products were separated by electrophoresis. Wild-type alleles resulted in 518 and 147 bp fragments and the variants alleles resulted in 665 bp fragment.
Real-time analysis of VEGF-A, VEGFR1 and sVEGFR1
We have recollected 19 paraffin samples of BC patients, taking into account the −710 C/T VEGFR1 genotype. We proceeded to the RNA extraction using the High Pure FFPE RNA Micro Kit by Roche®. A final elution volume of 20 μl was established. RNA quantity was measured by NanoDrop spectrophotometer, and RNA purity was evaluated. The samples were stored at −80°C. An aliquot of total RNA from each specimen was reverse transcribed into single-strand complementary DNA using the High Capacity cDNA Reverse Transcription Kit by Applied Biosystems®. Before amplification we performed a preamplification PCR considering a final volume of 25 μl with the TaqMan PreAmp Master Mix Kit Protocol by Applied Biosystems®. Absolute gene expression quantification for VEGFR1, sVEGFR1 and VEGF-A was performed using HPRT1 as internal reference gene, and the ABI Prism 7900 sequence detection system (Applied Biosystems) based on the TaqMan method. The PCR reaction mixture consisted of 5 μl of gene expression master mix, 0.5 μl of TaqMan probe, a final concentration of cDNA of 0.3 ηg/μl adding up to a final volume of 10 μl in a 384-well plate.
Statistical analysis
The statistical tools for genotype analysis of SNPs (Hardy–Weinberg equilibrium (HWE), allele and genotype distributions, association tests and linkage disequilibrium (LD) analysis) were provided by SNPStats [32]. χ2 analyses were used to determine the differences in distribution of genotype between cases and controls. For each polymorphism, HWE was tested by comparing the observed to expect genotype frequencies in controls using a χ2 test. Genotype frequencies were calculated for the cases and controls to determine their association with BC. The odds ratio (OR) and its 95% confidence interval (CI) were calculated as a measure of the association between the different genotypes and BC risk. The frequencies of expected haplotypes were estimated using the statistical methodologies implemented by SNPstats. The analysis of sVEGFR1/VEGF-A mRNA ratio was done by the non-parametric Mann–Whitney U test. Values were considered statistically significant when the p value was <0.05.
Results
Distribution of VEGF, VEGFR1 and FGF polymorphisms
Each of the polymorphisms was clearly discriminated using TaqMan SNP genotyping assays, or PCR–RFLP technique in the case of the −710 C/T VEGFR1. The distribution of VEGF, VEGFR1 and FGF genotypes among cases and controls and risk of BC due to these polymorphisms is listed Table 3. The distribution of the genotype frequencies in the selected polymorphisms among control group is in agreement with those expected under HWE with a p value >0.05. We also observed that the frequencies of the polymorphisms analysed in this study are similar to the frequencies previously reported in the Caucasian population described by HapMap. Despite that the age of the cases is higher than that of the controls, this does not affect our results since we found no association between genotype frequencies and age in either the cases or the controls (data not shown).
Concerning +936 C/T polymorphism the frequencies of the genotypes differed between BC patients and controls. The combination of the heterozygote +936 CT and the +936 TT homozygote was observed in lower frequency in the BC patients (18.1%) compared with the respective control group (24.9%). The +936 CT + TT genotypes showed a significantly protective effect to BC (p = 0.014; OR 0.67, 95% CI 0.48–0.92).
Regarding −710 C/T VEGFR1, our results showed that carriers of at least one T allele variant present approximately 45% reduction of the risk to develop BC (p = 0.039, OR 0.55, 95% CI 0.31–0.98). However, no statistically significant associations were found between the others polymorphisms analysed in this report and BC risk. We considered whether age at diagnosis modified the associations of the selected SNPs with BC risk (Table 4). Among women aged between 45 and 55 years we found a significant association between the carriers of the at least one T allele in the polymorphism rs3025039 and protection to BC. We also found the same tendency (borderline significant) at the group of women >55 years. These results are in agreement with the associations described in Table 3. In relation to the −710 C/T VEGFR1 polymorphism, women under 45 years of age and carriers of the minor allele had a reduced risk of BC (p = 0.017, OR 0.28, 95% CI 0.08–0.94). Conversely, in the others two groups of women for this SNP, risk was non-significantly altered. Also, the rs833052 genotype show an association with predisposition to develop BC in the subset of women younger than 45 years (p = 0.021; OR 1.85, 95% CI 1.10–3.11).
The effects of the polymorphisms were further analysed after the data were stratified by histological type, estrogen receptor (ER) status, menstrual history and lymph node affection. We detect an association between the ER status in patients and the polymorphism rs1109324. We found a modest statistical significance with a p = 0.041 (OR 2.11, 95% CI 1.00–4.43). It seems that carriers of at least one T allele are 2.11-fold more frequent in ER positive BC compared with the ER negative patients.
Distribution of VEGF haplotypes
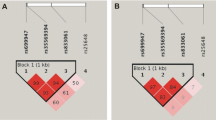
We established haplotypes for the four polymorphisms in VEGF gene in the 914 individuals to investigate their effects in BC risk. The results are described in Table 4. The analyses of haplotype frequency distributions show that a specific haplotype containing the variant allele for +936T VEGF was less frequent in patients than in controls. If we compare the common haplotype with the other possible haplotypes, we observed statistically significant differences regarding the second haplotype in Table 5 (p = 3e−04). The results suggest that carriers of this haplotype present a protective effect to developing BC (OR 0.48; 95% CI 0.32–0.72).
sVEGFR1/VEGF-A ratio in BC paraffin samples from different genotype carriers
The ratio of expression between sVEGFR1 and VEGF-A mRNA levels was calculated in C/T versus C/C genotype carriers for the −710 C/T VEGFR1 to gain better insight into the biological relevance of this polymorphism. The formalin-fixed paraffin-embedded samples from seven patients with C/T and twelve with C/C genotype where selected. The results showed that the sVEGFR1/VEGF-A ratio was approximately five times greater in C/T genotype carriers than in C/C genotype carriers, with median values of 1.62 (n = 7) versus 0.32 (n = 12), respectively (Fig. 1). The statistical analysis with the non-parametric U Mann–Whitney test shows a tendency to the significance (borderline significance with a p = 0.063).
Discussion
The individual susceptibility to the development of BC may be influenced by several factors [33]. It is now accepted that chemotherapy effectiveness in BC is limited by significant inter-individual variations, which are often associated to genetic variations in specific genes. Increasing evidences suggest that genetic polymorphisms may have a contribution on cancer susceptibility, particularly in genes of angiogenesis [34].
In this study, we evaluated four separate VEGF polymorphisms (rs3025039, rs1109324, rs1547651 and rs833052), one polymorphism at the promoter of VEGFR1 (−710 C/T) and another in the FGF gene (rs119683) in a group of BC patients as well as in matching controls. The allelic frequencies of our control group for the different polymorphisms are in accordance with the frequencies described by HapMap for the Caucasian population. There were no frequencies in that database for the −710 C/T VEGFR1, but our frequencies are similar to those described by Menendez et al. [26].
Angiogenesis is required for the growth of microscopic cancers into larger tumours and clearly plays a role in the pathogenesis of BC [35]. VEGF is believed to be an important factor for angiogenesis through various mechanisms, which has been proved to be a key step for tumour occurrence, progress and prognosis [16, 36]. Functional polymorphisms of VEGF gene can influence the expression of the protein, and then contribute to the susceptibility and severity of cancer [37]. Considering the polymorphism +936 C/T in VEGF, previous studies indicated that the C to T allele change led to the loss of a potential binding site for the activating enhancer binding protein 4 (AP-4) transcription factor, which increasing the expression of several viral and cellular genes [38, 39]. As result, +936 T allele may inhibit transcription of VEGF. This inhibition could explain why the +936 T allele carriers have lower risk developing cancer. Accordingly, the VEGF plasma levels in +936 T allele carriers were significantly lower than that in non-carriers [40]. Thus, VEGF +936 T allele carriers were considered to be associated with decreased risk of BC. This study explores the association between this and other polymorphisms in VEGF, VEGFR1 and FGF, and BC risk.
Our results indicate that the VEGF +936 T allele carriers were associated with decreased BC risk (p = 0.014; OR 0.67, 95% CI 0.48–0.92). These results are consistent with those from previous studies of VEGF polymorphisms and cancer risk that reported a protective association between the +936 T carriers and cancer [20, 25]. When we analyse the results by range of ages, we also found a significant association in the group of women between 45 and 55 years, and the same tendency in the group of woman older than 55 years (p = 0.046 and 0.067, respectively). In this stratified analysis we also detected an association between the rs833052 genotype and the BC risk in the subset of women younger than 45 years (p = 0.021; OR 1.85, 95% CI 1.10–3.11). To our knowledge, there was no previous work relating this polymorphisms and BC predisposition. However, a previous data published on bladder cancer shown an increment of the risk associated with this SNP [41]. The LD between polymorphisms region could be an important factor affecting the incidence of cancer in general, and BC, in particular. LD analyses showed that the four loci in VEGF were relatively strong. Thus, haplotype analysis would be an important tool to confirm the significance of these polymorphisms on BC susceptibility. We found a statistical significant association between haplotype 2 and BC (p = 3e−04; OR 0.48, 95% CI 0.32–0.72). This specific haplotype seems to have a protective effect in the development of BC.
The importance of VEGFR1 in angiogenesis is widely known but its exact role is not fully determined, especially in pathological processes as cancer. VEGFR1 is involved in the induction of matrix metalloproteinase 9 in lung cancer [26] and has been associated with cell growth and development of pathological angiogenesis in tumour cells. Variations in the expression of this receptor are expected to have a cellular impact.
Recent experiments of Menendez and collaborators show that VEGFR1 is involved in the p53 stress-response transcriptional network through the SNP −710 C/T. This SNP is located in a putative p53 response element sequence. This uncommon polymorphic variant was found in approximately the 6–7% of the general population. The authors prove in cell lines that only the T allele was p53 responsive. Based on the above knowledge, we hypothesized that the polymorphism −710 C/T VEGFR1 may affect the development of BC.
We assess the genotype of these polymorphisms in BC patients and in general population to know their relation with the risk to develop BC. We present here for the first time a significant association between −710 C/T VEGFR1 and BC risk. The genotype with at least one T allele associates with protection against BC. Indeed the percentages of C/T and T/T genotype carriers change from 7.4 in the general population to 4.2 in BC patients in our group of study (p = 0.039; C/C vs. C/T + TT; OR 0.55, 95% CI 0.31–0.98).
The results of Menendez et al. in cell lines show that the treatment with doxorubicin increases the levels of VEGFR1 in the cells with one or two T alleles. The p53 pathway is one possible mechanism, since this tumour suppressor gene is highly responsive to a variety of stress conditions. The treatment with doxorubicin or another chemotherapeutic agent increases the p53 level in the tumour cells and allows the binding to the specific response elements. Studies based on CHIP assay show that p53 is capable of binding to the responsive elements located in the promoter of VEGFR1 only when one of the alleles is T allowing the expression of the VEGFR1 protein [26, 42]. That means that the levels of this receptor could be increased in patients with the C/T or T/T genotype in the polymorphisms of VEGFR1 promoter treated with chemotherapeutic agents. The high levels of VEGFR1 that increase the angiogenesis in this context can diminish it. There is an alternative splice variant of VEGFR1 resulting in a soluble form that acts as negative modulator [28, 29, 43]. Our results of quantitative PCR show that the ratio sVEGFR1/VEGF-A was higher in C/T genotype carriers in −710 C/T VEGFR1 polymorphism than in patients with C/C genotype. High ratio levels have been associated with a favourable BC prognostic. Our data are in concordance with the published information about the promoter of VEGFR1 and reaffirm the idea of a protective role for the T allele in this polymorphism.
This genetic variant acts as low penetrance modifier of the risk. The data present above are also important because they have permitted to characterize this polymorphism in a control group population, so these frequencies can be useful as reference in other future works.
In our study, we did not observe association between the tumour type, grade or stage of the disease that could possibly indicate that the polymorphisms preferentially affect a subset of cancers. We only found a modest significance in ER positive patients and the polymorphism rs1109324. The results show that the carriers of at least one T allele are more frequent in this subgroup of BC with a p = 0.041 (OR 2.11, 95% CI 1.00–4.43).
In summary, we found three significant associations with predisposition to BC, in rs833052, rs3025039 and in −710 C/T VEGFR1 polymorphisms. Moreover, our VEGF haplotype analysis results suggest that the haplotype TGAC contributes to the protection of developing BC. As far as we know this is the first study that analyses and demonstrates a significant association of the polymorphism −710 C/T of VEGFR1 and BC risk. This study may have some limitations due to the fact that it is a case–control study restricted to the Spanish population in Valencia Province and also because we do not have the complete follow-up of all patients. Moreover, larger and preferably population-based case–control studies, as well as well-designed mechanistic studies are needed to validate our results. However, the genotype frequencies we observed among our controls were in agreement with HWE, suggesting that our subject sampling was sufficiently random. Additionally, it is noteworthy that we report for the first time the association of −710 C/T VEGFR1 genotype and protection to develop BC. Our case–control studies and also the experimental results support the protector effect of the T allele of this polymorphism since C/T genotype carriers associate with high levels of sVEGFR1/VEGF-A ratio that has been related with good prognostic in BC.
Conclusion
This study suggests that the rs833052, +936 C/T VEGF and −710 C/T VEGFR1 polymorphisms are important genetic markers of susceptibility to BC. Specifically, the +936 T and −710 T variant have a protective effect against the development of BC. Clearly, this predictive value needs to be validated in further epidemiologic and functional studies. Overall, our results support the hypothesis that polymorphisms in the VEGF and VEGFR1 may have important mechanistic implications in BC and reaffirm the belief that targets within VEGF pathway are potentially tools for BC prevention and treatment.
Abbreviations
- BC:
-
Breast cancer
- BRCA1:
-
Breast cancer 1
- BRCA2:
-
Breast cancer 2
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- FGF:
-
Fibroblast growth factor
- HWE:
-
Hardy–Weinberg equilibrium
- OR:
-
Odds ratio
- PCR–RFLP:
-
Polymerase chain reaction–restriction fragment length polymorphism
- SNP:
-
Single nucleotide polymorphism
- VEGF:
-
Vascular endothelial growth factor
- VEGFR1:
-
Vascular endothelial growth factor receptor 1
- sVEGFR1:
-
Soluble vascular endothelial growth factor receptor 1
References
Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M (2006) Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin 56:168–183
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Pollan M, Ramis R, Aragones N, Perez-Gomez B, Gomez D, Lope V, Garcia-Perez J, Carrasco JM, Garcia-Mendizabal MJ, Lopez-Abente G (2007) Municipal distribution of breast cancer mortality among women in Spain. BMC Cancer 7:78
Easton DF, Ford D, Bishop DT (1995) Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 56:265–271
Claus EB, Risch N, Thompson WD (1991) Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet 48:232–242
Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K (2000) Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343:78–85
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660
Shibuya M, Claesson-Welsh L (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymph angiogenesis. Exp Cell Res 312:549–560
Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M (2008) The FGF system has a key role in regulating vascular integrity. J Clin Invest 118:3355–3366
Murakami M, Nguyen LT, Hatanaka K, Schachterle W, Chen PY, Zhuang ZW, Black BL, Simons M (2011) FGF-dependent regulation of VEGF receptor 2 expression in mice. J Clin Invest 121:2668–2678
Uzzan B, Nicolas P, Cucherat M, Perret GY (2004) Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res 64:2941–2955
Linderholm B, Tavelin B, Grankvist K, Henriksson R (1998) Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J Clin Oncol 16:3121–3128
Brogan IJ, Khan N, Isaac K, Hutchinson JA, Pravica V, Hutchinson IV (1999) Novel polymorphisms in the promoter and 5′ UTR regions of the human vascular endothelial growth factor gene. Hum Immunol 60:1245–1249
Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP (1996) Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res 56:2013–2016
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA (1991) The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 266:11947–11954
Chae YS, Kim JG, Sohn SK, Cho YY, Moon JH, Bae HI, Park JY, Lee MH, Lee HC, Chung HY, Yu W (2006) Investigation of vascular endothelial growth factor gene polymorphisms and its association with clinicopathologic characteristics in gastric cancer. Oncology 71:266–272
Lin GT, Tseng HF, Yang CH, Hou MF, Chuang LY, Tai HT, Tai MH, Cheng YH, Wen CH, Liu CS, Huang CJ, Wang CL, Chang HW (2009) Combinational polymorphisms of seven CXCL12-related genes are protective against breast cancer in Taiwan. OMICS 13:165–172
Krippl P, Langsenlehner U, Renner W, Yazdani-Biuki B, Wolf G, Wascher TC, Paulweber B, Haas J, Samonigg H (2003) A common 936 C/T gene polymorphism of vascular endothelial growth factor is associated with decreased breast cancer risk. Int J Cancer 106:468–471
Wang K, Liu L, Zhu ZM, Shao JH, Xin L (2011) Five polymorphisms of vascular endothelial growth factor (VEGF) and risk of breast cancer: a meta-analysis involving 16,703 individuals. Cytokine 56:167–173
Jacobs EJ, Feigelson HS, Bain EB, Brady KA, Rodriguez C, Stevens VL, Patel AV, Thun MJ, Calle EE (2006) Polymorphisms in the vascular endothelial growth factor gene and breast cancer in the Cancer Prevention Study II cohort. Breast Cancer Res 8:R22
Kataoka N, Cai Q, Wen W, Shu XO, Jin F, Gao YT, Zheng W (2006) Population-based case-control study of VEGF gene polymorphisms and breast cancer risk among Chinese women. Cancer Epidemiol Biomarkers Prev 15:1148–1152
Jin Q, Hemminki K, Enquist K, Lenner P, Grzybowska E, Klaes R, Henriksson R, Chen B, Pamula J, Pekala W, Zientek H, Rogozinska-Szczepka J, Utracka-Hutka B, Hallmans G, Forsti A (2005) Vascular endothelial growth factor polymorphisms in relation to breast cancer development and prognosis. Clin Cancer Res 11:3647–3653
Jakubowska A, Gronwald J, Menkiszak J, Gorski B, Huzarski T, Byrski T, Edler L, Lubinski J, Scott RJ, Hamann U (2008) The VEGF_936_C>T 3′UTR polymorphism reduces BRCA1-associated breast cancer risk in Polish women. Cancer Lett 262:71–76
Menendez D, Krysiak O, Inga A, Krysiak B, Resnick MA, Schonfelder G (2006) A SNP in the flt-1 promoter integrates the VEGF system into the p53 transcriptional network. Proc Natl Acad Sci USA 103:1406–1411
Ciribilli Y, Andreotti V, Menendez D, Langen JS, Schoenfelder G, Resnick MA, Inga A (2010) The coordinated p53 and estrogen receptor cis-regulation at an FLT1 promoter SNP is specific to genotoxic stress and estrogenic compound. PLoS One 5:e10236
Kendall RL, Thomas KA (1993) Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90:10705–10709
Menendez D, Inga A, Snipe J, Krysiak O, Schonfelder G, Resnick MA (2007) A single-nucleotide polymorphism in a half-binding site creates p53 and estrogen receptor control of vascular endothelial growth factor receptor 1. Mol Cell Biol 27:2590–2600
Kato M, Okugawa G, Wakeno M, Takekita Y, Nonen S, Tetsuo S, Nishida K, Azuma J, Kinoshita T, Serretti A (2009) Effect of basic fibroblast growth factor (FGF2) gene polymorphisms on SSRIs treatment response and side effects. Eur Neuropsychopharmacol 19:718–725
Schulz S, Kohler K, Schagdarsurengin U, Greiser P, Birkenmeier G, Muller-Werdan U, Werdan K, Glaser C (2005) The human FGF2 level is influenced by genetic predisposition. Int J Cardiol 101:265–271
Sole X, Guino E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22:1928–1929
Dumitrescu RG, Cotarla I (2005) Understanding breast cancer risk—where do we stand in 2005? J Cell Mol Med 9:208–221
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10
Roy H, Bhardwaj S, Yla-Herttuala S (2006) Biology of vascular endothelial growth factors. FEBS Lett 580:2879–2887
Delli Carpini J, Karam AK, Montgomery L (2010) Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis 13:43–58
Schneider BP, Radovich M, Sledge GW, Robarge JD, Li L, Storniolo AM, Lemler S, Nguyen AT, Hancock BA, Stout M, Skaar T, Flockhart DA (2008) Association of polymorphisms of angiogenesis genes with breast cancer. Breast Cancer Res Treat 111:157–163
Hu YF, Luscher B, Admon A, Mermod N, Tjian R (1990) Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev 4:1741–1752
Mermod N, Williams TJ, Tjian R (1988) Enhancer binding factors AP-4 and AP-1 act in concert to activate SV40 late transcription in vitro. Nature 332:557–561
Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E (2000) A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res 37:443–448
Garcia-Closas M, Malats N, Real FX, Yeager M, Welch R, Silverman D, Kogevinas M, Dosemeci M, Figueroa J, Chatterjee N, Tardon A, Serra C, Carrato A, Garcia-Closas R, Murta-Nascimento C, Rothman N, Chanock SJ (2007) Large-scale evaluation of candidate genes identifies associations between VEGF polymorphisms and bladder cancer risk. PLoS Genet 3:e29
Menendez D, Inga A, Resnick MA (2006) The biological impact of the human master regulator p53 can be altered by mutations that change the spectrum and expression of its target genes. Mol Cell Biol 26:2297–2308
Shibuya M (2001) Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1). Int J Biochem Cell Biol 33:409–420
Acknowledgments
This work was supported in part by Grants from the Ministerio de Salud Carlos III and the Foundation Gent x Gent to A.Ll and Consellería de Sanidad [GE-004/09] to P.E. P.R. holds a Santiago Grisolia fellowship from the Conselleria de Sanidad Valenciana, J. F is founded from the RTICC RD06/0020/0080, E. T. from the Grant PS09/01700 and P. E. from the Instituto de Salud Carlos III under a ‘Miquel Servet’ contract [FIS03/0090]. We thank the Biobanco FIHCUV-INCLIVA for providing the paraffin-embedded samples.
Funding
There are no competing financial interests.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, P., Furriol, J., Tormo, E. et al. The single-nucleotide polymorphisms +936 C/T VEGF and −710 C/T VEGFR1 are associated with breast cancer protection in a Spanish population. Breast Cancer Res Treat 133, 769–778 (2012). https://doi.org/10.1007/s10549-012-1980-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-1980-1