Abstract
The incidences and clinical aggressiveness of intracranial metastases have not been as well characterized in patients with triple-negative (TN) breast cancer as in patients with human epidermal growth factor 2-positive (HER2+) breast cancer. Patients diagnosed with brain metastases from primary breast cancer, as determined by computed tomography and/or magnetic resonance imaging, at Asan Medical Center from January 1990 to July 2006 were identified and classified into three subtypes: TN, HER2+, and other. The clinical features and outcomes of these three groups were compared. Of the 7,872 patients diagnosed with primary breast cancer, 198 developed brain metastases; of these, 61 patients with unknown estrogen receptor, progesterone receptor, and HER2 status were excluded. Of the remaining 137 patients, 44 (32%) were classified as TN, 69 (50%) as HER2+, and 24 (18%) as other. Clinical parameters, including performance status and previous adjuvant chemotherapy and/or radiotherapy, were well balanced among groups, except that earlier staged tumors (I and II) were more prevalent in the TN than in the HER2+ and other (59 vs. 36 vs. 38%, P = 0.01). At a median follow-up of 99 months, the median times from initial diagnosis to brain metastasis (20 vs. 32 vs. 45 months, P = 0.01) and to first distant metastasis at any site (16 vs. 23 vs. 23 months, P = 0.005) were significantly shorter in TN than in the HER2+ and other. Median overall survival (OS) from primary cancer diagnosis was significantly shorter in the TN than in the HER2+ and other (31 vs. 39 vs. 57 months, P = 0.02), but survival after brain metastasis was similar (5.9 vs. 5.2 vs. 8.8 months, P = 0.31). Compared with other breast cancer phenotypes, TN breast cancer was characterized by earlier brain and other distant metastases and shorter OS, despite a higher proportion of tumors diagnosed at early stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancers are a heterogeneous group of tumors that can be divided into several subtypes according to their histopathological and molecular features [1, 2]. These subtypes have different biologic characteristics, clinical courses, and prognoses and require different treatment strategies. Although gene-expression signatures have been used as biomarkers, these methods are not always available. In contrast, immunohistochemical (IHC) staining for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) are well established clinical markers used to determine treatment of patients with breast cancer. Triple-negative (TN) breast cancer is a type of breast tumor that is negative for ER, PR, and HER2 on IHC staining and may be a clinical surrogate for the basal-like phenotype [3, 4]. Compared with other subtypes, TN breast cancer is characterized by a more aggressive clinical history and higher incidence rates of visceral metastasis and distant relapse, resulting in dismal outcomes [5–7].
Brain metastasis in patients with breast cancer is a catastrophic event that results in a poor prognosis, with a median survival of 4–6 months despite treatment [8–10]. Brain metastases occur in 10–16% of patients with metastatic breast cancer and in one-third of breast cancer patients treated with trastuzumab [11]. Risk factors for brain metastasis include younger age, ER-negative status, HER2-positive status, high-grade tumor, and node-positive status [12]. Studies assessing the association between HER2 over-expression and risk of brain metastasis have suggested that trastuzumab has survival benefit in patients with HER2-positive brain metastases [13–16].
We previously described the clinical features of brain metastases in 198 patients with breast cancer [10]. However, the incidence and clinical aggressiveness of intracranial metastasis have not been well characterized in patients with TN breast cancer as in patients with HER2-positive breast cancer. We therefore compared the clinical features, clinical course, and survival in patients with TN and HER2-positive breast cancer who developed brain metastases.
Methods and materials
Patients diagnosed with histologically confirmed breast cancer from January 1990 to July 2006 at a single institution were screened. All patients who developed solitary or multiple brain metastases and with IHC staining information for ER, PR, and HER2 were included in this retrospective analysis. Brain metastases were identified by computed tomography (CT) and/or magnetic resonance imaging (MRI) of the brain. All the patients were classified into three subtypes based on IHC staining for ER, PR, and HER2 receptor and HER2 FISH analysis. The TN subtype was defined as breast cancers with negative IHC staining for ER, PR, and HER2. The HER2-positive (HER2+) subtype was defined as breast cancer with positive IHC staining for HER2, regardless of ER/PR status. Breast cancer patients negative for HER2 but positive for hormone receptor (ER and/or PR) were classified into the other subtype. Samples were considered negative for ER and PR if <10% of tumor cells showed expression by IHC staining. As IHC staining for ER and PR was each scored from 0 to 7 in our institution, IHC scores of 0–3 were regarded as negative and scores of 4–7 were regarded as positive [17]. IHC staining for HER2 was scored as 0–3, with scores of 0–2 regarded as negative and a score of 3 regarded as positive. If the IHC staining score was 2+ and the sample was positive for HER2 by FISH, it was regarded as HER2 positive.
Categorical variables among subtypes were compared using the chi-square test or Fisher’s exact test. Survival parameters were calculated using the Kaplan–Meier method, and survival curves were compared using two-sided log-rank tests. All statistical analyses were performed with the Statistical Software Package for the Social Sciences (SPSS version 12.0 for Windows; SPSS, Chicago, IL, USA).
Results
Of the 7,872 patients diagnosed with breast cancer during the study period, 198 (2.5%) developed brain metastases. ER, PR, or HER2 status was not known in 61 patients, who were therefore excluded. Of the remaining 137 patients, 44 (32.1%) were classified as TN, 69 (50.4%) as HER2+, and 24 (17.5%) as other. The baseline patient characteristics of these subgroups are summarized in Table 1. Median ages at the time of breast cancer diagnosis in the TN, HER2+, and other subtype were 43, 45, and 37 years, respectively, (P = NS), and median tumor sizes were 2.5, 3.5, and 3.5 cm, respectively, (P = NS). The proportion of patients with lymph node metastases was lower in the TN (45%) than in the HER2+ (65%) and other (58%) subtype, whereas the proportion of patients with earlier stage (I and II) tumors was significantly higher in the TN than in the HER2+ and other subtype (59 vs. 36 vs. 38%, P = 0.01). We found that 81% of all the patients had received adjuvant chemotherapy, and about 40% received adjuvant radiotherapy, with similar distribution among the three subgroups.
The median ages at diagnosis of brain metastasis in the TN, HER2+, and other subtype were 46, 48, and 42 years (P = 0.14), respectively (Table 2). The three groups had similar distribution of performance status, features of brain metastasis, and treatment for brain metastasis. At a median follow-up of 99 months, the median time from initial diagnosis of primary breast cancer to brain metastases was significantly shorter in the TN than in the HER2+ and other subtype (20 vs. 32 vs. 45 months, P = 0.01, Table 3, Fig. 1), as was the median time from initial diagnosis to the first distant metastasis at any site (16 vs. 23 vs. 23 months, P = 0.005, Fig. 2).
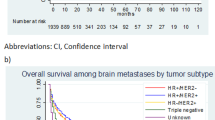
The median time from initial diagnosis of primary breast cancer to death was significantly shorter in the TN than in HER2+ and other subtype (31 vs. 39 vs. 57 months, P = 0.02, Fig. 3). However, overall survival (OS) after brain metastasis was similar in the three subgroups (5.9 vs. 5.2 vs. 8.8 months, P = 0.31).
Discussion
Although treatment of metastatic breast cancer has improved, brain metastases are associated with progressive neurological disability, resulting in devastating consequences with regard to both mortality and quality of life. The incidence of brain metastases from breast cancer has been reported to range from 3 to 5% [18–20], similar to the 2.5% rate we observed in 7,872 patients diagnosed at single institution. Classification of the 137 patients we identified with brain metastases based on IHC findings showed that 32.1, 50.4, and 17.5% could be classified as having the TN, HER2+, and other subtype, respectively. In comparison, other studies on patients with brain metastases from breast cancer found that 24–37% had the TN subtype [19, 21, 22]. If 15% of all breast cancer patients have the TN subtype and 25% have the HER2+ subtype [23], then brain metastases would occur in 3.7 and 3.5%, respectively, higher than the 0.5% of patients with the other subtype. Our findings are in good agreement with a previous report, showing that 6.7% of all patients with TN breast cancer developed brain metastases and that the TN phenotype was the greatest risk factor for brain metastasis by multivariate analysis [19]. These results indicate that TN breast cancer has a high predisposition to metastasize to the brain.
Interestingly, we found that the proportion of patients with early stage (I and II) tumors was higher in the TN than in the other two subtypes. Among the risk factors for brain metastasis from breast cancer are younger age, larger tumor size, nodal involvement, and high grade [11, 12], suggesting that large, advanced stage tumors with positive nodes have a tendency to metastasize to distant sites including the brain. HER2+ subtype breast cancers with brain metastases are at more advanced stages at initial diagnosis, in agreement with our findings. In contrast, we found that node positivity and advanced stage were less frequent in patients with TN breast cancers, whereas other clinical parameters, such as age at diagnosis, tumor size, tumor grade, and adjuvant chemotherapy and/or radiotherapy, were similarly distributed among the three subtypes. These results suggest that TN breast cancers, even at earlier stages, may have a special biologic ability to metastasize to the brain. To expatiate on adjuvant treatment in TN group, 8 patients (18%) in the TN group did not receive adjuvant chemotherapy. Two of them were stage IV at initial diagnosis. Six patients did not, because they had early staged cancer (4 were stage I and 2 were stage II), and they refused adjuvant chemotherapy. Twenty-seven (61%) patients in the TN group did not receive adjuvant radiotherapy. Two of them were stage IV at initial diagnosis. Until early of 21 century, most patients in Korea prefer to get modified radical mastectomy (MRM) instead of breast conserving surgery (BCO), when two options were explained. Twenty-three patients received MRM. Some of them with advanced nodal status and two patients treated with BCO refused adjuvant radiotherapy. For adjuvant hormonal therapy, 6 patients (14%) of the TN patients had received adjuvant hormonal therapy. One of the new ASCO/CAP (College of American Pathologists) guidelines for hormone receptor testing provide tumors with at least 1% positive tumor nuclei for ER and/or PR should be designated as hormone receptor positive [24]. Even a low number of cells stained positive (as low as 1% of tumor cells) identify a cohort of tumors having some responsiveness to endocrine therapies. Probably, as is typical biologic systems, a precise threshold does not exist. However, they are empirically chosen, about 10% positive staining of cells for either receptor might be considered as a reasonable threshold for both receptors confers definite endocrine non-responsiveness status. This crucial distinction implies the absolute necessity for the reporting of quantitative results of IHC staining with appropriate quality control [25]. Definition of hormone receptor positivity has been changed. Since St. Gallen Consensus in 2003, recommendation of endocrine therapy was widened to patients with at least 1% positive tumor nuclei in the sampled tissue. Before 2003, adjuvant endocrine therapy was not given usually in patients with weak-positive hormone receptors as weak positivity was considered negative.
We found that the median time from initial diagnosis of primary breast cancer to brain metastasis was significantly shorter in the TN (20 months) than in the HER2+ (32 months) and other (45 months) subtype. Prognosis after development of brain metastasis was poor in all subtypes, with OS times after brain metastasis in these 3 groups of 6, 5, and 9 months, respectively. Thus, OS from initial diagnosis of breast cancer was shorter in the TN than in the HER2+ and other subtype. In contrast, other studies have reported that survival after brain metastases was significantly shorter in patients with TN than with HER2+ tumors [21, 26]. This difference in results may have been due to treatment with trastuzumab, which has been shown to prolong survival in patients with brain metastasis from breast cancer by controlling both systemic disease and brain metastases [27]. Of our 69 patients with the HER2+ subtype, 14 had been treated with trastuzumab, 11 before brain metastases developed, and 9 afterward, including 6 who were treated both before and after. It was difficult to continue trastuzumab treatment in patients who developed brain metastases, because they are not covered by health insurance in Korea. Thus, the nine patients who were treated with trastuzumab after the development of brain metastases did so at their own expense. The median OS after brain metastases in these nine patients was 15 months, significantly longer than the OS of patients in the HER2+ (5 months) and TN (6 months) subtypes who were not treated with trastuzumab after brain metastases. Similar results were reported in an earlier study, which found that the median times from initial diagnosis to brain metastasis in the TN, HER2+, and other groups were 22, 30, and 64 months, respectively, indicating that subtype did not have a significant impact on survival after brain metastases [19].
Evaluation of CNS imaging screening and prophylactic cranial irradiation (PCI) at around 20 months in TN breast cancer may be warranted in high risk of subgroups of patients, those could potentially prolong survival of patients [28] and appropriate studies are underway on this issue. Diagnosis of CNS disease at an earlier stage with aggressive use of screening strategies sensitive to small volume brain metastases might be partly responsible for improved outcomes despite of high cost. This question is currently being addressed by a National Cancer Institute Study which randomizes women with Stage IV breast cancer to receive 4 or 12 monthly MRI brain scans to detect early cranial disease [29–31]. PCI is currently being investigated within the HER-PCI Anglo-Celtic VII Trial. Until the results of this are known, there is general reluctance to adopt a policy of PCI, and concern over potential neuro-cognitive effects and cost.
This study had several limitations, including the inclusion of patients at a single center and a retrospective design. Treatment with trastuzumab and lapatinib has been shown to improve the outcomes of patients with HER2+ metastatic breast cancer [32]. Targeted agents, including EGFR, VEGF, and PARP inhibitors, are currently under active investigation and hold promise in the treatment of TN breast cancer, including those with brain metastases.
In summary, patients with the TN phenotype developed brain metastases and distant metastases at other sites earlier and had a shorter OS, despite a higher proportion being initially diagnosed with earlier stage tumors, than patients with other breast cancer phenotypes. Prevention and early detection of brain metastases in patients with TN breast cancer are urgently required to improve survival and quality of life.
References
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Nielsen TO, Hsu FD, Jensen K et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10:5367–5374
Reis-Filho JS, Tutt AN (2008) Triple negative tumours: a critical review. Histopathology 51:108–118
Irvin WJ Jr, Carey LA (2008) What is triple-negative breast cancer? Eur J Cancer 44:2799–2805
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Foulkers WD, Brunet JS, Stefansson IM et al (2004) The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res 64:830–835
Haffty BG, Yang Q, Reiss M et al (2006) Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24:5652–5657
Di Sterano A, Yong Yap Y, Hortobagyi GN et al (1979) The natural history of breast cancer patients with brain metastases. Cancer 44:1913–1918
Fokstuen T, Wilking N, Rutqvist LE et al (2000) Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res Treat 62:211–216
Lee SS, Ahn JH, Kim MK et al (2008) Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat 111:523–530
Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22:3608–3617
Ryberg M, Nielsen D, Osterlind K et al (2005) Predictors of central nervous system metastasis in patients with metastatic breast cancer. A competing risk analysis of 579 patients treated with epirubicin-based chemotherapy. Breast Cancer Res Treat 91:217–225
Kirsch DG, Ledezma CJ, Mathews CS et al (2005) Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol 23:2114–2116
Bendell JC, Domchek SM, Burstein HJ et al (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast cancer. Cancer 97:2972–2977
Church DN, Modgil R, Guglani S et al (2008) Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol 31:250–254
Park IH, Ro J, Lee KS et al (2009) Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol 20:56–62
Reiner A, Reiner G, Spona J et al (1998) Histopathologic characterization of human breast cancer in correlation with estrogen receptor status. A comparison of immunocytochemical and biochemical analysis. Cancer 15:1149–1154
Barnholtz-Sloan JS, Sloan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872
Heitz F, Harter P, Lueck HJ et al (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 45:2792–2798
Tham YL, Sexton K, Kramer R et al (2006) Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 107:696–704
Nam BH, Kim SY, Han HS et al (2008) Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res 10(1):R20. doi:10.1186/bcr1870
Niwinska A, Murawska M, Pogoda K (2010) Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 21:942–948
Kim MJ, Ro JY, Ahn SH et al (2006) Clinicopathologic significance of basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol 37:1217–1226
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Eichler AF, Kuter I, Ryan P et al (2008) Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 112:459–469
Pienkowski T, Zielinski CC (2010) Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol 21:917–924
Clayton AJ, Danson S, Jolly S et al (2004) Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91:639–643
Miller KD, Weathers T, Haney LG et al (2003) Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on survival. Ann Oncol 14:1072–1077
Melisko ME, Glantz M, Rugo HS (2009) New challenges and opportunities in the management of brain metastases in patients with ErbB2-positive metastatic breast cancer. Nat Clin Pract Oncol 6:25–33
Lin NU, Winer EP (2007) Brain metastases: the HER2 paradigm. Clin Cancer Res 13:1648–1655
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 28:2733–2743
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, G., Lee, S.S., Ahn, JH. et al. Clinical features and course of brain metastases in triple-negative breast cancer: comparison with human epidermal growth factor receptor 2-positive and other type at single institution in Korea. Breast Cancer Res Treat 128, 171–177 (2011). https://doi.org/10.1007/s10549-011-1526-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1526-y