Abstract
Purpose
Discordances between the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), expression between primary breast tumors and their subsequent brain metastases (BM) were investigated in breast cancer patients.
Methods
We collected retrospective data from 11 institutions in 8 countries in a predefined-standardized format. Receptor status (positive or negative) was determined according to institutional guidelines (immunohistochemically and/or fluorescence in situ hybridization). The study was subject to each institution’s ethical research committee.
Results
A total of 167 breast cancer patients with BM were included. 25 patients out of 129 with a complete receptor information from both primary tumor and BM (ER, PR, HER2) available, had a change in receptor status: 7 of 26 (27%) ER/PR-positive/HER2-negative primaries (3 gained HER2; 4 lost expression of ER/PR); 10 of 31 (32%) ER/PR-positive/HER2-positive primaries (4 lost ER/PR only; 3 lost HER2 only; 3 lost both ER/PR and HER2); one of 33 (3%) ER/PR-negative receptor/HER2-positive primaries (gained ER); and 7 of 39 (18%) triple-negative primaries (5 gained ER/PR and 2 gained HER2).
Conclusions
The majority of breast cancer patients with BM in this series had primary HER2-enriched tumors, followed by those with a triple-negative profile. One out of 5 patients had a receptor discrepancy between the primary tumor and subsequent BM. Therefore, we advise receptor status assessment of BM in all breast cancer patients with available histology as it may have significant implications for therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second leading cause of brain metastases (BM) after lung cancer, and the leading cause of BM in women [1]. Up to 30% of the patients with progressive breast cancer will develop BM [1, 2], most often within an interval of 1–3 years after initial breast cancer diagnosis [1, 3]. In contrast, less than 0.5% of the patients are diagnosed with BM at time of their primary breast cancer diagnosis [4].
The primary breast cancer molecular subtype [as determined by the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2)] is a significant clinical factor that may predict systemic treatment efficacy and patient’s prognosis [5]. The current recommendations in the metastatic setting are to consider evaluation of receptor phenotype in a metastatic lesion to determine the possible discordance with the primary tumor, as such changes may have clinically relevant significance for treatment decision making [6]. However, there is limited data about the discordance rates between the breast cancer primary and the BM, [7,8,9] and the potential clinical implications of such findings.
A pooled analysis from 48 studies reported a discordance between the primary tumor and systemic metastases rate of 20% for ER [95% confidence interval (CI) 16–35%], 33% for PR (95% CI 29–38%), and 8% for HER2 (95% CI 6–10%). For all ER-, PR-, and HER2-enriched patients, the proportion of tumors shifting from positive expression to negative was higher than the rate of gaining expression of a certain receptor. However, the analysis did not indicate the site of the recurrent/metastatic tumor [10].
In the current study, we performed a multi-institutional data analysis to evaluate the discordance rates for the expression of ER, PR, and HER2 between the primary breast tumor and subsequent BM.
Methods
This was a retrospective study, pooling data collected by 11 institutions in 8 different countries. The centers were initially approached in June 2016, and data collection was completed in November 2016.
The data were collected from medical records of patients with breast cancer who were diagnosed with BM. Only patients for whom the pathology specimens and/or report from both the primary lesion and the BM were available (including evaluation of receptor status) were included in this study. The information was recorded in a predefined-standardized format, without patient identifiers. Each institution was responsible to follow their institutions’ ethical guidelines and gain ethics committee approval for the study.
Data collected included: patient-related factors (e.g., demographics); primary disease-related factors (e.g., histology type, receptor status, proliferation index, and stage at diagnosis); treatment-related factors (e.g., type of systemic therapy, given at diagnosis as well as for metastases); BM-related factors (e.g., date of occurrence, receptor status, proliferation index when available); and evaluation of receptor status from systemic metastases (i.e., extra-cranial) within the 3-month range from evaluation of BM (before or after).
Hormone receptor [HR, estrogen and/or progesterone] status (positive or negative), HER2 overexpression, and proliferation index rate (Ki67) were determined according to institutional guidelines [immunohistochemically (IHC) and/or fluorescence in situ hybridization (FISH)]. The The Mann–Whitney test was used to analyze the differences in the timing from the diagnosis of the primary tumor to develop BM. Bonferroni correction was applied, thus p < 0.025 was considered statistically significant. R version 3.2.3 was used for statistical analyses.
Results
A total of 167 breast cancer patients that developed BM was included. Patients’ demographics and disease-related information are listed in Table 1.
Out of 167 breast cancer patients with BM, 129 patients (77%) had complete receptor information (ER, PR, and HER2) of both primary tumor and BM. Of these, 25 patients (19%) had a change in receptor status (Table 2): 17/129 (58.6%) patients with HR-positive primary had ER/PR change, 11/129 (8.5%) with HER2-positive primary had HER2 change. The proportion of tumors shifting from positive expression to negative was higher than the rate of gaining expression of a certain receptor (13% vs. 8%). Table 3 summarizes loss or gain of receptor for BM compared to the primary tumor in the whole group (n = 167).
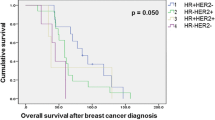
The median time to BM according to the primary tumor subtype was 5.6 years (range 0–13) for HR-positive/HER2-negative; 4.2 years (range 0–17) for HR-positive/HER2-positive; 3 years (range 0–47) for HR-negative/HER2-positive; and 2.3 years (range 0–17) for triple-negative primary breast tumors. These differences were not statistically significant for triple negative (TG) versus all others (p = 0.073), but were found significant for TG or HR negative/HER2 positive versus HR positive/HER2 negative or HR positive/HER2 positive (p = 0.0014).
Median time from diagnosis of primary breast cancer to BM in patients without receptor conversion was 3 years (range 0–47) compared to 2.45 years (range 0.6–17) in patients with conversion (p = 0.31).
Of the total 167 patients, 51 had an available Ki67 index for the primary tumor, of which 44 patients (86%) showed a Ki67 index of > 20% and 7 patients (14%) of < 20%. A total of 36 patients had a Ki67 index for both the primary tumor and the BM. Of these, 29 patients (80.5%) had a Ki67 index > 20% in both primary and BM tumors. Discordance in Ki67 was found in 7 patients: in 5 patients (14%) the primary tumor Ki67 index was < 20% while the BM Ki67 index was > 20%, and in 2 patients (5.5%) the primary tumor Ki67 index was > 20% while the BM Ki67 index was < 20%.
Change in receptor expression in relation to Ki67 index was seen in 5 patients [out of the 29 patients (17%) with both primary and BM with Ki67 > 20%]: 4 had loss of HR and 1 had gain of HR and in 2 patients [out of 5 patients (40%) with higher Ki67 index in BM]: 1 had loss of HR and 1 had gain of HR. None of the patients in which the Ki67 index in the BM was lower than the primary tumor had changes in receptor expression.
Out of 167 patients, five patients (3%) have had an assessment of receptor status from systemic metastases within a 3-month period from the evaluation of BM. Data of full receptor status of the primary tumor, systemic metastases (extra-cranial, within a 3-month period from BM), and BM was available for four patients; one had all 3 receptors in agreement (positive expression of all three receptors in primary, systemic metastases, and BM); one patient had a TN phenotype of the primary and gained HER2 overexpression in both systemic and brain metastasis.
Out of the 25 patients with changes in BM receptor expression, data about systemic therapy changes were available for 15 patients (60%). Of these, for 12 patients (80%), the systemic therapy was modified according to the BM receptor status.
Discussion
Breast cancer is one of the most common cancers and comprises a large fraction of our patients. It is widely accepted that receptor discrepancies between the primary breast tumor and its systemic metastases (extra-cranial) exist, and that it might have significance for treatment decisions [6]. Yet, the molecular phenotype of BM has received little attention in the scientific literature, even though craniotomy/biopsy (therefore accessibility to evaluate the receptor status of the BM) is often part of the diagnosis and treatment of these patients.
Our study represents the largest cohort evaluating discrepancies in biological marker expression of primary breast cancer and subsequent BM. A major finding of our study is that one out of five (20%) patients with breast cancer BM had a receptor discrepancy between the primary tumor and the subsequent BM, with loss of hormone receptors (ER and/or PR) expression, and gain of HER2 overexpression as the most commonly observed changes.
Several studies have reported similar findings with up to a 30% of discordance rate between the primary breast tumor and subsequent BM [2, 8, 9, 11, 12]. Gaining expression of HER2 in the BM was estimated to be in up to 16–18% of the cases [8, 9].
The observation that the common changes are loss of hormone receptor expression and gain of HER2 overexpression in BM compared to the primary tumor was also supported by a recent novel report published by Priedigkeit and colleagues using gene expression testing [7]. In that report, HER2 alteration was the most frequent observed change, showing an at least twofold increase in mRNA expression in BM compared to the primary breast tumor [7]. The authors indicated that there was a robust and significant enrichment of HER2 alterations, specifically in breast cancer BM (24%) and that these changes were not found to be significantly overexpressed in other metastatic sites (13%) [7]. Moreover, the Estrogen Receptor Gene 1 (ESR1) was the most recurrently down-regulated gene, with twofold and fourfold expression decrease in 9 out of 20 samples evaluated [7]. These findings might indicate a clonal selection favoring TN- and HER2-positive cancer cells that preferentially seed the CNS and develop BM [13,14,15].
Similar to other studies, the predominant histological primary tumor in our cohort was invasive ductal carcinoma, and 86% of the primary tumors had high proliferation index (Ki67 > 20%), suggesting an aggressive behavior of highly proliferating primaries [9, 15, 16]. However, our small sample size did not allow us to further analyze whether this finding is important for receptor conversion.
A limitation of our study is that a central review of the specimens was not performed; therefore, some of the reported discordances might be a result of inter- and intra-observer variability in assays between the two sets of samples tested (primary versus metastases). Yet, the rate of such variabilities has been estimated in the literature to be only around 6% [17] and our results were supported by the findings using gene expression testing of BM versus primary breast cancer [7].
It is well-accepted that breast cancer patients are a heterogenous group and that the molecular subtype is an important prognostic and predictive factor. The primary subtype has also significance for the timing of developing BM from primary diagnosis. With shortest time to develop BM seen in the TG and for HR-negative/HER2-positive patients. Moreover, a recent population study showed that the incidence of BM at time of breast cancer diagnosis was highest in these two groups and suggested to consider brain-imaging as screening for this population [4].
Additionally, the primary subtype is a known prognostic for survival in breast cancer patients with BM, and integrated in the graded prognostic assessment [18]. Therefore, we should strive to better understand if discrepancies in receptor expression contribute to these differences in patients’ survival. In some cases, these discrepancies may represent an undiagnosed extra-cranial metastatic phenotype that necessitate treatment modification, while in others, it might explain the unresponsiveness of intracranial disease to systemic therapy. There are numerous preclinical and clinical studies evaluating the activity of systemic therapy for controlling breast cancer BM, with early reports that systemic therapy may contribute to intracranial disease control [19,20,21], therefore, exploring these changes and better understanding resistance pathways may provide clinical benefit for these patients.
At this time, we advise receptor status assessment of BM in all breast cancer patients with available histology as it may have significant implications for therapy. For patients with receptor conversion (e.g., HER2 gain), we advise to consider adapting systemic therapy accordingly. Further studies are needed to understand the clinical implications of these changes and potential treatments.
References
Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G (2011) Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 22(1):1–6 v
Yonemori K, Tsuta K, Shimizu C, Hatanaka Y, Hashizume K, Ono M, Nakanishi Y, Hasegawa T, Miyakita Y, Narita Y et al (2008) Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol 90(2):223–228
Graesslin O, Abdulkarim BS, Coutant C, Huguet F, Gabos Z, Hsu L, Marpeau O, Uzan S, Pusztai L, Strom EA et al (2010) Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 28(12):2032–2037
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA, Alexander BM, Lin NU, Aizer AA (2017) Brain metastases in newly diagnosed breast cancer: A population-based study. JAMA Oncol 3(8):1069–1077
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, HJ Senn, Panel M (2015) Tailoring therapies–improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncology 26(8):1533–1546
Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso MJ et al (2017) 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 28:16–33
Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, Thomas R, Leone JP, Lucas PC, Bhargava R et al (2017) Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol 3(5):666–671
Thomson AH, McGrane J, Mathew J, Palmer J, Hilton DA, Purvis G, Jenkins R (2016) Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br J Cancer 114(7):793–800
Duchnowska R, Dziadziuszko R, Trojanowski T, Mandat T, Och W, Czartoryska-Arlukowicz B, Radecka B, Olszewski W, Szubstarski F, Kozlowski W et al (2012) Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res 14(4):R119
Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, Adamoli L, Munzone E, Sciandivasci A, De Vita F et al (2014) A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer 50(2):277–289
Duchnowska R, Sperinde J, Chenna A, Huang W, Weidler JM, Winslow J, Haddad M, Paquet A, Lie Y, Trojanowski T et al (2015) Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro Oncol 17(9):1241–1249
Bachmann C, Grischke EM, Fehm T, Staebler A, Schittenhelm J, Wallwiener D (2013) CNS metastases of breast cancer show discordant immunohistochemical phenotype compared to primary. J Cancer Res Clin Oncol 139(4):551–556
Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B (2006) Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 24(36):5658–5663
Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A, du Bois A (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 45(16):2792–2798
Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R (2006) Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer 107(4):696–704
Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH (2007) Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol 20(8):864–870
Griggs JJ, Hamilton AS, Schwartz KL, Zhao W, Abrahamse PH, Thomas DG, Jorns JM, Jewell R, Saber ME, Haque R et al (2016) Discordance between original and central laboratories in ER and HER2 results in a diverse, population-based sample. Breast Cancer Res Treat 161:375–384
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J et al (2012) Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 82(5):2111–2117
Cortes J, Rugo HS, Awada A, Twelves C, Perez EA, Im SA, Gomez-Pardo P, Schwartzberg LS, Dieras V, Yardley DA et al (2017) Prolonged survival in patients with breast cancer and a history of brain metastases: results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat 1–3
Zagar TM, Van Swearingen AE, Kaidar-Person O, Ewend MG, Anders CK (2016) Multidisciplinary management of breast cancer brain metastases. Oncology 30(10)
Van Swearingen AED, Sambade MJ, Siegel MB, Sud S, McNeill RS, Bevill SM, Chen X, Bash RE, Mounsey L, Golitz BT, Santos C, Deal A, Parker JS, Rashid N, Miller CR, Johnson GL, Anders CK (2017) Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro Oncol. doi:10.1093/neuonc/nox052
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kaidar-Person, O., Meattini, I., Jain, P. et al. Discrepancies between biomarkers of primary breast cancer and subsequent brain metastases: an international multicenter study. Breast Cancer Res Treat 167, 479–483 (2018). https://doi.org/10.1007/s10549-017-4526-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4526-8