Abstract
Adjuvant endocrine treatment-related adverse effects have a strong impact on patients’ quality of life and thereby limit therapy’s risk benefit ratio resulting in morbidity and treatment discontinuation. Still, many AI adverse effects remain untreated given that they are unrecognized by conservative methods (e.g., proxy ratings). The ability of complementary patient-reported outcomes (PROs) to provide a more comprehensive assessment of side-effects is to be explored. A cross-sectional study sample of 280 postmenopausal, early stage breast cancer patients was subjected to a comprehensive PRO assessment (FACT-B/+ES) at their after-care appointment. Prevalence and severity of patient-reported physical side-effects and psychosocial burden related to adjuvant AI therapy were compared with prevalences derived from pivotal phase IV trials (ATAC 2005, BIG1-98 2005). Across all symptom categories, highest prevalence rates were found for joint pain (59.6%), hot flushes (52%), lost interest in sexual intercourse (51.4%), and lack of energy (40.3%). Overall, PROs resulted in significantly higher prevalence rates as compared to physician ratings for all symptoms published in pivotal clinical trials except vaginal bleeding and nausea. The treatment duration exerted no significant impact on symptom frequency (P > 0.05). Established prevalence rates of endocrine treatment-related toxicity seem to be underestimated. The incorporation of PRO data should be mandatory or at least highly recommended in clinical treatment planning to arrive at a more accurate assessment of a patient’s actual symptom burden enabling improved individualized management of side-effects and mediating the preservation of treatment adherence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Adjuvant endocrine therapy has been proven to significantly reduce recurrence rates in breast cancer patients [1, 2]. Substantial research has shown improved disease-free survival and reduced incidence of contralateral breast cancer under endocrine treatment. To date, after 20 years of gold-standard tamoxifen treatment, aromatase inhibitors (AIs) have become the first choice option in the treatment of postmenopausal breast cancer [3–5].
While efficacy of treatment with AIs has been extensively evaluated, research has focused to a lesser extent on toxicity profiles. The terms toxicity or adverse drug reaction hereby refer to “…a response to a medicine which is noxious and unintended, and which occurs at doses normally used in man” [6].Yet, phases III and IV studies indicate a distinct prevalence of musco-skeletal complaints, arthralgia, fractures, vaginal dryness, loss of libido, dyspareunia, and diarrhea [4, 7–9]. These treatment-related adverse effects have a strong impact on patients’ quality of life (QOL) and limit the therapy’s risk benefit ratio resulting in morbidity and treatment discontinuation [3, 10, 11].
Still many AI adverse effects are underrecognized, underreported and thereafter, are undertreated in breast cancer patients undergoing AI therapy [10]. Available data on toxicities are generally based on clinicians’ impressions and proxy ratings [12]. Although health care providers are supposed to be at the best position to report on toxicity [6], several studies on endocrine strategies in breast cancer treatment [9, 13–15] have shown a poor concordance of expert ratings and patient-reported frequencies for treatment-related non-life threatening symptoms. In the only study to date comparing patient-reported outcomes (PROs) in breast cancer patients receiving endocrine therapy with expert ratings [16], numerous symptoms (e.g., hot flushes/sweats, low energy, and vaginal dryness) have been reported in a significantly higher percentage by the patients than by physicians. There is a lack of prevalence data generated in prospective studies specifically aiming for the assessment of adverse effects in this treatment group. Admission trials alone, given their non-naturalistic setup, might not fully reflect the actual condition of patients. In addition, reported toxicities are frequently incomplete and show great variability in both definition of adverse effects and toxicities as well as the criteria of reporting them if reported at all [17].
The implementation of PROs, a measurement based on a patient’s report about his or her own health condition coming directly from the patient without amendment or interpretation by a clinician or anyone else (e.g., self-report questionnaires such as The Functional Assessment of cancer therapy—FACT G and supplements assessing quality of life) [18], could provide a feasible supplement to common proxy rating-based toxicity evaluation. Enabling a more comprehensive assessment of side-effects, PROs may essentially contribute to medication evaluation and in conjunction, to individualized clinical decision making [12, 19].
Objectives
The objective of this study is to determine prevalence and severity of patient-reported physical side-effects and psychosocial burden (referred to as quality of life impairments) related to adjuvant AI therapy in postmenopausal breast cancer patients. In detail, we aim to address the following research questions:
-
To what degree do breast cancer patients experience QOL impairments related to AI therapy?
-
How concordant is the prevalence in our study compared with prevalences derived from the pivotal phase IV trials?
-
Does age or treatment duration have an impact on the degree of QOL impairment?
This study is part of the comprehensive research project PRO-BETh (PROs in breast cancer patients undergoing endocrine therapy) conducted by the Department of Psychiatry and Psychotherapy in cooperation with the Department of Gynecology and Obstetrics and the Department of Legal Medicine, Innsbruck Medical University, Austria. The PRO-BETh study aims for a multi-method assessment of adherence and QOL among breast cancer patients at all stages of adjuvant endocrine therapy.
Ethical approval for this project was obtained from the Ethics Committee of Innsbruck Medical University.
Patients and methods
Sample
The PRO-BETh was conducted at the out-patient unit of the Department of Gynecology and Obstetrics at Innsbruck Medical University.
Data were derived from the cross-sectional study sample which includes breast cancer patients with the following characteristics:
-
a diagnosis of breast cancer stages I–III
-
upfront adjuvant endocrine therapy with AIs for at least 1 month after primary surgery for breast cancer (no prior tamoxifen)
-
postmenopausal state
-
maximum age of 85 years
-
written informed consent
-
no recurrent or metastatic disease
-
no overt cognitive impairment
-
no previous treatment with anti-hormonal medication.
Procedure
Eligible patients were identified by searching the medical records and were approached at their routine after-care appointments with their breast cancer specialist. Within after-care, breast cancer patients routinely attend the University Clinic of Gynecology and Obstetrics in 3-month interval after-care appointments over a period of 5 years. The patients within all treatment phases (from the start of endocrine treatment to the last day) were included at one of their 3-month-after-care appointments and asked to complete this cross-sectional survey. While waiting for their follow-up medical examinations, the patients were introduced to study details and asked to provide written informed consent. Afterward, the patients completed a comprehensive PRO assessment. Data collection was computerized using a software called “Computer based Health Evaluation System” (CHES) [20]. PRO data were entered by the patients themselves via a Tablet-PC, a study assistant explaining the procedure and supervising the assessment.
Assessment instruments
To evaluate the impact of therapy on patients’ QOL, we administered the “Functional assessment of Cancer therapy—Breast and Endocrine Subscale” (FACT-B/+ES).
Functional assessment of cancer therapy-breast (Fact-B)
The FACT-B is a well validated and widely used self-report questionnaire composed of 36 items assessing QOL in breast cancer patients. It is a multi-dimensional tool covering general QOL domains (FACT-G: physical, emotional, functional, and social well-being) and a specific domain for additional concerns related to breast cancer [21]. The questionnaire employs a five-point-Likert scale and refers to the time-frame of the last 7 days. Response scales range from 0 (not at all) to 4 (very much). Maximum scoring for global well-being ranges from 0 to 108, for emotional well-being 0–24 and for physical, functional and social well-being 0–28. High scores indicate good QOL.
Functional assessment of cancer therapy-endocrine subscale (Fact-ES)
The Fact-B is supplemented by the endocrine subscale (FACT-ES) measuring symptoms and side-effects related to endocrine therapy for breast cancer e.g., hot flushes, vaginal discharge, or loss of libido [22]. The FACT-ES comprises 19 items and applies to the FACT-B regarding format and response scale.
Pivotal trials and studies included for comparison
Trials and studies included for comparison were selected based on comparability regarding patient group and endocrine agent. Two trials [3, 4] and a subprotocol of the ATAC trial [23] were identified as eligible.
ATAC [3]. The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, a double-blind randomized trial, compared 5 years of primary adjuvant treatment with anastrozole alone, tamoxifen alone or the combination of both in 9366 postmenopausal women with localized early breast cancer. Primary objectives were to evaluate the efficacy and tolerability of anastrazole compared with tamoxifen. Prevalence data of side-effects included in our study are derived from analysis of data after a median follow-up of 68 months [3].
In a subprotocol of the ATAC trial [23], prospectively investigated the impact of anastrozole and tamoxifen on health-related QOL of a sub-sample of women enrolled into the trial (n = 1105). QOL data was assessed by means of FACT-B/+ES in the patients receiving anastrazole (n = 335) or tamoxifen (n = 347) at baseline, 3 and 6 months, and every 6 months thereafter, up to and including confirmation of disease recurrence, cessation of trial therapy or withdrawal from the subprotocol.
BIG 1-98 [4]. The Breast International Group (BIG) 1-98 study is a randomized, phase 3, double-blind trial comparing 5 years of treatment with four different adjuvant endocrine therapy regimens (i.e., letrozole, letrozole followed by tamoxifen, tamoxifen, and tamoxifen followed by letrozole) in postmenopausal women with hormone-receptor-positive breast cancer.
Prevalence and frequencies of side-effects found in our study were compared with data from the BIG 1-98 patient group included in the safety analysis (n = 3,975 for letrozole monotherapy). Adverse events were recorded by the checking of specific boxes on the case report forms, grades were determined according to the Common Toxicity Criteria of the National Cancer Institute (version 2.0) or according to criteria defined by a senior oncologist in the protocol otherwise.
Statistical analysis
Sample characteristics are given as percentages, ranges, means, and standard deviations. The prevalence of QOL impairment is presented as percentages and 95% confidence intervals.
We calculated the frequency of each symptom by summarizing percentages of the categories “somewhat”, “quite a bit”, and “very much” on single item level of the FACT-G/+ES. Confidence intervals were calculated using the modified Wald method [24]. Similar to Cella et al. [23], items of the endocrine subscale were grouped as (a) vasomotor symptoms, (b) gynecologic symptoms, (c) gastrointestinal symptoms, (d) pain, and (e) psychological symptoms (see Table 3).
The χ2 test was used to compare symptom frequencies found in this study with those derived from the literature. The impact of age was as well analyzed using the χ2 test. For the purpose of comparison, we defined age groups according to the MA.17 trial (<60 years, 60–69 years, >70) [8]. To investigate the impact of treatment duration on side-effects, treatment duration was grouped into three categories: patients within the 1 and 2 year of treatment, 3–4 and >4 years. These three groups were compared with regard to symptom frequencies by use of the χ2 test. Moreover, group differences between substances (anastrazole vs. letrozole) with regard to toxicity rates were investigated using the χ2 test.
Statistical analyses were performed using the software SPSS (version 15.0).
Results
Patient characteristics
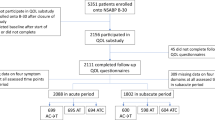
Between June 2009 and May 2010 a total of 280 breast cancer patients were identified as eligible for inclusion in this analysis.
The patients varied in age between 46 to 85 years, with a mean age of 65.3 years. Mean treatment duration was 27.8 months on average. Almost half of the participants (49.4%) has been receiving endocrine therapy for less than 2 years. Anastrazole was the agent most frequently prescribed (83.1%), followed by letrozole. Only 1.1% of patients were taking exemestane.
The most frequent histopathologic cancer type in this study sample was invasive ductal carcinoma and grade II carcinomas. For further details see Tables 1 and 2.
Frequency of symptoms and comparison with pivotal trials
Quality of life and frequency of symptoms
The patients scored high regarding FACT-B global well-being (mean 87.3, SD 13.7), physical well-being (mean 23.9, SD 4.2), emotional well-being (mean 19.4, SD 3.7, functional well-being (mean 21.4, SD 5.0) and social well-being (mean 22.0, SD 5.8). The frequency of endocrine symptoms experienced by this patient population amounted to a mean of 15.8, SD 9.2.
Across all symptom categories, highest prevalence rates for adverse events with AI treatment were found for joint pain (59.6%), hot flushes (52%), lost interest in sexual intercourse (51.4%), and lack of energy (40.3%). Vaginal dryness (31.9%) and breast sensitivity/tenderness (26.4%) were the most prevalent gynecological symptoms. Gastrointestinal symptoms were reported less often with one-third of the patients having stated to suffer from weight gain (33%) presenting as the highest score in this group. Psychological symptoms appeared most frequently as lack of energy (40.3%) and mood swings (36.2%). Table 3 shows details on symptoms and comparative studies.
Frequency of symptoms was not significantly associated with the type of substance prescribed (results not shown). The patients receiving exemestane (1.1%) were excluded for this analysis due to the small sample size.
Comparison with pivotal trials [3, 4]
Overall, PROs resulted in higher prevalence rates as compared to physician ratings for all symptoms published in pivotal clinical trials except for vaginal bleeding (3.3% in BIG 1-98; 5.4% in ATAC; 0.4% in PRO-BETh) and nausea (12.7% in ATAC; 5.3% in PRO-BETh). We found significantly higher prevalence rates of hot flushes, night sweats, joint pain, lack of energy, and mood swings based on the PRO assessment than derived from pivotal trials.
A comparison of the frequencies of adverse events showed agreement on highest prevalence for joint pain (20.3% in BIG 1-98; 35.6% in ATAC) as well as hot flushes (33.5% in BIG 1-98; 37.7% in ATAC). Lack of energy (18.6%) almost equaled mood swings (19.3%) in frequency in the ATAC trial, while lost interest in sexuality was assessed in neither clinical trial. Despite the congruence regarding the most frequent symptoms, their prevalence rates still rank below the scores recorded in our study.
Comparison with ATAC subprotocol [23]
Lower prevalences for 17 out of 19 symptoms were found in the ATAC subprotocol (n = 335) by Cella et al. [23], of which 15 differed significantly. Lack of energy was more than twice as frequent in PRO-BETh study patients (40.3 vs. 15.9%), followed by hot flushes (52 vs. 26.6%) and lost interest in sexual intercourse (51.4 vs. 34%). Analyses revealed a markedly increased prevalence of all psychological symptoms. Sleeping difficulties (16.4% in PRO-BETh vs. 19% in ATAC) and pain or discomfort with sexual intercourse (16% in PRO-BETh vs. 17.3% in ATAC) were the only two symptoms showing a pattern contrary to the overall trend. No reference data could be found for joint pain and some other symptoms.
Impact of age and treatment duration
Comparison of three age groups (<60, 60–69, and 70 years) with respect to symptom burden revealed a significantly higher frequency of vasomotor symptoms, vaginal discharge, vaginal dryness, breast sensitivity, mood swings, and being irritable in the youngest group (Table 4).
Moreover, we investigated the association of treatment duration with levels of side-effects comparing patients in the first and second year of endocrine treatment, in the third to fourth year and with more than 4 years of therapy. Regarding all side-effects, observed differences in symptom prevalences ranged from 0 to 18.3% (median 4.2) and did not reach statistical significance (P > 0.05).
Discussion
Adjuvant endocrine therapy with aromatase inhibitors is considered to exert moderate toxicity and good tolerability. Yet, results on toxicity profiles are derived from proxy ratings in clinical trial settings which might not adequately reflect the toxicity burden experienced by the patients.
Hence, in some contrast to current opinion based large phase III and IV adjuvant studies on AI treatment symptom burden, this large scale PRO study here revealed a higher level of psychosocial burden and physical side-effects in breast cancer patients undergoing endocrine therapy with older patients apparently suffering from significantly less symptoms. Despite the agreement on symptoms with highest prevalence rates (i.e., joint pain, vasomotor symptoms, loss of interest in sexual intercourse) pivotal trials report significantly lower rates [3, 4]. Our results, thereby, confirm the presumption that PROs reveal a higher level of symptom burden than conventional pivotal clinical trials [11, 12, 25, 26].
Ruhstaller et al. [16] reported comparable results in their PRO-based study. Though differing sample characteristics (i.e., treatment with tamoxifen) precluded direct comparison with our study results, their findings support the notion that the use of PROs leads to reporting of increased symptom frequency. Similar findings were obtained by Fellowes et al. [13], who found significant differences between physician-recorded side-effects of endocrine agents with interview-based PROs. Also Basch [12] claimed that established methods for treatment evaluation in clinical trials such as proxy ratings lack sensitivity; e.g., physicians tend to downgrade their patient’s actual symptom burden resulting in deficient toxicity profiles. Moreover, some patients might not report disturbing side-effects to their full extent in routine clinical examinations because of their fear of being perceived as overly needy or their subjective perceptions of the physicians not interested in the matter. In fact, side-effects found most problematic by the patients often differ from those that most concern clinicians [25].
One may, however, hypothesize that presented differences in symptom frequencies between PRO assessments and pivotal trials are not to be solely explained by the use of self-report instruments but also by the impact of the methodologically different features of implemented study designs (as such non-trial designs). This might explain the difference between the reported symptom frequencies in the naturalistic after-care setting of our study and the significantly lower rates registered by Cella et al. [23] who investigated patient-reported side-effects of adjuvant anastrazole therapy in a subprotocol of the ATAC trial. Although randomized-controlled clinical trials (RCTs) provide higher internal validity [27], the generalizability of results from RCTs is limited due to a lack of representativity of the study population and clinical setting [28]. Fraser et al. [29] have highlighted in their community-based population study the problems of applying clinical trial data to a generalized, non-trial population and revealed a distinct difference to the PACS 01 trial results regarding toxicity of a chemotherapeutic regime. Moreover, the patients change their medication intake behavior outside clinical trial settings resulting in divergent outcomes [10].
An implemented study design might reduce the selection bias evident in clinical trials. In this study, a minimal refusal rate of overall 7–10% enabled us to assess a broad cross section of breast cancer cohorts minimizing the risk for a selection bias and, thus, enhancing generalizability of results.
Moreover, direct comparisons for toxicity rates with the ATAC PRO-subprotocol are to be interpreted with caution due to the different substances administered. The sample population in our study received any type of AIs compared to anastrozole as the only substance prescribed in the ATAC subprotocol. We did not find significant group differences between the types of substances prescribed concerning side-effect frequency. This is in accordance with the literature proving the non-steroidal aromatase inhibitors to cause very similar symptom burden.
Findings concerning impact of age on levels of side-effects experienced were consistent with the current literature [8, 30]. Given recent knowledge about the manifestation of adverse effects in association with treatment duration [31, 32], the lack of impact of the latter on side-effect frequency was, however, unexpected. Our differing results may be explained by the cross-sectional design of our study solely allowing comparisons between different duration groups at one assessment time point. A longitudinal study design provides another approach to capture the issue of time dependent symptom prevalence. Baseline-data, shorter time intervals, and a higher frequency of assessments, particularly in the first 2 years, might provide further insight into this issue.
Interpretation of results presented in this article is limited by the fact that an indirect comparison of symptom frequencies with prevalence rates reported in the literature cannot replace a direct comparison of PROs and expert ratings in the same patient population. In addition, lack of baseline-data due to the cross-sectional design prevents us from concluding the observed relationship as a causal one. Nevertheless, this approach illustrates the relevance of the issue in question and demonstrates the need for further research on this matter. Moreover, given the importance of clinical trials in the development of clinical guidelines, it seems necessary to be aware of their limitations regarding reporting of treatment toxicity. Correspondingly, the FDA [18] has recommended the use of PROs as primary endpoints in trials [33] and underscores their relevance to secure labeling claims [34].
To obtain a more accurate picture of the true extent of toxicity caused by adjuvant endocrine therapy, direct comparison of all available treatment strategies within a longitudinal study design should be considered, which, however, requires larger sample size to ensure statistical comparability. Furthermore, the impact of other variables (e.g., co-morbidities, marital status, genetic variables, depression, or menopausal state) on side-effects should be explored in more detail aiming for reevaluation of toxicity profiles in both post- and pre-menopausal women by use of the patient-based instruments.
Regarding clinical practice, our findings emphasize the necessity of increased application of self-report instruments given that outcomes voiced by the patients provide essential information on treatment impact (e.g., pain intensity and pain relief) that are difficult to obtain from other sources [26]. Screening for self-reported data could minimize potential negative consequences of the patient attitudes toward their physician and/or symptom experience and could help identify the patients suffering from side-effects otherwise missed. This is even more important in the light of 33% of breast cancer patients undergoing adjuvant endocrine therapy outside a clinical trial setting are assumed to not to adhere to treatment recommendations over the 5-year treatment period due to symptom burden [10]. This patient non-adherence not only compromises treatment efficacy but diminishes the patient QOL [35–37] and could be mediated by improved side-effect management based on PROs. This entails considering potential barriers to their implementation [12, 38].
In conclusion, according to our findings, established prevalence rates of endocrine treatment-related toxicity seem to be underestimated. Hence, monitoring a patient’s subjective perception of his or her side-effects complementing clinician ratings, may provide a more comprehensive picture of the patient experience. The incorporation of PRO data should be mandatory or at least highly recommended in clinical treatment planning to arrive at a more accurate assessment of a patient’s actual symptom burden enabling improved individualized management of side-effects and mediating preservation of treatment adherence.
References
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467
Jonat W, Gnant M, Boccardo F et al (2006) Effectiveness of switching from adjuvant tamoxifen to anastrazole in postmenopausal women with hormone-sensitive early-stage breast cancer. Lancet Oncol 7:991–996
Howell A, Cuzick J, Baum M et al (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
The Breast International Group 1-98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26):2747–2757
Cuzick J, Sestak I, Baum M et al (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11(12):1135–1141. doi:10.1016/S1470-2045(10)70257-6
World Health Organization (2002) Safety of Medicines. A guide to detecting and reporting adverse drug reactions. Why health professionals need to take action. http://whqlibdoc.who.int/hq/2002/WHO_EDM_QSM_2002.2.pdf. Accessed 19 Oct 2010
The ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Muss HB, Tu D, Ingle JN et al (2008) Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with letrozole or placebo after 5 years of tamoxifen: NCIC CTG Intergroup Trial MA.17. J Clin Oncol 26(12):1956–1964
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350(11):1081–1092
Fallowfield L (2007) Quality of life issues in relation to the aromatase inhibitor. J Steroid Biochem Mol Biol 106:168–172
Cella D, Fallowfield LJ (2008) Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 107:167–180
Basch E (2010) The missing voice of patients in drug-safety reporting. N Engl J Med 362(10):865–869
Fellowes D, Fallowfield LJ, Saunders CM et al (2001) Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments? Breast Cancer Res Treat 66:73–81
Savage C, Pater JL, Tu D et al. (2002) He said/she said: how much agreement is there on symptoms between common toxicity criteria and quality of life? Proc Am Soc Clin Oncol 21(409):1540 (abstr)
Fallowfield LJ (2007) Why patient recorded outcomes should be mandatory in and outside clinical trials to guide management of patients with metastatic breast cancer. Breast Cancer Res 9(2):S7. doi:10.1186/bcr1805
Ruhstaller T, von Moos R, Rufibach K et al (2009) Breast cancer patients on endocrine therapy reveal more symptoms when self-reporting than in pivotal trials: an outcome research study. Oncology 76:142–148
Gibson L, Lawrence D, Dawson C et al. (2009) Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev 4 Art No: CD003370. doi: 10.1002/14651858.CD003370.pub3
US Department of Health and Human Services Food and Drug Administration Guidance for Industry (2009) Patient-reported outcome measures. Use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 22 Oct 2010
Giesinger J, Kemmler G, Meraner V et al (2009) Towards the implementation of quality of life monitoring in daily clinical routine: methodological issues and clinical implication. Breast Care 4:148–154
ESD Inc (2010) E.S.D., Computer-based health evaluation system (CHES). Innsbruck, Austria
Brady MJ, Cella DF, Mo F et al (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15:974–986
Fallowfield LJ, Leaity SK, Howell A et al (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55:189–199
Cella D, Fallowfield L, Barker P et al (2006) Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100:273–284
Vollset SE (1993) Confidence intervals for a binomial proportion. Stat Med 12:809–827
Fallowfield LJ (2008) Treatment-decision making in breast cancer: the patient–doctor relationship. Breast Cancer Res Treat 112:5–13
Marquis P, Arnould B, Acquadro C, Roberts WM (2006) Patient-reported outcomes and health-related quality of life in effectiveness studies: pros and cons. Drug Develop Res 67:193–201
Cole SR, Stuart EA (2010) Generalizing evidence from randomized clinical trials to target populations: the ACTG 320 trial. Am J Epidemiol 172:107–115
Greenhouse JB, Kaizar EE, Kelleher K et al (2008) Generalizing from clinical trial data: a case study. The risk of suicidality among pediatric antidepressant users. Stat Med 27:1801–1813
Fraser J, Steele N, Zaman A, Yule A (2011) Are patients in clinical trials representative of the general population? Dose intensity and toxicities associated with FE100C-D chemotherapy in a non-trial population of node positive breast cancer patients compared with PACS-01 trial group. Eur J Cancer 47:215–220
Freedman OC, Verma S, Clemons MJ (2006) Pre-menopausal breast cancer and aromatase inhibitors: treating a new generation of women. Breast Cancer Res Treat 99:241–247
Fallowfield LJ, Cella D, Cuzick J et al (2004) Quality of life of postmenopausal women in the arimidex, tamoxifen, alone or in combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 22(21):4261–4270
Jin J, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97(1):30–39
Cleeland CS, Sloan JA (2010) Assessing the symptoms of cancer using patient-reported outcomes (ASCPRO): searching for standards. J Pain Symptom Manage 39(6):1077–1085
Doward LC, Gnanasakthy A, Baker MG (2010) Patient reported outcomes: looking beyond the label claim. Health Qual Life Outcomes 8:89
Partridge AH (2006) Non-adherence to endocrine therapy for breast cancer. Ann Oncol 17:183–184
Partridge AH, Avorn J, Wang PS et al (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94(9):652–661
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Snyder CF, Jensen RE, Geller G et al (2010) Relevant content for a patient-reported outcomes questionnaire for use in oncology clinical practice: putting doctors and patients on the same page. Qual Life Res 19:1045–1055
Acknowledgments
We would like to thank Prof. Dr. Wolfgang Fleischhacker from the University Clinic of Biological Psychiatry, Innsbruck Medical University for insightful comments. Monika Sztankay MSc and Anne Oberguggenberger MSc were financially supported by the Innsbruck Leopold-Franzens-University Innsbruck, Austria. This project was partly funded by AstraZeneca and Novartis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Anne Oberguggenberger and Michael Hubalek have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Oberguggenberger, A., Hubalek, M., Sztankay, M. et al. Is the toxicity of adjuvant aromatase inhibitor therapy underestimated? Complementary information from patient-reported outcomes (PROs). Breast Cancer Res Treat 128, 553–561 (2011). https://doi.org/10.1007/s10549-011-1378-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1378-5